Back to Journals » Infection and Drug Resistance » Volume 16
Similarities and Differences Between Diabetes-Related and Trauma-Related Calcaneal Osteomyelitis: Comparisons Based on 681 Reported Cases
Authors Liu GQ, Chen P, Huang MZ, Song MR, Song CS, Zhu RJ, Xiong J, Jiang N , Yu B
Received 26 August 2023
Accepted for publication 29 November 2023
Published 8 December 2023 Volume 2023:16 Pages 7547—7557
DOI https://doi.org/10.2147/IDR.S437211
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Guan-Qiao Liu,1,2,* Peng Chen,1,3,* Mou-Zhang Huang,1,4 Ming-Rui Song,2 Chen-Sheng Song,1 Run-Jiu Zhu,1 Jun Xiong,3 Nan Jiang,1,2 Bin Yu1,2
1Division of Orthopaedics and Traumatology, Department of Orthopaedics, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Bone and Cartilage Regenerative Medicine, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China; 3Department of Orthopaedics, Hainan General Hospital, Hainan Hospital affiliated to Hainan Medical University, Haikou, People’s Republic of China; 4Department of Orthopaedics and Traumatology, Ganzhou Hospital Affiliated to Nanfang Hospital, Southern Medical University, Ganzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Nan Jiang; Bin Yu, Division of Orthopaedics & Traumatology, Department of Orthopaedics, Nanfang Hospital, Southern Medical University, No. 1838, Guangzhou Avenue North, Baiyun District, Guangzhou, 510515, People’s Republic of China, Email [email protected]; [email protected]
Background: Current information were still limited regarding clinical characteristics, diagnosis, and treatment efficacy of calcaneal osteomyelitis (CO). The present study summarized similarities and differences between diabetes-related CO (DRCO) and trauma-related CO (TRCO) based on synthesis analysis of literature-reported cases.
Methods: We searched the PubMed, Embase, and Cochrane Library databases to find English studies reporting DRCO and TRCO published between January 2000 and December 2021. Effective data were extracted and synthesized for comparisons.
Results: Altogether 108 studies with 278 DRCO and 403 TRCO patients were analyzed. The ratio of females among the DRCO patients was significantly higher than that of the TRCO patients (37.4% vs 24.3%, P < 0.001). The median age at diagnosis of the DRCO patients was statistically older than the TRCO patients (56 vs 44 years, P < 0.001). The median symptom duration of the DRCO patients was longer than the TRCO patients (4 vs 2 months, P = 0.136), with ulcer and sinus as the top symptoms for the DRCO and TRCO patients, respectively. The positive rate of pathogen culture for the DRCO patients was significantly higher than that for the TRCO patients (94.8% vs 69.5%, P < 0.001). The DRCO patients had higher risks of infection relapse (32.3% vs 16.3%, P < 0.001) and amputation (24.8% vs 1.4%, P < 0.001), and a higher all-cause mortality (4.9% vs 1.3%, P = 0.03) than the TRCO patients.
Conclusion: DRCO and TRCO shared similar and different clinical features and diagnostic issues. However, compared with TRCO, the clinical efficacy and prognosis of DRCO were worse.
Keywords: calcaneal osteomyelitis, diabetic foot, post-traumatic osteomyelitis, fracture-related infection, synthesis analysis
Introduction
Calcaneal osteomyelitis (CO), defined as the osseous tissue infection of the calcaneus, represents 3% to 11% of all the bone infections.1–3 Despite great advances in surgical techniques, currently, successful treatment of CO is still a great challenge as absolute eradication of infection with limb preservation is always difficult. On the one hand, the anatomic location and function of the calcaneus are unique, with limited soft tissue coverage and poor blood supply.4 In case of infection, such a situation may get even worse, adding the difficulty of treatment. On the other hand, the CO etiology is complex, which often occurs following trauma, orthopaedic surgery, hematogenous spread, and diabetic foot. In addition, pressure sores, nervous system problems, and immunosuppression can also lead to CO.3 Such a wide range of etiology will definitely expand the heterogeneity of this disorder, making it more difficult to treat.5
Currently, surgical intervention remains the mainstay of CO treatment. Nonetheless, clinical efficacy is far from satisfying. According to a systematic review,5 the risk of infection relapse following only bone treatment for CO ranged from 0% to 35%, with the amputation rate ranging from 0% to 29%. The both rates ranged between 0% and 24% even if additional soft tissue coverage was conducted. These data suggest unsatisfactory and poor prognosis of CO. Clinical efficacy of CO is influenced by multiple factors, such as patient age, comorbidities, and the American Society of Anesthesiologists (ASA) score.4 Among these, etiology is one of the most overlooked factors. A previous study6 found that patients diagnosed of chronic osteomyelitis (COM) with different etiologies displayed different outcomes of clinical characteristics and diagnostic indicators. This implies that etiology may be an important objective factor that influencing clinical characteristics, diagnosis and even treatment efficacy.
As aforementioned, although the etiology of CO is complex, diabetes and trauma are the top two causes. Diabetes-related CO (DRCO) and trauma-related CO (TRCO) may share similarities and display differences. However, to the best of our knowledge, there still lacks of such studies focusing on comparing DRCO with TRCO. In order to better recognize the two types of CO, we summarized clinical characteristics, diagnosis and treatment efficacy between DRCO and TRCO patients, based on comparisons of literature-reported cases.
Materials and Methods
Literature Search and Study Registration
A literature search was performed by the two independent authors in the PubMed, Embase and Cochrane Library databases to identify English studies reporting clinical characteristics, diagnosis and treatment efficacy of DRCO and TRCO, published between January 1st, 2000 and December 31st, 2021. The following search term was used: “(“osteomyelitis” OR “osteitis” OR “bone infection” OR “osteoarticular infection”) AND (“calcaneal” OR “calcaneus” OR “heel”)”. This study protocol had been registered in the PROSPERO database with the registration number CRD42022301091.
Inclusion and Exclusion Criteria
As revealed in Table 1, the PICOS principle was applied to display the detailed inclusion and exclusion criteria.
 |
Table 1 Inclusion and Exclusion Criteria of the Current Study: The PICOS Framework |
Study Identification and Data Extraction
Two authors independently screened titles, abstracts, and even full texts to confirm that the recruited studies strictly satisfied the PICOS inclusion criteria. Two authors independently extracted effective data from all the eligible studies. Disagreement was resolved by discussion and when necessary, the corresponding authors’ opinion was consulted for final decision.
Statistical Analysis
Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS) 17.0 software (SPSS Inc., Chicago, IL, USA). Distributions of continuous variables were initially assessed for normality using the Kolmogorov–Smirnov test. Results were presented as mean with range and median with interquartile range (IQR) for normally and abnormally distributed data, respectively. For normally distributed data, Student’s t-test was used to compare differences between the DRCO and the TRCO patients. Otherwise, the Mann–Whitney test was selected. Dichotomous variables were expressed as percentages with events and totals. The chi-square test was used to compare rate differences between the two groups. A statistically significant difference was defined as a P-value ≤ 0.05.
Results
Study Identification and Characteristics
Altogether 1109 potentially relevant studies were identified initially. After removing the duplicates, screening titles, and evaluating the abstracts and/or the full texts, we finally included 108 studies7–114 with 681 patients. There were 53 studies reported 278 DRCO cases and 55 studies with 403 TRCO cases, with 4 studies8,39,44,106 reported DRCO and TRCO at the same time. The eligibility selection process is revealed in Figure 1. The specific data information of the 108 included studies were attached in the supplementary material.
 |
Figure 1 Flow chart of eligibility selection. |
Clinical Characteristics of the Included Patients
Sex Ratio and Age at Diagnosis
Among the included patients, the proportion of females among the DRCO patients was significantly higher than that among the TRCO patients (37.4% vs 24.3%, P = 0.001). In addition, the median age at diagnosis of the DRCO patients was significantly older than that of the TRCO patients (56 years vs 44 years, P < 0.001) (Table 2).
 |
Table 2 Similarities and Differences Regarding Clinical Characteristics, Diagnosis, and Treatment Between DRCO and TRCO Patients |
Infection Duration and Body Side Distribution
The median infection duration of the DRCO patients [median: 4, IQR (2, 6) months] was longer than that of the TRCO patients [median: 2, IQR (1, 8) months], though no statistical difference was found (P = 0.136). Also, no statistical difference was found regarding the body side distribution between the two groups of patients (TRCO patients: left: 140, right: 137, bilateral: 1; DRCO patients: left: 23, right: 20, bilateral: 2) (P = 0.065) (Table 2).
Clinical Symptoms and Microorganism Culture Outcomes
Local ulcer, wound sinus and pain were top three symptoms for the both DRCO and TRCO patients. However, local ulcer (77.5%) was the most frequently reported symptom for the DRCO patients, while it was wound sinus (52.9%) for the TRCO patients. Significant difference was identified regarding the distributions of clinical symptoms between DRCO and TRCO (P < 0.001). To be specific, the proportion of local ulcer was significantly higher, while the percentages of wound sinus, pain, swelling and limited activity were significantly lower among the DRCO patients than those among the TRCO patients (P < 0.05) (Table 2).
The positive rate of pathogen culture of the DRCO patients was significantly higher than that of the TRCO patients (94.8% vs 69.5%, P < 0.001). In addition, the proportion of polymicrobial infections among the DRCO patients was significantly higher than that among the TRCO patients (34.3% vs 18.4%, P = 0.029). Regarding the distributions of detected pathogens for monomicrobial infection, Staphylococcus aureus and Pseudomonas aeruginosa were the top two for both DRCO and TRCO patients. Distributions of pathogens for the DRCO and TRCO patients were displayed in Figure 2.
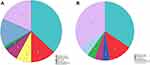 |
Figure 2 Distributions of the pathogens for DRCO (A) and TRCO (B). |
Infection Recurrence, Amputation and All-Cause Mortality
The risk of infection recurrence of the DRCO patients following surgical intervention was significantly higher than that of the TRCO patients (32.3% vs 16.3%, P < 0.001). In addition, the incidence of limb amputation of the DRCO patients was significantly higher than that of the TRCO patients (24.8% vs 1.4%, P < 0.001). Furthermore, the all-cause mortality of the DRCO patients was statistically higher than that of the TRCO patients (4.9% vs 1.3%, P = 0.030). These outcomes suggest that compared with TRCO, clinical efficacy and prognosis of DRCO were worse.
Discussion
Outcomes of the current study demonstrated that DRCO and TRCO shared both similarities and differences with regard to clinical characteristics, diagnosis and treatment efficacy. Compared with TRCO, DRCO displayed unique features, a higher proportion of female patients, an older median age at diagnosis, a longer median symptom duration. Although local ulcer, wound sinus and pain were the top symptoms for both DRCO and TRCO, local ulcer and wound sinus were the most frequently reported symptoms for DRCO and TRCO, respectively. Although the positive rate of culture of DRCO was higher, its efficacy and prognosis were worse, with high incidences of infection relapse, limb amputation, and a higher all-cause mortality, than TRCO. Our findings can be discussed with the following aspects.
First, we found that the proportion of females among the DRCO patients was significantly higher than that among the TRCO patients. Meanwhile, the median age at diagnosis of the DRCO patients was older than that of the TRCO patients. These results were in consistent with our previous study analyzing COM.6 In that cohort, the ratios of the females patients with diabetic-foot OM (DFOM) and post-traumatic OM (PTOM) were 39.1% and 17.2%, with the median ages at diagnosis being 62 years (DFOM) and 38 years (PTOM), respectively. Among the included studies analyzing TRCO, the investigation with the largest sample size (127 patients) was reported by Huang et al,15 who summarized clinical features of TRCO. They concluded that young (mean age: 46.8 years) and males (male/female = 4.47) were dominated, which was in line with our results (TRCO: median age: 44 years, male/female = 3.12). Aside from these, the median infection symptom duration of the DRCO patients was longer than that of the TRCO patients. This is probably because diabetes is a chronic disorder, and DROM is classified as vascular insufficiency-related infection.115,116 These determine that DRCO patients usually experience a long disease term. Quite different from DRCO patients, TRCO patients usually have a wound in the calcaneus before infection occurrence, changes of the wound can be easily noted in case of infection, and thus, the symptom duration of OM is often not long.
Second, we found that the included DRCO and TRCO patients shared similarities and differences in the fields of clinical symptoms and pathogen identification. The top symptoms for both DRCO and TRCO patients were ulcer, sinus and pain. However, the most frequently reported and dominant symptom for DRCO was local ulcer (78%), while sinus was the top among the TRCO patients (53%). The positive rate of culture for DRCO was significantly higher than that for TRCO (95% vs 70%), which revealed similar outcomes with our previous study.6 In that cohort, the positive rates of culture for DFOM and PTOM patients were 91% and 68%, separately. Regarding potential reasons accounting for such differences between DRCO and TRCO, we considered that it may be linked to the fact that the blood supply to the calcaneus of the DRCO patients may be worse, rendering the antibiotics less effective or even ineffective. Meanwhile, persistent ulcer will definitely increase the detection rate of pathogen. As for the detailed types of pathogens identified, the top two bacteria for both DRCO and TRCO were Staphylococcus aureus and Pseudomonas aeruginosa, which agrees with previous studies.6,117 With respect to polymicrobial infection, the percentage of polymicrobial infection of the DRCO patients was statistically higher than that of the TRCO patients, implying the complexity in bacteria species of DRCO.
Third, we found that the treatment efficacy and prognosis of DRCO were worse than TRCO. The overall incidences of infection relapse and limb amputation of the DRCO patients were higher than those of the TRCO patients. Such outcomes may be correlated with several factors. First, the DRCO patients included were relatively older and having diabetes, demonstrating their probably not-so-good immune status. Second, the blood supply to the calcaneus gets even worse in case of infection and diabetes, influencing the efficacy of antibiotics. Third, most of the reported DRCO patients had an ulcer, indicating that the status of their soft tissues was poor. Aside from the above-mentioned factors, infection range, pathogen type and virulence, surgical strategy, and antibiotic selection also affect the treatment efficacy. Previous systematic reviews also analyzed the efficacy of CO. In a 2012 study, Schade VL118 evaluated the efficacy of partial or total calcanectomy for the management of CO. They concluded that such an alternative strategy to amputation was a viable selection for limb salvage. Later in 2019, Sabater-Martos et al5 analyzed surgical treatment outcomes of CO among the adult patients. They found that the risks of infection relapse and amputation ranged from 0% to 35% and 0% to 29% among the studies with bone treatment only. Such reinfection and amputation rates ranged from 0% to 24% if additional soft tissue coverage was needed. These outcomes were in consistent with our findings regarding high rates of infection recurrence and limb amputation of the CO patients.
As for mortality, the DRCO patients suffered from a higher risk of all-cause of mortality than the TRCO patients. However, most of the studies did not mention the detailed death causes. One potential reason for such a higher mortality among the DRCO patients rests with the diabetes itself, which not only means a metabolic-related disorder, but also means increased susceptibilities to other system disorders. A previous study119 found that even COM itself significantly increased the long-term risk of mortality in the elderly. In a recent systematic review and meta-analysis, Yammine et al120 evaluated the efficacy of partial and total calcanectomies for treatment of diabetic heel ulcers with OM. They found that the mortality rate of the calcanectomy was as high as 13.4%. In addition, in an observational and retrospective study, Merlet et al4 analyzed the prognostic factors of CO, and indicated that TRCO is one of the favorable prognostic factors of CO patients. These outcomes support our findings that clinical efficacy and prognosis of DRCO were worse than TRCO.
Our study also has limitations. First, although we included all available studies with different types to lower the risk of selection bias to the minimum, most of the recruited studies were presented as a case report or case series. Thus, the evidence level was not high. Second, the numbers of the DRCO and TRCO patients with available data for analysis were limited, leading to the unavailability of comparisons. Third, we did not evaluate the risk factors for infection recurrence, limb amputation and death, as potentially required data (eg, body mass index, comorbidities, diabetes duration, and detailed surgical strategy with follow-up outcomes) provided by the included studies were also quite limited. Nonetheless, this study provided the useful information on the similarities and differences between DRCO and TRCO, which may help readers better recognize the two types of CO.
Conclusion
In summary, outcomes of the current study demonstrated that, DRCO and TRCO shared similarities and differences regarding clinical characteristics and diagnostic issues. However, compared with TRCO, clinical efficacy and prognosis of DRCO were worse, with higher risks of infection relapse and amputation, and also a higher all-cause mortality. These suggest that DRCO should arouse wide attention and effective preventive measures should be taken to lower the prevalence of DRCO.
Acknowledgments
The authors would like to thank the funding support by the National Natural Science Foundation of China and Joint Program on Health Science & Technology Innovation of Hainan Province.
Funding
This study was supported by the National Natural Science Foundation of China [grant no. 82172197, 82272517] and Joint Program on Health Science & Technology Innovation of Hainan Province [grant no. SO2023WSJK0215].
Disclosure
The authors report no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might conflict with the submitted article.
References
1. Antoniou D, Conner AN. Osteomyelitis of the calcaneus and talus. J Bone Joint Surg Am. 1974;56(2):338–345. doi:10.2106/00004623-197456020-00014
2. Wang EH, Simpson S, Bennet GC. Osteomyelitis of the calcaneum. J Bone Joint Surg Br. 1992;74(6):906–909. doi:10.1302/0301-620x.74b6.1447256
3. McCann MJ, Wells A. Calcaneal Osteomyelitis: current Treatment Concepts. Int J Lower Extremity Wounds. 2020;19(3):230–235. doi:10.1177/1534734619895187
4. Merlet A, Cazanave C, Dauchy FA, et al. Prognostic factors of calcaneal osteomyelitis. Scandinavian j Infect Dis. 2014;46(8):555–560. doi:10.3109/00365548.2014.914241
5. Sabater-Martos M, Sigmund IK, Loizou C, McNally M. Surgical Treatment and Outcomes of Calcaneal Osteomyelitis in Adults: a Systematic Review. J Bone Joint Infection. 2019;4(3):146–154. doi:10.7150/jbji.34452
6. Jiang N, Ma YF, Jiang Y, et al. Clinical Characteristics and Treatment of Extremity Chronic Osteomyelitis in Southern China: a Retrospective Analysis of 394 Consecutive Patients. Medicine. 2015;94(42):e1874. doi:10.1097/MD.0000000000001874
7. Xu L, Song H, Ren Y, et al. Antibiotic-Impregnated Calcium Sulfate vs. Wound Irrigation-Suction to Treat Chronic Calcaneal Osteomyelitis. Foot Ankle Int. 2022;43(3):331–342. doi:10.1177/10711007211049768
8. Pereira PF, Silva MR, Simao RS, Negrao P, Sousa A, Neves N. Total calcanectomy in calcaneal osteomyelitis: an alternative to major amputation. Foot. 2022;51:101896. doi:10.1016/j.foot.2021.101896
9. Tsuihiji K, Daniel BW, Kageyama T, et al. Free tensor fascia lata true-perforator flap transfer for reconstruction of the calcaneal soft tissue defect complicated with osteomyelitis in a patient with alcohol-induced Charcot foot: a case report and literature review. Microsurgery. 2021;41(5):473–479. doi:10.1002/micr.30724
10. Shah S, Nicolau DP, McManus D, Topal JE. A Novel Dosing Strategy of Ceftolozane/Tazobactam in a Patient Receiving Intermittent Hemodialysis. Open Forum Infect Dis. 2021;8(6):ofab238. doi:10.1093/ofid/ofab238
11. Phyo N, Tang W, Kavarthapu V. Medium-term outcomes of multi-disciplinary surgical management of non-ischemic diabetic heel ulcers. J Clin Orthop Trauma. 2021;17:30–36. doi:10.1016/j.jcot.2021.01.012
12. Peng P, Dong ZG, Liu L, Wei JW, Luo Z, Cao S. An Effective Technique for Managing the Calcaneus Osteomyelitis Combined with Soft-Tissue Defect. Int J Lower Extremity. 2021;15347346211016696. doi:10.1177/15347346211016696
13. Liang X, Nong S, Zhou L, Hochwald S, Huang H. Non-Tuberculous Mycobacteria Caused Calcaneal Osteomyelitis after Ankle Local Injection Therapy. Clin Lab. 2021;67. doi:10.7754/Clin.Lab.2020.201143
14. Huchital MJ, Saleh A, Patel R, Subik M. Cancelloplasty for Treatment of Osteomyelitis of the Calcaneus: a Novel Technique and Case Report. Foot Ankle Spec. 2021;14(3):255–265. doi:10.1177/1938640020975885
15. Huang K, Guo Q, Zhu Y. The epidemiology and clinical features of calcaneus osteomyelitis following calcaneus fracture: a retrospective study of 127 cases. Ann Palliative Med. 2021;10(3):3154–3161. doi:10.21037/apm-21-208
16. Capuzzi M, Laco N, Ford T. Novel Technique for the Treatment of a Tongue-Type Calcaneal Fracture in the Setting of Chronic Osteomyelitis. J Am Podiatr Med Assoc. 2021;111. doi:10.7547/20-064
17. Brodell JD, Kozakiewicz LN, Hoffman SL, Oh I. Intraoperative Site Vancomycin Powder Application in Infected Diabetic Heel Ulcers With Calcaneal Osteomyelitis. Foot Ankle Int. 2021;42(3):356–362. doi:10.1177/1071100720962480
18. Whisstock C, Volpe A, Ninkovic S, et al. Multidisciplinary Approach for the Management and Treatment of Diabetic Foot Infections with a Resorbable, Gentamicin-Loaded Bone Graft Substitute. J Clin Med. 2020;9(11):3586. doi:10.3390/jcm9113586
19. Tatsukawa T, Kikuchi S, Tochikubo A, et al. A Case of Chronic Limb-Threatening Ischemia with Heel Ulcers Cured by Revascularization and Partial Calcanectomy. Ann Vasc Dis. 2020;13(1):86–89. doi:10.3400/avd.cr.19-00122
20. Qin CH, Zhou CH, Ren Y, et al. Extensive eggshell-like debridement technique plus antibiotic-loaded calcium sulphate for one-stage treatment of chronic calcaneal osteomyelitis. Foot Ankle Surg. 2020;26(6):644–649. doi:10.1016/j.fas.2019.08.008
21. Nguyen MTT, Ahern NR, Train MK. Staphylococcus schleiferi diabetic foot osteomyelitis and bacteraemia in an immunocompromised host. BMJ Case Rep. 2020;13(11):e238302. doi:10.1136/bcr-2020-238302
22. Jiang N, Zhao XQ, Wang L, Lin QR, Hu YJ, Yu B. Single-stage debridement with implantation of antibiotic-loaded calcium sulphate in 34 cases of localized calcaneal osteomyelitis. Acta Orthop. 2020;91(3):353–359. doi:10.1080/17453674.2020.1745423
23. Alkhatieb M, Mortada H, Aljaaly H. Management of a Difficult-to-Treat Diabetic Foot Wound Complicated by Osteomyelitis: a Case Study. Case Rep Surg. 2020;2020:3971581. doi:10.1155/2020/3971581
24. Wang JS, Gunsch C, Thompson C, Nigam M, Evans KK, Attinger CE. Proximally Based Split Abductor Hallucis Turnover Flap for Medial Hindfoot Reconstruction: a Case Report. J Foot Ankle Surg. 2019;58(6):1072–1076. doi:10.1053/j.jfas.2019.06.004
25. Mohd Khalid SA, Bajuri MY. Unexpected sequelae of plantar fasciitis: iatrogenic calcaneal osteomyelitis following plantar heel injection. Malaysian Family Physician. 2019;14(3):80–83. doi:10.1177/107110079601700903
26. Mata-Ribeiro L, Casal D, Ferreira JA, Costa DS, Lacerda J. The use of free fibula-flexor hallucis longus osteomuscular flap for calcaneal reconstruction after partial calcanectomy for the chronic osteomyelitis: a case report. Int J Surgery Case Rep. 2019;65:213–216. doi:10.1016/j.ijscr.2019.10.046
27. Elmarsafi T, Pierre AJ, Wang K, et al. The Vertical Contour Calcanectomy: an Alternative Surgical Technique to the Conventional Partial Calcanectomy. J Foot Ankle Surg. 2019;58(2):381–386. doi:10.1053/j.jfas.2018.08.040
28. Brucato MP, Wachtler MF, Nasser EM. Osteomyelitis of the Calcaneus With Pathologic Fracture. J Foot Ankle Surg. 2019;58(3):591–595. doi:10.1053/j.jfas.2018.09.016
29. Tuzun HY, Kurklu M, Kulahci Y, Turkkan S, Arsenishvili A. Case Report: late Reconstruction of the Land Mine-Injured Heel With an Osteomyocutaneous Composite Fibular Flap. J Foot Ankle Surg. 2018;57(3):627–631. doi:10.1053/j.jfas.2017.10.032
30. Solanki DMR, Shah DL. Case study of calcaneal osteomyelitis in Type 2. Int J Diabetes Dev Countries. 2018;38:S156. doi:10.1007/s13410-018-0702-6
31. Mikami T, Kaida E, Yabuki Y, Kitamura S, Kokubo K, Maegawa J. Negative Pressure Wound Therapy Followed by Basic Fibroblast Growth Factor Spray as a Recovery Technique in Partial Necrosis of Distally Based Sural Flap for Calcaneal Osteomyelitis: a Case Report. J Foot Ankle Surg. 2018;57(4):816–820. doi:10.1053/j.jfas.2017.11.011
32. Kim Y, Inori F, Yamanaka K, et al. A Case of Osteomyelitis after Calcaneal Fracture Treated by Antibiotic-Containing Calcium Phosphate Cements. Case Rep Orthop. 2018;2018:9321830. doi:10.1155/2018/9321830
33. Drampalos E, Mohammad HR, Kosmidis C, Balal M, Wong J, Pillai A. Single stage treatment of diabetic calcaneal osteomyelitis with an absorbable gentamicin-loaded calcium sulphate/hydroxyapatite biocomposite: the Silo technique. Foot. 2018;34:40–44. doi:10.1016/j.foot.2017.11.011
34. Yaita K, Akiyoshi H, Nakae I, et al. Disseminated Mycobacterium intracellulare infection with multiple abscesses on extremities in a woman with chronic corticosteroid therapy. J Gen Fam Med. 2017;18(6):425–427. doi:10.1002/jgf2.99
35. Karr JC. An Overview of the Percutaneous Antibiotic Delivery Technique for Osteomyelitis Treatment and a Case Study of Calcaneal Osteomyelitis. J Am Podiatr Med Assoc. 2017;107(6):511–515. doi:10.7547/13-047
36. Faleiro TB, de Meirelles AV, Rezende RG, Ferreira HR, Daltro GC, Schulz RDS. Subtotal Calcanectomy for the Treatment of Chronic Ulcer Associated with Osteomyelitis: a Case Report. J Orthop Case Rep. 2017;7(5):71–74. doi:10.13107/jocr.2250-0685.902
37. Akkurt MO, Demirkale I, Öznur A. Partial calcanectomy and Ilizarov external fixation may reduce amputation need in severe diabetic calcaneal ulcers. Diabetic Foot Ankle. 2017;8(1):1264699. doi:10.1080/2000625x.2017.1264699
38. Tural Kara T, Erat T, Ozdemir H, et al. Calcaneus osteomyelitis secondary to Guthrie test: case report. Arch Argent Pediatr. 2016;114(4):e260–263. doi:10.5546/aap.2016.eng.e260
39. Pappalardo M, Jeng SF, Sadigh PL, Shih HS. Versatility of the Free Anterolateral Thigh Flap in the Reconstruction of Large Defects of the Weight-Bearing Foot: a Single-Center Experience with 20 Consecutive Cases. J Reconstructive Microsurgery. 2016;32:562–570. doi:10.1055/s-0036-1584204
40. Nicolosi N, Pratt C. Infectious Spondylodiscitis, Epidural Phlegmon, and Psoas Abscess Complicating Diabetic Foot Infection: a Case Report. J Foot Ankle Surg. 2016;55(2):267–271. doi:10.1053/j.jfas.2014.06.022
41. Mohan R, Gopakumar TS. Clinico-radiological improvement in an immunocompetent patient presented with scedosporium apiospermum osteomyelitis. J Clin Orthop Trauma. 2016;7:134–137. doi:10.1016/j.jcot.2016.02.006
42. Evran M, Sert M, Tetiker T, Akkus G, Bicer OS. Spontaneous calcaneal fracture in patients with diabetic foot ulcer: four cases report and review of literature. World J Clin Cases. 2016;4(7):181–186. doi:10.12998/wjcc.v4.i7.181
43. Dalla Paola L, Carone A, Boscarino G, Scavone G, Vasilache L. Combination of Open Subtotal Calcanectomy and Stabilization With External Fixation as Limb Salvage Procedure in Hindfoot-Infected Diabetic Foot Ulcers. Int J Lower Extremity Wounds. 2016;15(4):332–337. doi:10.1177/1534734616667865
44. Babiak I, Pedzisz P, Kulig M, Janowicz J, Maldyk P. Comparison of Bone Preserving and Radical Surgical Treatment in 32 Cases of Calcaneal Osteomyelitis. J Bone Joint Infection. 2016;1(1):10–16. doi:10.7150/jbji.14342
45. Allen LL, Kalmar G, Driver VR. Treatment of a High-Risk Diabetic Patient with Peripheral Vascular Disease and Osteomyelitis. Tech Vasc Interv Radiol. 2016;19(2):96–100. doi:10.1053/j.tvir.2016.05.001
46. Paisley AN, Grecian SM, Chadwick PJ, Haycocks SJ, Chadwick PR, Young RJ. Characteristics of calcaneal osteomyelitis: an unusual and severe form of diabetic foot disease. Medicine. 2015;32:150. doi:10.1111/dme.12668_1
47. Ocampo-Garza J, Ramos-Jimenez J, Ruiz-Lujan R, et al. Association of botryomycosis and cutaneous and bone coccidioidomycosis of the foot. J Am Acad Dermatology. 2015.
48. Monaco SJ, Pearson K, Wukich DK. Squamous Cell Carcinoma With Chronic Osteomyelitis: a Case Report. Foot Ankle Spec. 2015;8(6):529–531. doi:10.1177/1938640015569766
49. Lin CT, Chen CY, Chen SG, Chen TM, Chang SC. Preserve the lower limb in a patient with calcaneal osteomyelitis and severe occlusive peripheral vascular disease by partial calcanectomy. J Med Sci. 2015;35(2):74–78. doi:10.4103/1011-4564.156016
50. Leclere FM, Casoli V. Reconstruction of a traumatic plantar foot defect with a novel free flap: the medial triceps brachii free flap. J Cosmet Laser Ther. 2015;17(5):286–289. doi:10.3109/14764172.2015.1022188
51. Kim YC, Ahn JH, Kim MS. Infectious Achilles Tendinitis After Local Injection of Human Placental Extracts: a Case Report. J Foot Ankle Surg. 2015;54(6):1193–1196. doi:10.1053/j.jfas.2015.04.028
52. Karns M, Dailey SK, Archdeacon MT. Treatment of Calcaneal Fracture With Severe Soft Tissue Injury and Osteomyelitis: a Case Report. J Foot Ankle Surg. 2015;54(5):973–977. doi:10.1053/j.jfas.2014.05.010
53. Janjua SA, Pastar Z. A case of malum perforans pedis complicated by chronic osteomyelitis. Acta Dermatovenerol Croat. 2015;23(1):72–73.
54. Babamahmoodi F, Shokohi T, Ahangarkani F, Nabili M, Afzalian Ashkezari E, Alinezhad S. Rare Case of Aspergillus ochraceus Osteomyelitis of Calcaneus Bone in a Patient with Diabetic Foot Ulcers. Case Rep Med. 2015;2015:509827. doi:10.1155/2015/509827
55. Waryasz GR, Bariteau JT. Trichophyton rubrum osteomyelitis after calcaneus external fixation pin stabilization of a pilon fracture. J Foot Ankle Surg. 2014;53(4):480–484. doi:10.1053/j.jfas.2014.02.015
56. Wang CY, Sun LY, Chai YM, Han P, Wen G, Chen H. A “hybrid” sural flap for treatment of chronic calcaneal osteomyelitis. J Reconstructive Microsurgery. 2014;30(07):457–462. doi:10.1055/s-0034-1372480
57. Suresh SS, Zaki H, Shalamzari JE, Bhatnagar G. Osteomyelitis calcaneum due to a scorpion sting. J Foot Ankle Surg. 2014;53(3):340–343. doi:10.1053/j.jfas.2014.01.004
58. Shirol SS, Nimbaragi G, Prabhu M, Ratkal J. Abductor digiti minimi muscle flap in reconstruction of diabetic foot ulcers: a case series. Eur J Plastic Surgery. 2014;37(4):227–232. doi:10.1007/s00238-013-0923-3
59. Sayers AE, Bramhall RJ, Akali A. Flap within a flap: the benefit of a musculocutaneous flap over a pure muscle flap. J Plastic Reconstructive Aesthetic Surgery. 2014;67(2):286–287. doi:10.1016/j.bjps.2013.08.010
60. Mills RD, Whitehouse F. Hypoglycemia due to tigecycline therapy for a non-healing foot wound. Endocrine Reviews. 2014;35.
61. Memis A, Mutluoglu M, Ozturk S, Kara K, Ay H. Calcaneal Osteomyelitis Associated With a Severe Abscess. J Am Coll Clin Wound Spec. 2014;6(3):53–56. doi:10.1016/j.jccw.2016.03.002
62. Mastroianni M, Leto Barone AA, Shanmugarajah K, et al. Lower extremity soft tissue defect reconstruction with the serratus anterior flap. Microsurgery. 2014;34(3):183–187. doi:10.1002/micr.22191
63. Lu S, Chai Y, Wang C, Wen G. Complex heel reconstruction with a sural fasciomyocutaneous perforator flap. J Reconstructive Microsurgery. 2014;30:83–90. doi:10.1055/s-0033-1357270
64. Loder BG, Dunn KW. Functional reconstruction of a calcaneal deficit due to osteomyelitis with femoral head allograft and tendon rebalance. Foot. 2014;24(3):149–152. doi:10.1016/j.foot.2014.03.010
65. Iwakura T, Lee SY, Niikura T, et al. Gentamycin-impregnated calcium phosphate cement for calcaneal osteomyelitis: a case report. J Orthop Surg. 2014;22(3):437–439. doi:10.1177/230949901402200335
66. Imirzalioglu C, Sethi S, Schneider C, et al. Distinct polymicrobial populations in a chronic foot ulcer with implications for diagnostics and anti-infective therapy. BMC Res Notes. 2014;7(1):196. doi:10.1186/1756-0500-7-196
67. Hamada Y, Hibino N, Kobayashi A. Expanding the utility of modified vascularized femoral periosteal bone-flaps: an analysis of its form and a comparison with a conventional-bone-graft. J Clin Orthop Trauma. 2014;5(1):6–17. doi:10.1016/j.jcot.2014.01.002
68. Fikri R, Bravis V, Gedroyc WM, et al. Conservative management of neuropathic heel ulceration with calcaneal osteomyelitis and avulsion fracture in a cohort with diabetic foot disease. Diabetologia. 2014;57:S472. doi:10.1007/s00125-014-3355-0
69. Ensat F, Hladik M, Larcher L, Mattiassich G, Wechselberger G. The distally based peroneus brevis muscle flap-clinical series and review of the literature. Microsurgery. 2014;34(3):203–208. doi:10.1002/micr.22172
70. Al-Otaibi FE, Al-Mohizea MM. Non-vertebral Veillonella species septicemia and osteomyelitis in a patient with diabetes: a case report and review of the literature. J Med Case Rep. 2014;8(1):365. doi:10.1186/1752-1947-8-365
71. Yang C, Geng S, Fu C, Sun J, Bi Z. A minimally invasive modified reverse sural adipofascial flap for treating posttraumatic distal tibial and calcaneal osteomyelitis. Int J Lower Extremity Wounds. 2013;12(4):279–285. doi:10.1177/1534734613511637
72. Wang HF, Gao YS, Yuan T, Yu XW, Zhang CQ. Chronic calcaneal osteomyelitis associated with soft-tissue defect could be successfully treated with platelet-rich plasma: a case report. Int Wound J. 2013;10(1):105–109. doi:10.1111/j.1742-481X.2012.00951.x
73. Pietsch C, Osterhoff G, Böni T, Berli M. Surgical treatment of calcaneal osteomyelitis in the diabetic patient. Swiss Medical Weekly. 2013;143:14S.
74. Mounasamy V, Fulco P, Desai P, Adelaar R, Bearman G. The successful use of vancomycin-impregnated cement beads in a patient with vancomycin systemic toxicity: a case report with review of literature. Eur J Orthopaedic Surgery Traumatology. 2013;23 Suppl 2(S2):S299–302. doi:10.1007/s00590-012-1062-4
75. Lykoudis EG, Gantsos A, Dimou AO. Complex calcaneal defect reconstruction with osteotomized free fibula-flexor hallucis longus osteomuscular flap. Microsurgery. 2013;33(1):63–68. doi:10.1002/micr.22053
76. Hoops K, Gran K. A case of concomitant osteomyelitis, sepsis, and meningitis presenting to the pediatric emergency department. J Investigative Med. 2013;61:436.
77. Faglia E, Clerici G, Caminiti M, Vincenzo C, Cetta F. Heel ulcer and blood flow: the importance of the angiosome concept. Int J Lower Extremity Wounds. 2013;12(3):226–230. doi:10.1177/1534734613502043
78. Faglia E, Clerici G, Caminiti M, Curci V, Somalvico F. Influence of osteomyelitis location in the foot of diabetic patients with transtibial amputation. Foot Ankle Int. 2013;34(2):222–227. doi:10.1177/1071100712467436
79. Endo J, Kuniyoshi K, Mochizuki M, et al. Two-staged hindfoot reconstruction with vascularized fibula graft for calcaneal osteomyelitis caused by methicillin-resistant Staphylococcus aureus: a case report. Microsurgery. 2013;33(3):232–235. doi:10.1002/micr.22070
80. Boffeli TJ, Collier RC. Near total calcanectomy with rotational flap closure of large decubitus heel ulcerations complicated by calcaneal osteomyelitis. J Foot Ankle Surg. 2013;52(1):107–112. doi:10.1053/j.jfas.2012.06.018
81. Aragón-Sánchez J, Lázaro-Martínez JL, Quintana-Marrero Y, Sanz-Corbalán I, Hernández-Herrero MJ, Cabrera-Galván JJ. Super-oxidized solution (Dermacyn Wound Care) as adjuvant treatment in the postoperative management of complicated diabetic foot osteomyelitis: preliminary experience in a specialized department. Int J Lower Extremity Wounds. 2013;12(2):130–137. doi:10.1177/1534734613476710
82. Aragón-Sánchez J, Lázaro-Martínez JL, Quintana-Marrero Y, Álvaro-Afonso FJ, Hernández-Herrero MJ. Charcot neuroarthropathy triggered and complicated by osteomyelitis. How limb salvage can be achieved. Diabetic Med. 2013;30(6):e229–232. doi:10.1111/dme.12191
83. Wronka KS, Sinha A. Calcaneal osteomyelitis following steroid injection for plantar fasciitis: a case report. Foot Ankle Spec. 2012;5(4):253–255. doi:10.1177/1938640012451313
84. Ozer K, Kankaya Y, Baris R, Bektas CI, Kocer U. Calcaneal osteomyelitis due to Achromobacter xylosoxidans: a case report. J Infection Chemotherapy. 2012;18(6):915–918. doi:10.1007/s10156-012-0373-z
85. Goudie EB, Gendics C, Lantis JC. Multimodal therapy as an algorithm to limb salvage in diabetic patients with large heel ulcers. Int Wound J. 2012;9(2):132–138. doi:10.1111/j.1742-481X.2011.00869.x
86. Ghods M, Grabs R, Kersten C, Chatzopoulos PP, Geomelas M. A modified free muscle transfer technique to effectively treat chronic and persistent calcaneal osteomyelitis. Ann Plast Surg. 2012;68(6):599–605. doi:10.1097/SAP.0b013e31821ee359
87. Fraccalvieri M, Pristerà G, Zingarelli E, Ruka E, Bruschi S. Treatment of chronic heel osteomyelitis in vasculopathic patients. Can the combined use of Integra, skin graft and negative pressure wound therapy be considered a valid therapeutic approach after partial tangential calcanectomy? Int Wound j. 2012;9(2):214–220. doi:10.1111/j.1742-481X.2011.00878.x
88. Bibbo C, Stough JD. Reduction calcaneoplasty and local muscle rotation flap as a salvage option for calcaneal osteomyelitis with soft tissue defect. J Foot Ankle Surg. 2012;51(3):375–378. doi:10.1053/j.jfas.2011.12.001
89. Temmen TM, Perez J, Smith DJ. Transverse splitting of the gracilis muscle free flap: maximal use of a single muscle. Microsurgery. 2011;31(6):479–483. doi:10.1002/micr.20907
90. Guner S, Ceylan MF, Isik D, Guner SI, Ediz L. A case of wooden foreign body retained in the calcaneus. Pakistan J Med Sci. 2011;27:932–934.
91. Barbour J, Saunders S, Hartsock L, Schimpf D, O’Neill P. Calcaneal reconstruction with free fibular osteocutaneous flap. J Reconstructive Microsurgery. 2011;27(06):343–348. doi:10.1055/s-0031-1278713
92. Pepper M, Willick S, Beals T, Randall RL. An unusual cause of heel pain in a young athlete: a case report. PM R. 2010;2(12):1145–1148. doi:10.1016/j.pmrj.2010.05.016
93. Ignatiadis IA, Tsiampa VA, Arapoglou DK, Georgakopoulos GD, Gerostathopoulos NE, Polyzois VD. Surgical management of a diabetic calcaneal ulceration and osteomyelitis with a partial calcanectomy and a sural neurofasciocutaneous flap. Diabetic Foot Ankle. 2010;1(1):5544. doi:10.3402/dfa.v1i0.5544
94. Fisher TK, Armstrong DG. Partial calcanectomy in high-risk patients with diabetes: use and utility of a “hurricane” incisional approach. Eplasty. 2010;10:e17.
95. Fathinul F, Nordin A. F-FDG PET/CT as a potential valuable adjunct to MRI in characterising the Brodie’s abscess. Biomed Imaging Intervention j. 2010;6(3). doi:10.2349/biij.6.3.e26
96. Xu XY, Zhu Y, Liu JH. Treatment of calcaneal osteomyelitis with free serratus anterior muscle flap transfer. Foot Ankle Int. 2009;30(11):1088–1093. doi:10.3113/FAI.2009.1088
97. Steinberg JS, Meyr AJ, Taylor CR. Limb salvage versus amputation in the setting of an amended calcaneal bone biopsy report. J Foot Ankle Surg. 2009;48(4):518–521. doi:10.1053/j.jfas.2009.04.007
98. Kalinchenko S, Zemlyanoy A, Gooren LJ. Improvement of the diabetic foot upon testosterone administration to hypogonadal men with peripheral arterial disease. Report of three cases. Cardiovascular Diabetol. 2009;8(1):19. doi:10.1186/1475-2840-8-19
99. Freeman A. Bilateral heel ulcers- A complex case for topical negative pressure wound therapy. Repair Regeneration. 2009;17:A56. doi:10.1111/j.1524-475X.2009.00519.x
100. Brinker MR, Loncarich DP, Melissinos EG, O’Connor DP. Calcaneogenesis. J Bone Joint Surg Br. 2009;91(5):662–665. doi:10.1302/0301-620x.91b5.21938
101. Sendi P, Friedl A, Graber P, Zimmerli W. Reactivation of dormant microorganisms following a trauma. Pneumonia, sternal abscess and calcaneus osteomyelitis due to Mycobacterium tuberculosis. Neth J Med. 2008;66(8):363–364.
102. Karr J. Utilization of living bilayered cell therapy (Apligraf) for heel ulcers. Adv Skin Wound Care. 2008;21(6):270–274. doi:10.1097/01.ASW.0000323504.68401.d6
103. Athans W, Stephens H. Open calcaneal fractures in diabetic patients with neuropathy: a report of three cases and literature review. Foot Ankle Int. 2008;29(10):1049–1053. doi:10.3113/FAI.2008.1049
104. Yuksel S, Yuksel G, Oncel S, Divanli E. Osteomyelitis of the calcaneus in the newborn: an ongoing complication of Guthrie test. Eur J Pediatr. 2007;166(5):503–504. doi:10.1007/s00431-006-0268-z
105. Hawley JS, Murray CK, Jorgensen JH. Development of Colistin-Dependent Acinetobacter baumannii-Acinetobacter calcoaceticus Complex. Antimicrob. Agents Chemother. 2007;51(12):4529–4530. doi:10.1128/aac.01115-07
106. Al-Qattan MM. The reverse sural artery fasciomusculocutaneous flap for small lower-limb defects: the use of the gastrocnemius muscle cuff as a plug for small bony defects following debridement of infected/necrotic bone. Ann Plast Surg. 2007;59(3):307–310. doi:10.1097/SAP.0b013e31802e094e
107. Vidyadhara S, Rao SK. Thorn prick osteomyelitis of the foot in barefoot walkers: a report of four cases. J Orthop Surg. 2006;14(2):222–224. doi:10.1177/230949900601400225
108. Schwabegger AH, Shafighi M, Gurunluoglu R. Versatility of the abductor hallucis muscle as a conjoined or distally-based flap. J Trauma. 2005;59(4):1007–1011. doi:10.1097/01.ta.0000187967.15840.15
109. Paik E, Wissman RD. Distinguishing imaging characteristics of the diabetic foot. Infect Dis Clin Practice. 2005;13(6):303–305. doi:10.1097/01.idc.0000189981.77551.7c
110. Kneser U, Bach AD, Polykandriotis E, Kopp J, Horch RE. Delayed reverse sural flap for staged reconstruction of the foot and lower leg. Plastic Reconstructive Surgery. 2005;116(7):1910–1917. doi:10.1097/01.prs.0000189204.71906.c2
111. Chen SL, Chen TM, Chou TD, Chang SC, Wang HJ. Distally based sural fasciomusculocutaneous flap for chronic calcaneal osteomyelitis in diabetic patients. Ann Plast Surg. 2005;54(1):44–48. doi:10.1097/01.sap.0000141377.00807.16
112. Cetinus E, Ciragil P, Uzel M, et al. Calcaneal osteomyelitis after puncture wound to foot: case report and review of the literature. J Orthopaedics Traumatol. 2005;6(4):194–196. doi:10.1007/s10195-005-0108-3
113. Abdelwahab IF, Klein MJ, Hermann G, Abdul-Quader M. Focal tuberculous osteomyelitis of the calcaneus secondary to direct extension from an infected retrocalcaneal bursa. J Am Podiatr Med Assoc. 2005;95(3):285–290. doi:10.7547/0950285
114. Antonio L, Fulvio F. Post-traumatic osteomyelitis of the foot. A report from a developing country in Africa. Ankle Surgery. 2003;9(2):119–122. doi:10.1016/S1268-7731(03)00030-4
115. Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Eng J Med. 1970;282(6):316–322. doi:10.1056/NEJM197002052820606
116. Lew DP, Waldvogel FA. Osteomyelitis. Lancet (London, England). 2004;364(9431):369–379. doi:10.1016/s0140-6736(04)16727-5
117. Wang X, Yu S, Sun D, et al. Current data on extremities chronic osteomyelitis in southwest China: epidemiology, microbiology and therapeutic consequences. Sci Rep. 2017;7(1):16251. doi:10.1038/s41598-017-16337-x
118. Schade VL. Partial or total calcanectomy as an alternative to below-The-knee amputation for limb salvage: a systematic review. J Am Podiatr Med Assoc. 2012;102(5):396–405. doi:10.7547/1020396
119. Huang CC, Tsai KT, Weng SF, et al. Chronic osteomyelitis increases long-term mortality risk in the elderly: a nationwide population-based cohort study. BMC Geriatrics. 2016;16(1):72. doi:10.1186/s12877-016-0248-8
120. Yammine K, El-Alam A, Assi C. Outcomes of partial and total calcanectomies for the treatment of diabetic heel ulcers complicated with osteomyelitis. A systematic review and meta-analysis. Foot Ankle Surg. 2021;27(6):598–605. doi:10.1016/j.fas.2020.07.014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
