Back to Journals » Journal of Pain Research » Volume 16
Spinal Anaesthesia as an Adjunct to General Anaesthesia for Laparoscopic Abdominoperineal Rectal Amputation
Authors Antunes M, Baumgärtel A, Gjessing PF, Ytrebø LM
Received 21 March 2023
Accepted for publication 16 May 2023
Published 31 May 2023 Volume 2023:16 Pages 1855—1865
DOI https://doi.org/10.2147/JPR.S410019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Marisa Antunes,1 Aleksander Baumgärtel,2 Petter Fosse Gjessing,3 Lars Marius Ytrebø1
1Department of Anaesthesiology, University Hospital of North Norway and Institute of Clinical Medicine, Acute and Critical Care Research Group, UiT – the Arctic University of Norway, Tromsø, Norway; 2Institute of Clinical Medicine, Acute and Critical Care Research group, UiT – the Arctic University of Norway, Tromsø, Norway; 3Department of Digestive Surgery, University Hospital of North Norway and Institute of Clinical Medicine, Gastro Surgery Research group, UiT – the Arctic University of Norway, Tromsø, Norway
Correspondence: Lars Marius Ytrebø, Department of Anaesthesiology, University Hospital of North Norway and Acute and Critical Care Research Group, UiT – the Arctic University of Norway, Tromsø, Norway, Tel +47 90788058, Fax +4777626192, Email [email protected]
Background: Spinal anaesthesia as an adjunct to general anaesthesia may reduce postoperative pain and opioid consumption after laparoscopic abdominoperineal rectal amputation. We designed a randomized double blinded pilot study with two objectives: 1) to explore potential benefits of spinal anaesthesia as an adjunct to general anaesthesia and 2) to provide power and sample size estimations for potential differences between the groups. Primary outcome measures were postoperative pain and oral morphine equivalent (OMEq) consumption.
Methods: Patients scheduled for elective laparoscopic abdominoperineal rectal amputation at the University Hospital of North Norway were randomised to spinal (n=5) or a sham spinal procedure (n=5). Numeric rating scale (NRS) and OMEq were monitored postoperatively for 72 h.
Results: Age, sex, body mass index, and ASA were not significantly different between the groups. During surgery, patients in the spinal group received less remifentanil (p=0.06). NRS was lower in the spinal group 1 hr after admittance to the post-anaesthesia care unit (PACU) (p=0.06) and on the first postoperative day at 8 AM (p=0.03). OMEq consumption in the PACU was lower in the spinal group (p=0.008), but no differences between the groups were detected after discharge to the ward. Sample size estimations revealed that eight patients in each group would be needed to study potential NRS differences after admission to the PACU and 23 patients in each group to study potential differences in OMEq consumption on day 1.
Conclusion: Spinal anaesthesia as an adjunct to general anaesthesia reduces postoperative pain and opioid consumption after laparoscopic abdominoperineal rectal amputation. Data from the current study should be followed up by a sufficiently powered randomized controlled trial.
Clinical Trial Registration: Trial registered at https://clinicaltrials.gov (NCT05406765).
Keywords: anesthesiology, opioid consumption, rectal amputation, spinal anesthesia, surgery
Background
Colorectal cancer carries the third highest incidence rate and accounts for 11% of all deaths per year from cancer in Norway.1 Every patient diagnosed with colorectal cancer is considered for surgical treatment with curative or palliative outcome in mind. A multimodal approach for postoperative pain management is fundamental in enhanced recovery after surgery (ERAS) protocols.2 Spinal anaesthesia as an adjunct to general anaesthesia is recommended for laparoscopic colorectal surgery by the ERAS programme.2 However, a beneficial effect has only been studied in patients undergoing laparoscopic colon surgery. Wongyingsinn et al showed that an intrathecal mixture of bupivacaine and morphine was associated with less postoperative opioid consumption after laparoscopic colonic resection.3 In a more recent study by Koning et al, intrathecal morphine within an ERAS program reduced postoperative pain in laparoscopic colonic surgery.4 Spinal anaesthesia as an adjunct to general anaesthesia has to our knowledge not been studied in laparoscopic abdominoperineal rectal amputation. We hypothesized that spinal anaesthesia as an adjunct to general anaesthesia may reduce postoperative pain and opioid consumption. A prospective randomized double blinded pilot study was designed with two objectives: 1) to explore the potential effectiveness of spinal anaesthesia as an adjunct to general anaesthesia and 2) to provide power and sample size estimations for the study of potential differences between the two groups in future clinical trials. Primary outcome measures were postoperative pain and opioid consumption. Secondary outcome measures were postoperative nausea and vomiting (PONV), time to first flatus, and first bowel movement.
Methods
The research protocol was approved by the regional committee for medical and health research ethics (REC 334882) and registered at https://clinicaltrials.gov (NCT05406765). The study complies with the Declaration of Helsinki and was conducted at the University Hospital of North Norway from January to September 2022. It was approved by the Institutional Regulatory Board (Record ID 2805).
Patients scheduled for elective laparoscopic abdominoperineal rectal amputation from January to September 2022 were screened for inclusion by one of the authors (PG). Inclusion criteria were rectal cancer and age 18–100 years. Exclusion criteria were American Society of Anaesthesiologists Classification (ASA) IV, contraindications to spinal anaesthesia, allergy to any of the drugs used in the study protocol, chronic use of opioids or steroids, liver or renal impairment, and patients scheduled for synchronous laparoscopic liver metastatic surgery. Patients in need of extra rectal en bloc resections (ie prostate, seminal vesicles, etc.) and extra wide perineal excision with reconstructive surgery were excluded. Written informed consent was obtained from all patients included.
Included patients were assessed and interviewed by the attending anaesthesiologist (MA) in the preoperative clinic, receiving instructions about the numeric rating scale (NRS) and how to manage the patient-controlled analgesia (PCA) device. Patients were block randomized by one of the authors who did not take part in the treatment of the patients (LMY). The randomization code was put into 10 envelopes and stored outside the operating room. Patients were allocated upon arrival by the attending anaesthesiologist (MA). Only the anaesthetic team was aware of the allocation code, and they were instructed to strictly keep this information to themselves. Accordingly, patients, surgical teams, post-anaesthesia care nurses, nurses on the ward, and the assessor (AB) were all blinded to the actual treatment arm.
Surgical Procedure
All operations were performed using a robot-assisted laparoscopic approach and four laparoscopy-trained colorectal consulting surgeons. Surgery time was defined as the time interval from the start of the first incision made by the surgeon to the completion of the last suture. Pneumoperitoneum was established with a constant pressure of 12 mm Hg. The abdominal part was performed in supine position with total mesorectal excision and high ligation of the inferior mesenteric artery and construction of a colostomy in the left lower abdominal quadrant. After repositioning of the patient in the prone position and after anal closure, trans-perineal rectal amputation was performed including excision of the external anal sphincter followed by three-layer closure of the levator ani muscle, subcutaneous fat, and skin. Antithrombotic stockings were applied, and a urinary bladder catheter was inserted. None of the patients received any abdominal drain or nasogastric tube. Dalteparin 5000 U was administered subcutaneously once daily.
Anaesthesia Procedure
According to ERAS guidelines, all patients received 400 mL of a carbohydrate-loaded drink (ProvideXtra®, Fresenius Kabi, Norway) in the evening before surgery.2 Two hours before induction of anesthesia, all patients received orally paracetamol 1.5 g, doxycycline 400 mg, metronidazole 1.2 g, and 200 mL of a carbohydrate-loaded drink (ProvideXtra®, Fresenius Kabi, Norway).2 Anesthesia was induced and maintained with propofol and remifentanil using a target-controlled infusion protocol (TCI). Rocuronium 0.6–1.2 mg/kg (ideal weight) was given to facilitate tracheal intubation. Standard target-controlled infusion protocols based on weight, height, age and sex were applied. All patients were monitored using bispectral index (BIS) monitors, and anesthesia was adjusted based on BIS data and sound clinical judgement.
Vasoactive medication was given at the discretion of the attending anaesthetist (ie, phenylephrine, ephedrine, and norepinephrine) to maintain mean arterial pressure (MAP) >65 mm Hg. Ringer’s acetate was infused to target zero fluid balance during surgery. Thermal blanket was used to preserve body temperature. Dexamethasone 8 mg was administered after induction of anaesthesia, and 4 mg ondansetron was given intravenously 30 min before emerging from anesthesia. By end of surgery, a total of 20 mL bupivacaine 2.5 mg/mL with adrenalin 5 µg/mL was injected into the laparoscopy ports. Fentanyl 1 µg/kg (ideal weight) was given intravenously 15 min before end of surgery.
Treatment Arms
Patients were randomized to either intrathecal injection of bupivacaine/morphine or a sham spinal procedure. In both cases, patients were placed in an upright sitting position, and the skin over the lumbar region of the back was cleaned with chlorhexidine and draped sterile. For the intrathecal injection, the skin was infiltrated with 5 mL of lidocaine 10 mg/mL. A sterile 27-gauge pencil-point needle (Pajunk, GA, USA) was used to enter the intrathecal space at the L2–3 or L3–4 interspace. After obtaining cerebrospinal fluid, isobaric bupivacaine 5 mg/mL, 3 mL and morphine 200 µg/mL, 0.5 mL, in a total volume of 3.5 mL was injected intrathecally.2 Sensory level was tested before induction of general anaesthesia, and all patients experienced loss of sensation caudal to the Th4-Th6 dermatomes. For the sham procedure, skin was infiltrated with 5 mL of lidocaine 10 mg/mL. After that, the attending anaesthesiologist pressed one finger at the skin and talked as if she was given an intrathecal injection at the L3–4 interspace.
Postoperative Pain Management
Patients in both groups received postoperatively oral paracetamol 1 gr × 4. Morphine 2.5–5 mg was administered intravenously by the post-anaesthesia-care-unit (PACU) nurse if NRS>3. PCA with 3 mg morphine and 15 min lockout was instituted as soon as patients scored NRS<4. The PCA pump did not provide any background infusion of morphine. Droperidol 0.625 mg i.v. served as a rescue PONV medication. Rescue pain medication at the surgical ward was given intravenously as 5 mg morphine if PCA failed to provide NRS<4 at rest. PCA was discontinued after 72 h.
Outcome Measures
The assessor (AB) was blinded for the actual treatment arm and performed all postoperative assessments and patient interviews. The quality of postoperative pain control was assessed using NRS, which was assessed on admission to the PACU and subsequently every hour, while the patients were treated in the PACU. At the surgical ward, NRS and morphine consumption data were collected daily at 8 AM and 4 PM. Intravenous opioid consumption was converted to oral morphine equivalents (OMEq). OMEq day 1 was calculated as opioid consumption after discharge from PACU up to 16:00 on day 1. OMEq day 2 was calculated as opioid consumption from 16:00 on day 1 to 16:00 on day 2. OMEq day 3 was calculated as opioid consumption from 16:00 on day 2 to 16:00 on day 3. The OMEq conversion factor was adapted from Nielsen et al5 1 mg oral morphine=0.5 mg oral oxycodone = 1 OMEq. 1mg morphine i.v. = 3 OMEq.
Statistics
The Mann–Whitney U-test was applied to test for differences between the two groups at pre-specified time points using SPSS version 28.0 (IBM® SPSS statistics®, New York, USA). P≤0.05 was considered significant. Power and sample size calculations were performed in R version 4.1.0 (https://www.r-project.org/). Main objectives for the power and sample size estimations were to calculate the probability of observing the true effect and the probability of type I errors (α, false positive) and type II errors (β, false negative) in the design of future studies.
We used Mann–Whitney U-tests to test whether continuous or discrete variables were significantly different between the spinal and control groups. This non-parametric test was primarily used due to the low number of observations in each group, and we used SPSS version 28.0 (IBM® SPSS statistics®, New York, USA) for these tests. P≤0.05 was considered significant.
Furthermore, using R version 4.1.1, for each variable tested previously we calculated one power table with the package “pwr” and function “pwr.t2n.test”. For each power table, we then calculated the power for different levels of additional patients (from 0 to 20 extra in each sample; ie increment by two) for four different significance levels at 0.1, 0.05, 0.01 and 0.001. We believe these power tables will help future researchers assess how likely they would be to correctly reject a false nil hypothesis for a certain significance level. Unfortunately, for a few variables these power tables could not be computed due to equal means or a standard deviation being equal to zero. We also used SPSS and assuming two-sample t-tests to calculate the exact sample sizes required, assuming 80% power, 5% significance level and observed effect sizes, to detect a significant difference between the two groups.
Results
Patients were consecutively recruited in accordance with the inclusion and exclusion criteria (Figure 1). Two patients did not meet the inclusion criteria because they were in need for an extended abdominoperineal resection. Intraoperative complications and additional procedures occurred in two patients in the spinal group. One was due to perforation during dissection of the rectum. In this patient, the pelvic cavity was immediately rinsed with sterile sodium chloride, hydrogen peroxide, and gentamicin solutions. The other patient received a minimal en block resection of the vaginal wall with primary closure; one patient had additional correction of a small left-flank hernia after abdominal surgery was completed. All patients were treated in accordance with the study protocol, and all data were included in the final analyses.
 |
Figure 1 Consort flow diagram. |
Age, sex, BMI, and ASA were not significantly different between the two groups (Table 1). Patients in the spinal group received less, although not significant, intraoperative remifentanil compared to the control group (Table 2, P=0.06). Intraoperative dose of propofol did not differ between the study groups (P=0.19).
 |
Table 1 Patient Demographics. Median (Range) |
 |
Table 2 Intraoperative Data. Median (Range) |
Pain assessment 1 hour after admittance to the PACU revealed lower NRS scores in the spinal group, but without reaching significance (Figure 2, p=0.06). On average, the patients in the spinal group spent 186 min in the PACU compared to 155 min in the control group (P=0.42). On postoperative day 1, however, NRS at rest was significantly lower in the spinal group at 8 AM (p=0.03), but NRS was not different at subsequent time points (Figure 2). Pain while moving was not different at any time point on day 1–3 (Table 3).
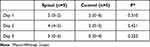 |
Table 3 NRS While Moving at 8 AM on Postoperative Day 1–3. Median (Range) |
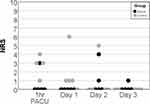 |
Figure 2 Scatter plot of resting numeric rating scale (NRS) 1 hr after admission to the post anaesthesia care unit (PACU) and at 8 AM on subsequent day 1–3. |
OMEq consumption during the PACU stay was lower in the spinal group compared to the control group (p=0.008), but without any differences between the groups, while they were treated at the surgical ward (Figure 3).
 |
Figure 3 Scatter plot showing consumption of oral morphine equivalents (OMEq) in the post anaesthesia care unit (PACU) and at the surgical ward on day 1–3. |
Time to flatus and first bowel movement were not different between the groups, p=1.00 and p=0.31, respectively.
We used the current data set to perform power and sample size estimation for clinically relevant outcome variables. With a power of 0.80 and α=0.05 we would need six patients in each group to study potential differences in total intraoperative remifentanil dose (Table 4), eight patients in each group to study potential differences in NRS 1 hr after admission to the PACU (Table 5) and 23 patients in each group to study potential differences in OMEq consumption on day 1 (Table 6). Furthermore, we would need 631 patients in each group to study potential differences in OMEq consumption on day 2, 1589 patients in each group to study potential differences in OMEq consumption on day 3, and 536 patients in each group to study potential differences in pooled OMEq consumption data for day 1–3 (data not shown).
Discussion
The main findings in this pilot study were that NRS was significantly lower at rest in the spinal group on the first postoperative day and OMEq consumption was significantly lower during PACU admission. However, spinal anaesthesia as an adjunct to general anaesthesia did not reduce pain scores or lower OMEq consumption beyond 24 hr. Power and sample size estimations for remifentanil dose, NRS 1 hr after admittance to the PACU, and OMEq consumption at day 1 indicated that a total of 23 patients in each group would be required to test the nil hypothesis of no differences between the groups for all these variables. Power and sample size estimations for OMEq consumption beyond day 1 indicated that a very large number of patients would be required.
A multimodal approach for pain management is fundamental in all ERAS protocols,2 and effective analgesia without delayed recovery is the primary goal.6 Current ERAS recommendation on the use of spinal as an adjunct to general anaesthesia in colorectal surgery is mainly based on two former studies of patients undergoing laparoscopic colon resection.3,4 In the first study by Wongyingsinn et al, postoperative opioid consumption in the spinal group was significantly less over the first three postoperative days. They also found that the quality of analgesia at rest in the first 24 hr was better in the spinal group. No other advantages over systemic opioids were reported. Konig et al found that patients who received a spinal were earlier fit for discharge, used less opioid, and reported lower pain scores on the first postoperative day. Although patients in both studies were prepared and treated in accordance with an ERAS protocol, only the study by Wongyingsinn et al3 is actually comparable and thus relevant to discuss. Wongyingsinn et al used isobaric bupivacaine 0.5% (10 mg) together with preservative-free morphine for the spinal. The dose of morphine was 200 µg in patients aged ≤75 yr and 150 µg in patients aged >75 yr. The spinal group received oxycodone as a rescue pain medication, while control patients received i.v. morphine delivered via a PCA pump. Both groups received postoperatively oral paracetamol and naproxen for 5 days. Favourable pain scores during the first 24 hr and lower opioid consumption for up to 72 hr may be explained by administration of a higher intrathecal dose of morphine and postoperative prescription of both paracetamol and naproxen. Our data indicate a similar pattern with less pain and reduced opioid consumption during the first 24 hr.
In the current study, both groups received multimodal analgesia consisting of oral paracetamol, intravenous dexamethasone, bupivacaine with adrenaline injected into the laparoscopy ports and intravenous fentanyl administered at the end of surgery. Data show that even control patients reported relatively low pain scores, which means that the current multimodal analgesia works quite well. It is interesting though that we actually were able to demonstrate beneficial effects of spinal analgesia on top of this multimodal analgesia protocol. However, statistical significance does not automatically imply clinically meaningful effects. Only one control patient reported NRS 4 as the worst pain score during PACU admission and another patient reported NRS 6 as the worst pain score on day 1. The remaining control patients reported NRS<4 at all other time points (Figure 2). It is therefore appropriate to question the clinical rational for adding a spinal to general anesthesia in the first place. An alternative strategy could simply be to wait and aggressively treat breakthrough pain in patients who score NRS>3. It would also be relevant to review the current multimodal pain management programme in this context. Postoperative prescription of a non-steroidal inflammatory drug (NSAID) may have further reduced postoperative pain and opioid consumption, yet prescription of NSAIDs after abdominal surgery remains controversial.7,8
Concentration and volume of bupivacaine and morphine is relevant to discuss in relation to analgesic potency of a spinal anaesthetic.9,10 Based on local clinical tradition and experience, we decided to use 3 mL of isobaric bupivacaine 5 mg/mL and 0.5 mL of morphine 200 µg/mL in this study. Clinical assessment revealed adequate dermatome levels of analgesia in all patients allocated to the spinal group. However, different local anaesthetics and concentrations may have given different results. Intrathecal morphine as an adjuvant to a spinal is well-known to increase potency and prolong pain relief.11 A higher intrathecal morphine dose could potentially prolong the time window for adequate pain relief, but the clinical effects of spinal analgesia are known to be highly variable.12 Increased concentrations and volumes may be more effective but should be balanced against the probability of causing unwanted side effects.13,14
This pilot study has several limitations. First, inclusion criteria were rectal cancer and age 18–100 years. Age may be important in this context as pain experience may differ.15,16 Separate cohorts of younger and elderly patients may have given different results. Second, patients in need of extra rectal en bloc resections (ie prostate, seminal vesicles, etc.) and extra wide perineal excision with reconstructive surgery were excluded. More extensive surgery is one factor that may increase postoperative pain,17,18 and the effects of adding a spinal to general anesthesia in that group of patients remain to be studied. Third, this pilot study did not monitor patients beyond 72 hr. The potential impact of spinal analgesia on outcome measures like surgical complications, readmission rate, long-term opioid consumption, morbidity, and mortality are of interest and should be studied in future clinical trials.
In conclusion, spinal anaesthesia as an adjunct to general anaesthesia reduces postoperative pain and opioid consumption after laparoscopic abdominoperineal rectal amputation. Data from the current study should be followed up by a sufficiently powered randomized controlled trial.
Ethics Approval and Consent to Participate
The research protocol was approved by the regional committee for medical and health research ethics (REC 334882) and registered at https://clinicaltrials.gov (NCT05406765). The study was conducted at the University Hospital of North Norway, Tromsø with approval from the Institutional Regulatory Board (Record ID 2805). A written informed consent was obtained before inclusion.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available as individual deidentified participant data. All data will be shared on request for 3 years after the study has been published. Please contact the corresponding author if you would like to receive data from this study.
Institutional Review Board Contact Information
The regional committee for medical and health research ethics. Phone: +4777644000 (switch board) Email: [email protected] Uit- The Arctic University of Norway, 9038 Tromsø, Norway.
Key Messages Regarding Feasibility
- It was uncertain whether patients would benefit from a spinal anesthesia
- Spinal anesthesia as an adjunct to general anesthesia provides better pain control
- Data should be confirmed in a properly powered randomized clinical study
Acknowledgments
Statistician Lars Hindenes is acknowledged for his expert advice on statistical methods. Name of Departments and Institutions to which this work should be attributed:
Department of Anaesthesiology, University Hospital of North Norway and Acute and Critical Care Research Group, UiT – The Arctic University of Norway, Tromsø, Norway.
Author Contributions
All authors met the authorship criteria, read and approved the final version of this manuscript. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study has received departmental funding only.
Disclosure
The authors report no competing interests related to this work.
References
1. Larsen IKMB, Johannesen TB, Robsahm TE, et al. Cancer Incidence, Mortality, Survival and Prevalence in Norway. Oslo: Cancer Registry of Norway; 2022.
2. Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg. 2019;43(3):659–695. doi:10.1007/s00268-018-4844-y
3. Wongyingsinn M, Baldini G, Stein B, Charlebois P, Liberman S, Carli F. Spinal analgesia for laparoscopic colonic resection using an enhanced recovery after surgery programme: better analgesia, but no benefits on postoperative recovery: a randomized controlled trial. Br J Anaesth. 2012;108(5):850–856. doi:10.1093/bja/aes028
4. Koning MV, Teunissen AJW, van der Harst E, Ruijgrok EJ, Stolker RJ. Intrathecal Morphine for Laparoscopic Segmental Colonic Resection as Part of an Enhanced Recovery Protocol: a Randomized Controlled Trial. Reg Anesth Pain Med. 2018;43(2):166–173. doi:10.1097/AAP.0000000000000703
5. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733–737. doi:10.1002/pds.3945
6. Feldheiser A, Aziz O, Baldini G, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60(3):289–334. doi:10.1111/aas.12651
7. Hakkarainen TW, Steele SR, Bastaworous A, et al. Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg. 2015;150(3):223–228. doi:10.1001/jamasurg.2014.2239
8. Modasi A, Pace D, Godwin M, Smith C, Curtis B. NSAID administration post colorectal surgery increases anastomotic leak rate: systematic review/meta-analysis. Surg Endosc. 2019;33(3):879–885. doi:10.1007/s00464-018-6355-1
9. Gwirtz KH, Young JV, Byers RS, et al. The safety and efficacy of intrathecal opioid analgesia for acute postoperative pain: seven years’ experience with 5969 surgical patients at Indiana University Hospital. Anesth Analg. 1999;88(3):599–604. doi:10.1097/00000539-199903000-00026
10. Pirie K, Doane MA, Riedel B, Myles PS. Analgesia for major laparoscopic abdominal surgery: a randomised feasibility trial using intrathecal morphine. Anaesthesia. 2022;77(4):428–437. doi:10.1111/anae.15651
11. Gehling M, Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anaesthesia: a meta-analysis. Anaesthesia. 2009;64(6):643–651. doi:10.1111/j.1365-2044.2008.05817.x
12. Drasner K. Spinal anaesthesia: a century of refinement, and failure is still an option. Br J Anaesth. 2009;102(6):729–730. doi:10.1093/bja/aep085
13. Sultan P, Gutierrez MC, Carvalho B. Neuraxial morphine and respiratory depression: finding the right balance. Drugs. 2011;71(14):1807–1819. doi:10.2165/11596250-000000000-00000
14. Koning MV, Reussien E, Vermeulen BAN, et al. Serious Adverse Events after a Single Shot of Intrathecal Morphine: a Case Series and Systematic Review. Pain Res Manag. 2022;2022:4567192. doi:10.1155/2022/4567192
15. van Dijk JFM, Zaslansky R, van Boekel RLM, et al. Postoperative Pain and Age: a Retrospective Cohort Association Study. Anesthesiology. 2021;135(6):1104–1119. doi:10.1097/ALN.0000000000004000
16. Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. Age changes in pain perception: a systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev. 2017;75:104–113. doi:10.1016/j.neubiorev.2017.01.039
17. Coppes OJM, Yong RJ, Kaye AD, Urman RD. Patient and Surgery-Related Predictors of Acute Postoperative Pain. Curr Pain Headache Rep. 2020;24(4):12. doi:10.1007/s11916-020-0844-3
18. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. doi:10.1097/ALN.0b013e31828866b3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



