Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Sacubitril/Valsartan Improves Progression of Early Diabetic Nephropathy in Rats Through Inhibition of NLRP3 Inflammasome Pathway
Authors Pan Y, Liu L, Yang H, Chen W, Chen Z, Xu J
Received 17 March 2022
Accepted for publication 2 August 2022
Published 13 August 2022 Volume 2022:15 Pages 2479—2488
DOI https://doi.org/10.2147/DMSO.S366518
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Yan Pan, Lei Liu, Huijuan Yang, Weidong Chen, Zheng Chen, Jing Xu
Department of Nephrology, First Affiliated Hospital of Bengbu Medical College, Bengbu City, People’s Republic of China
Correspondence: Yan Pan, Department of Nephrology, First Affiliated Hospital of Bengbu Medical College, No. 287, Changhuai Road, Longzihu District, Bengbu City, Anhui Province, 233000, People’s Republic of China, Tel +86 13865030612, Email [email protected]
Purpose: Diabetic nephropathy (DN), a global disease, is the leading cause of end-stage renal disease. There is a lack of specific treatment for this disease, and early intervention in disease progression is essential. In this paper, we used a rat model of early diabetic nephropathy to explore the therapeutic mechanism of sacubitril/valsartan in rats with early diabetic nephropathy.
Materials and Methods: Rats were grouped into 1 normal group; 2. Model group (DN group): STZ (45 mg/kg/d) induced early diabetic nephropathy rats; 3. Sac group: DN rats + Sac group (orally, 60 mg/kg/d) for 6 weeks. After 6 weeks, the levels of serum albumin (ALB), glucose (GLU), creatinine (Cr), urea nitrogen (BUN) and 24-h urinary protein excretion were measured. In renal tissue homogenates, NLRP3 inflammasome, proinflammatory factors IL1-β and TNF-α, oxidative stress MDA and pro-fibrotic cytokine TGF-β 1 were performed. Histological analysis of kidneys by hematoxylin and eosin (HE), PAS and Masson trichrome staining.
Results: 1. Sacubitril/valsartan (Sac) significantly improved renal hypertrophy, proteinuria and serum albumin levels in rats with early diabetic nephropathy (P < 0.001), and decreased GLU, Scr (P< 0.001), and BUN levels (P < 0.01).2. Light microscopy of renal tissues showed glomerular hypertrophy and interstitial inflammatory cell infiltration, and mean glomerular area (MGA) and mean glomerular volume (MGV) were crucially increased in early diabetic nephropathy (P < 0.001), and the Sac group showed reduced renal pathology and improved MGA and MGV (P < 0.001).3. Kidney tissue homogenate levels of NLRP3, Caspase-1, IL1-β, TNF-α, MDA and TGF-β 1 were critically, increased in DN rats (P < 0.001), and SOD was significantly decreased. All these indicators above were improved after treatment (P < 0.0001).
Conclusion: Nlrp3-inflammasome promote progression of diabetic nephropathy through inflammation, fibrosis and oxidative stress; sacubitril/valsartan ameliorated early diabetes-induced renal damage by inhibiting NLRP3 pathway activation.
Keywords: diabetic nephropathy, Nlrp3-inflammasome, sacubitril/valsartan, oxidative stress, inflammation, fibrosis
Introduction
Diabetic nephropathy (DN) is a common complication of type 2 diabetes mellitus characterised clinically by persistent proteinuria, decreased glomerular filtration rate, elevated blood pressure and increased cardiovascular risk,1 with pathology manifested by glomerular basement membrane thickening, thylakoid expansion, Kimmelstiel-Wilson, lesions and glomerulosclerosis, ultimately leading to tubulointerstitial fibrosis,2,3 with poor clinical outcome and an increased risk of all-cause mortality.4 Early intervention in disease progression is therefore an urgent clinical need. It is widely accepted that glomerular damage is the main pathological change in DN; However, in recent years, researchers have found that interstitial lesions may be an independent factor in DN progression and may occur prior to glomerular lesions,5 with early interstitial lesions including interstitial inflammatory cell infiltration and later stages including tubular atrophy and interstitial fibrosis. Current studies suggest that NLRP3 inflammation-related molecular mechanisms are thought to participate in the development of inflammation and fibrosis and oxidative stress in the diabetic kidney.6–8 In contrast, Sacubitril/Valsartan has been shown to have a better effect in controlling diabetic nephropathy proteinuria, delaying the decline in eGFR and improving renal pathological changes.9–11 In this paper, we explored the biochemical and renal pathological characteristics and the expression of Nlrp3-inflammasome in rats with early diabetic nephropathy, and also explored the possible mechanisms of Sacubitril/Valsartan treatment in rats with early diabetic nephropathy, using existing studies as hypotheses.
Materials and Methods
Animals
Healthy male SD rats, clean grade, about 8 weeks old, body mass 200–220g, were purchased from the Experimental Animal Centre of Bengbu Medical College (experimental animal license number: SCXK (Lu) 20190003). The rats were housed in an animal room with suitable temperature and humidity, and were subjected to a 12-h light and dark cycle, and fed and watered freely. The study was approved by the Ethics Committee of Bengbu Medical College (Ethics No.: Lundeko Approval No. [2020] No. 227).
Experimental Materials
Streptozotocin (STZ) was purchased from Sollerbauer, Sacubitril/Valsartan 100mg (Sacubitril 49mg/Valsartan 51mg) from Novartis Pharma Stein AG, Malondialdehyde (MDA) and Superoxide Dismutase (SOD) assay kits, Hematoxylin Eosin dye were purchased from Shanghai Biyuntian, Trizol was purchased from Invitrogen, Reverse Transcription kits were purchased from Thermo Scientific, RT-PCR kits were purchased from Takara. NLRP3, Caspase-1, IL-1β, TNF-α, TGF-β1 and GAPDH primers were synthesized by Shanghai Bioengineering Company and their sequences are as follow: see Table 1 for details.
 |
Table 1 Primer Sequences Used for RT-PCR Analysis |
Experimental Methods
Rats were adaptively fed for 1 week and randomly divided into normal control (NC) group (n = 10), type 2 diabetes mellitus (T2DM) model group (n = 40), normal group was fed basal diet; T2DM model group was fed high sugar and high fat diet (67% basal diet, 10% lard, 20 sucrose). The T2DM model group was fed with a high sugar and high fat diet (67% basal diet, 10% lard, 20 sucrose, 2.5% cholesterol, 0.5% sodium cholate) and after 4 weeks of feeding, fasting for 12h.. The T2DM model group was given a single intraperitoneal injection of 45 mg/kg of STZ, while the normal group was given an equal dose of citrate buffer intraperitoneally for control. 72 h later, fasting blood glucose (FBG) was collected from the tail vein and fasting blood glucose ≥16.7 mmol/L was used to determine the success of the T2DM model. The rats were divided into Sacubitril/Valsartan group and Diabetic nephropathy (DN) group. The Sac group was given Sacubitril/Valsartan (0.5% sodium carboxymethylcellulose + Sacubitril valsartan agent prepared into a 20mg/mL solution) by gavage at a dose of 60mg/kg/d; the normal group and the T2DM group were gavaged with an equal amount of sodium carboxymethylcellulose solution. After 6 weeks of continuous gavage, the rats were executed and blood, urine and kidney tissue samples were collected.
Body Weight Measurement
Rat weight measurements were arranged on the last day of the experiment, while weight changes were recorded.
Blood and Urine Chemistry Analysis
Serum and urine samples were collected on the last day of the experiment. The detection indicators include ALB, GLU, Scr, BUN and 24-h urinary protein excretion.
Renal Tissue Examination
1. Kidney weight/body weight and light microscopy of kidney tissue glomeruli, interstitium and renal vessels were examined. After execution of the rats, kidney tissues were removed, rinsed twice in pre-cooled PBS and blotted dry on filter paper, weighed for kidney weight (KW) and the kidney hypertrophy index (KW/BW) % was calculated. One kidney tissue was stored in a −80°C refrigerator for subsequent oxidative stress and PT-PCR assays. The other kidney tissue was fixed in 4% paraformaldehyde, embedded in paraffin and sectioned for HE staining, PAS staining, Masson staining and read under the microscope by at least 2 senior pathologists. Twenty glomeruli were taken, with vascular and/or urinary poles in each layer of the section, and magnified 400 times. Glomeruli from 20 randomly selected areas were analysed semi-quantitatively using the Image-Pro Plus image analysis system. Glomerular area (GA) and glomerular volume (GV) were measured and calculated. Five glomerular areas were averaged for this specimen as MGA, and according to the formula, MGV = 1. 25 × [MGA]3 /2.
Two renal tissue PCR assay inflammatory factors (NLRP3, Caspase-1, IL1-β, TGF-β1, TNF-α); oxidative stress (SOD, MDA) mRNA expression: total RNA was extracted from kidney tissue by Trizol reagent. 2µg of total RNA was reverse transcribed into cDNA using a reverse transcription kit using SYBR Green Super Mix kit with 2µL cDNA as template in a total volume of 20µL in a system with the following conditions: pre-denaturation, 95°C, 180s; denaturation: 95°C, 30s; annealing: 60°C, 30s; extension 72°C, 15s, 45 cycles of reaction. Cq values were analysed using a PT-PCR instrument and the relative expression of the target gene was calculated using 2-ΔΔCq.
Statistical Analysis
All data were analyzed and are shown as the mean ± SD from three independent repeats. To compare a normal distribution and a global ANOVA F-test was used. Data did not conform to a normal distribution and a rank-sum test was used. This result is significant at the p = 0.05 level.
Results
Comparison of the General Condition and Biochemical Parameters of the Rats in the Three Groups
In the study, we observe that the rats in the DN group had significantly reduced BW (P < 0.001) and KI was greatly increased (p < 0.001) compared to the NC rats. Rats in the Sac group had increased body weight (P < 0.001) and decreased kidney/body weight ratio (P < 0.001) compared to DN rats. Compared with the NC group, the DN group had low albumin (P < 0.001) and significantly higher UTP (P < 0.001); compared with the DN group, the SAC group had improved high proteinuria (P < 0.001) and low albumin (P < 0.001). Compared with the NC group, the levels of GLU, Scr and BUN in the DN group were importantly higher than those in the NC group (P < 0.001); compared with the DN group, the levels of GLU, Scr and BUN in the Sac group decreased significantly (P < 0.001) and BUN decreased significantly (P < 0.01). The results indicated that urinary protein was significantly increased in diabetic rats, which were considered as an early model of type 2 diabetic kidney. Sac improved renal hypertrophy, proteinuria, serum albumin, glucose metabolism, Scr and BUN levels in early DN rats (see Figure 1 for details).
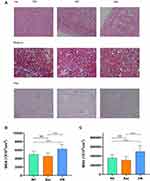
Comparison of Renal Pathological Features in Three Groups of Rats
In our study, HE staining showed that MGA and MGV were obviously larger (P < 0.001) in the DN group compared to the NC group (see Figure 2B and C for details), and the tubulointerstitial inflammatory cells were infiltrated, but no interstitial fibrosis was observed; Masson and PAS staining showed mild thickening of the glomerular basement membrane (see Figure 2A for details). It was demonstrated that Sac could interfere with the early renal pathological changes in DN. To further clarify the mechanistic study, we performed PCR on renal tissues for inflammation, oxidative stress and fibrosis indicators.
Comparison of the Expression Levels of NLRP3-Caspase-1 in the Kidney Tissues of Three Groups of Rats
To further investigate the mechanism of Sac’s role in early DN, we performed NLRP3 inflammatory vesicle assays on kidney tissues from the DN and NC groups. We found that the indexes were significantly higher in the DN group compared to the NC group (P < 0.001), while the indexes decreased in the Sac group (P < 0.001). The results showed that the expression of Nlrp3-inflammasome was significantly increased in the DN group compared to the NC group (p < 0.001), and NLRP3-Caspase-1 plays an important role in the early stage of diabetic nephropathy; the indexes in the Sac group were significantly decreased compared to the DN group (p < 0.001) (see Figure 3 for details).
Comparison of the Expression Levels of Inflammation, Oxidative Stress and Fibrosis Indicators in the Kidney Tissues of Three Groups of Rats
In order to clarify the downstream factors of NLRP3-Caspase-1 pathway, we conducted tests for inflammation, oxidative stress and fibrosis indicators. The study revealed that the expression levels of IL-1β, TNF-a, TGF-β1 and MDA were significantly upregulated in the DN group compared to the NC group (p < 0.001); the expression level of SOD was decreased (p < 0.001). The results suggest that inflammation, oxidative stress and fibrosis play an important role in the early stage of diabetic nephropathy. The expression levels of all indicators improved in the Sac group compared to the DN group (p < 0.001). Sacubitril/Valsartan may play a vital role in improving DN lesions by inhibiting the NLRP3-Caspase-1 pathway and suppressing downstream inflammation, oxidative stress and fibrotic responses (see Figure 3 for details).
Discussion
The inflammasome of pyrrole structural domain 3 (NLRP3) is a multi-protein complex located in the cytoplasm and consists of the pattern recognition receptor NLRP3, apoptosis-associated spot-like protein (ASC) and caspase-1. In response to persistent high glucose stimulation, NLRP3 triggers the active expression of inflammatory factors through caspase-1 effector proteins,12 which in turn are involved in the pathogenesis of DN.13 First developed for use in cardiovascular disease, sacubitril valsartan is a 1:1 ratio of sacubitril and valsartan. The former antagonises enkephalinase, increases natriuretic peptide levels and exerts diuretic and antiproliferative effects;14 the latter acts on the RAAS system, inhibiting vasoconstriction and sympathetic excitation, reducing aldosterone secretion and suppressing inflammatory responses and oxidative stress.15,16 DN is one of the most common end-stage renal diseases, and current studies have found that inflammatory response and oxidative stress play a central role in the pathology of DN,17,18 and controlling these pathological processes can effectively mitigate the progression of DN.19 In the STZ-induced diabetic rat model, members of our group found elevated kidney/body weight ratio, hyperproteinuria, hypoproteinemia, higher glucose, creatinine and urea nitrogen levels; renal pathology highlighted by enlarged glomeruli and interstitial inflammatory cell infiltration; renal tissue was seen with Nlrp3-inflammasome (NLRP3, Caspase-1) and inflammatory markers (IL-1β, TNF-a), oxidative stress (MDA) and fibrogenic factor (TGF-β1) were upregulated. NLRP3 vesicles were considered to be involved in early diabetic nephropathogenesis. In our study, we focused on exploring the therapeutic mechanism of Sac, and found that after 6 weeks of drug intervention, the biochemical indexes and light microscopic features of renal tissues in the intervention group of rats were improved; the expression of renal NLRP3 inflammatory vesicle pathway was down-regulated. The results demonstrated that Sacubitril/Valsartan could improve the renal lesions in rats with early diabetic nephropathy by down-regulating the expression of the above mentioned parameters.
Numerous studies have shown that activation of inflammatory signaling and inflammatory cell infiltration are critical for the development of DN20,21 and that inflammatory factors may be present throughout the pathogenesis of diabetic nephropathy.22 NLRP3 and caspase-1 expression was upregulated in the renal tissues of rats in the DN group, suggesting that NLRP3 inflammasomes are involved in early diabetic kidney injury, consistent with previous in vivo and in vitro studies.23 Nlrp3-inflammasome may trigger multiple inflammatory factors, such as IL-1β and TNF-α.25,26 During the experiment, we observed significantly elevated proteinuria in DN rats with glomerular hyperfiltration manifestations; renal tissue expression of IL-1β and TNF-α was significantly increased, accompanied by glomerular hypertrophy and interstitial inflammatory cell infiltration. The present study demonstrates that diabetes causes increased expression of renal NLRP3 inflammasome, IL-1β and TNF-α, suggesting that NLRP3 inflammasome upregulates IL-1β and TNF-α expression and that the latter two play a crucial role in renal hypertrophy, the levels of which are closely associated with microalbuminuria. This is consistent with previous studies.27,28 IL-1β and TNF-α play an important role in the early progression of DN. In contrast, Sac reduced proteinuria filtration in the intervention group of rats; significantly inhibited the infiltration of inflammatory cells in renal tissue and down-regulated the expression of NLRP3 inflammasome, IL-1β and TNFα. It showed that Sac reduced the expression levels of IL-1β and TNF-α by inhibiting the NLRP3-caspase-1 pathway, thereby reducing proteinuria and creatinine levels, improving renal pathological manifestations and improving the early stage of DN.
So many researchs suggests that oxidative stress and inflammatory responses go hand in the progression of diabetic nephropathy.21,29 Diabetes, as a metabolic disease, can induce excessive reactive oxygen species (ROS) production, which when exceeded by the endogenous antioxidant system, results in a weakened oxidative defence barrier.21 The imbalance of oxidative status in renal tissues is manifested by elevated MDA levels, which is consistent with previous reports.30–32 In our study, we found that DN rats accompanied by upregulation of NLRP3 inflammasome expression exhibited a significant oxidative stress state, such as elevated MDA and reduced antioxidant enzyme SOD, accompanied by altered renal tissues, consistent with previous studies,32 considering the involvement of NLRP3 inflammasomes in the oxidative stress response in glycogenic renal tissues. In contrast, Sac treatment successfully down-regulated MDA expression by down-regulating inflammasome expression, blocking the renal oxidative stress response and reducing renal tissue damage. These results confirm that Sacubitril valsartan can attenuate oxidative stress in hyperglycaemia-induced diabetic nephropathy.
Current studies have found infiltration and fibrosis of inflammatory cells (including monocytes and macrophages) in post-injury renal tissue,33 and inflammatory activity in early renal injury eventually leads to renal fibrosis. Interstitial fibrosis in DN is associated with a variety of inflammatory cytokines, such as TGF-β 1.15,34 Also, NLRP3 was found to upregulate TGF-β1 expression in DN mice,24 which acts as a pro-fibrotic cytokine, accompanied by fibronectin and collagen, and is one of the pathological features of renal fibrosis in DN.35 In vitro and in vivo experiments have demonstrated that by interfering with TGF-β1 expression in renal tissue, tubulointerstitial lesions can be reduced and ultimately DN tubulointerstitial fibrosis can be reduced.36–38 In our experiments, the DN group did not show significant interstitial fibrosis, considering that our experimental period was short and the renal pathology was mainly early histological changes in the glycogen kidney-glomerular hypertrophy and interstitial inflammatory cell infiltration;39 however, renal tissue TGF-β1 expression was upregulated, considering that the inflammatory response in the early stage of kidney injury would contribute to stromal deposition and later progression to interstitial fibrosis.40 In our experiments, we considered that fibrotic factors, such as TGF-β1, are expressed early in DN, and the increase in its expression level may directly promote interstitial fibrosis in late DN. TGF-β1 has also been previously demonstrated to be elevated in urine, serum and glomerular tissue in the early stages of diabetes.41 Upregulation of TGF-β1 has also been reported to correlate directly with the degree of thylakoid expansion and interstitial fibrosis.42 This is consistent with the results of our study. In the present study, a significant decrease in TGF-β1 was seen in renal tissue in the Sac group, suggesting that Sac can down-regulate TGF-β1 expression and may have an ameliorative effect on subsequent renal fibrosis in DN rats.
In this study, we found that early diabetic renal damage was dominated by proteinuria, and renal pathology showed glomerular hypertrophy and interstitial inflammatory cell infiltration, along with inflammation, oxidative stress and fibrotic response. The levels of NLRP3, Caspase-1, IL-1β, TNF-a, MDA and TGFβ1 in the renal tissues of rats with diabetic nephropathy were decreased in the Sac group compared with the DN group. Therefore, Sacubitril valsartan may play a role in improving the renal tissues of rats with diabetic nephropathy by inhibiting the NLRP3 inflammatory vesicle signaling pathway and thus down-regulating the expression of IL-1β, TNF-a, MDA and TGFβ1, inflammatory response, oxidative stress and renal interstitial fibrosis in rats with diabetic nephropathy. However, there are some limitations of our work. The present study demonstrated that Sac could treat DN by inhibiting the NLRP3 inflammasome pathway to improve inflammation, oxidative stress and fibrosis, but experimental limitations prevented us from specifying the cell type of action. The current study suggests that the pathogenesis of DN involves a variety of cell types, including podocytes, glomerular endothelial cells, glomerular thylakoid cells and tubular epithelial cells, and that oxidative stress and inflammation can affect these cells and together contribute to the progression of DN. In the future, we may be able to identify the cells that are affected by high glucose-induced foot cells, glomerular thylakoid cells and renal tubular cells in vitro. In the meantime, members of the team plan to follow up with immunohistochemical examination of the three groups of rats to identify the site of cellular action.
Conclusion
In conclusion, the results of this study suggest that sacubitril valsartan can reduce the hyperfiltration state in DN rats and can improve renal pathological progression. The treatment results indicate that sacubitril valsartan has therapeutic potential to inhibit DN progression by inhibiting the NLRP3 inflammatory vesicle pathway and suppressing the inflammatory response, oxidative stress and fibrotic response in DN renal tissues.
Data Sharing Statement
Data supporting the results of this study are not publicly available and can be obtained from Corresponding author Yan Pan ([email protected]).
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of Bengbu Medical College (Ethics Committee No. [2020] 227) and that the experimental animals and husbandry conditions met the requirements of the Regulations on the Administration of Laboratory Animals and complied with the 3R principles of Laboratory Animal Welfare: Reduction, Substitution, Optimization.
Consent for Publication
All authors agree to the publication of this paper.
Acknowledgment
We are deeply grateful to all participants of this study.
Funding
Natural Science Foundation of Bengbu Medical College Project (BYKY1838ZD)Key Special Project on Translational Medicine, Bengbu Medical College, (BYTM2019038).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Nicola LD, Gabbai FB, Liberti ME, et al. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am J Kidney Dis. 2014;64(1):16–24. doi:10.1053/j.ajkd.2014.02.010
2. Lane PH, Steffes MW, Mauer SM. Renal histologic changes in diabetes mellitus. Semin Nephrol. 1990;10(3):254–259.
3. Eugenia E, Irene A, Meritxell I, et al. Renal biopsy in Type 2 diabetic patients. J Clin Med. 2015;4(5):998–1009. doi:10.3390/jcm4050998
4. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in Type 2 diabetes. Jasn. 2013;24(2):302–308.
5. Shimizu M, Furuichi K, Yokoyama H, et al. Kidney lesions in diabetic patients with normoalbumi- nuric renal insufficiency. Clin Exp Nephrol. 2014;18(2):305–312.
6. Chi HH, Hua KF, Lin YC, et al. IL-36 signaling facilitates activation of the NLRP3 inflammasome and IL-23/IL-17 axis in renal inflammation and fibrosis. J Am Soc Nephrol. 2017;28(7):2022–2037.
7. Krishnan SM, Dowling JK, Ling YH, et al. Inflammasome activity is essential for one kidney/ deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol. 2016;173(4):752–765.
8. Kadoya H, Satoh M, Sasaki T, et al.Excess aldosterone is a critical danger signal for inflammasome activation in the development of renal fibrosis in mice. FASEB J. 2015;29(9):3899–3910.
9. Packer M, Claggett B, Lefkowitz MP, et al. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2018;6(7):547–554.
10. Habibi J, Aroor AR, Das NA, et al. The combination of a neprilysin inhibitor (sacubitril) and angiotensin-II receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker Obese rat. Cardiovasc Diabetol. 2019;18(1):40. doi:10.1186/s12933-019-0847-8
11. Mohany M, Alanazi AZ, Alqahtani F, et al. LCZ696 mitigates diabetic-induced nephropathy through inhibiting oxidative stress, NF-κB mediated inflammation and glomerulosclerosis in rats. PeerJ. 2020;8:e9196. doi:10.7717/peerj.9196
12. Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi:10.1038/s41577-019-0165-0
13. Hutton HL, Ooi JD, Holdsworth SR, et al. NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology. 2016;21(9):736–744. doi:10.1111/nep.12785
14. Muskiet MH, Smits MM, Morsink LM, et al. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol. 2014;10(2):88–103. doi:10.1038/nrneph.2013.272
15. Li Y, Liu J, Liao G, et al. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int J Mol Med. 2018;41(5):2629–2639. doi:10.3892/ijmm.2018.3501
16. Habib HA, Heeba GH, Khalifa MMA. Effect of combined therapy of mesenchymal stem cells with GLP-1 receptor agonist, exenatide, on early-onset nephropathy induced in diabetic rats. Eur J Pharmacol. 2021;892:173721. doi:10.1016/j.ejphar.2020.173721
17. Yaribeygi H, Farrokhi FR, Rezaee R, et al. Oxidative stress induces renal failure: a review of possible molecular pathways. J Cell Biochem. 2018;119(4):2990–2998. doi:10.1002/jcb.26450
18. Rayego-Mateos S, Morgado-Pascual JL, Opazo-Ríos L, et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int J Mol Sci. 2020;21(11):3798. doi:10.3390/ijms21113798
19. Pickering RJ, Rosado CJ, Sharma A, et al. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin Transl Immunol. 2018;7(4):e1016. doi:10.1002/cti2.1016
20. Gnudi L, Coward RJM, Long DA. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab. 2016;27(11):820–830. doi:10.1016/j.tem.2016.07.002
21. Jha JC, Banal C, Chow BS, et al. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25(12):657–684. doi:10.1089/ars.2016.6664
22. Lampropoulou IT, Stangou Μ, Sarafidis P, et al. TNF-α pathway and T-cell immunity are activated early during the development of diabetic nephropathy in Type II diabetes mellitus. Clin Immunol. 2020;215:108423. doi:10.1016/j.clim.2020.108423
23. Zhang C, Zhu X, Li L, et al. A small molecule inhibitor MCC950 ameliorates kidney injury in diabetic nephropathy by inhibiting NLRP3 inflammasome activation. Diabetes Metab Syndr Obes. 2019;12:1297–1309. doi:10.2147/DMSO.S199802
24. Wu M, Han W, Song S, et al. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol Cell Endocrinol. 2018;478:115–125. doi:10.1016/j.mce.2018.08.002
25. Peiró T, Patel DF, Akthar S, et al. Neutrophils drive alveolar macrophage IL-1β release during respiratory viral infection. Thorax. 2018;73(6):546–556.
26. Zhang QB, Zhu D, Dai F, et al. MicroRNA-223 suppresses IL-1β and TNF-α production in gouty inflammation by targeting the NLRP3 inflammasome. Front Pharmacol. 2021;12:637415. doi:10.3389/fphar.2021.637415
27. Aleissa MS, Alkahtani S, Abd Eldaim MA, et al. Fucoidan ameliorates oxidative stress, inflammation, DNA damage, and hepatorenal injuries in diabetic rats intoxicated with Aflatoxin B1. Oxid Med Cell Longev. 2020;2020:9316751. doi:10.1155/2020/9316751
28. Zhang Y. MiR-92d-3p suppresses the progression of diabetic nephropathy renal fibrosis by inhibiting the C3/HMGB1/TGF-β1 pathway. Biosci Rep. 2021;41(9):BSR20203131. doi:10.1042/BSR20203131
29. Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol. 2016;12(1):13–26.
30. Liu H, Chen W, Lu P, et al. Ginsenoside Rg1 attenuates the inflammation and oxidative stress induced by diabetic nephropathy through regulating the PI3K/AKT/FOXO3 pathway. Ann Transl Med. 2021;9(24):1789.
31. Ma L, Wu F, Shao Q, et al. Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des Devel Ther. 2021;15:3207–3221.
32. Sun Z, Ma Y, Chen F, et al. Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-κB/NLRP3 inflammasome pathway. Chem Biol Interact. 2018;293:11–19.
33. Lu H, Bai Y, Wu L, et al. Inhibition of macrophage migration inhibitory factor protects against inflammation and matrix deposition in kidney tissues after injury. Mediators Inflamm. 2016;2016:2174682.
34. Bai Y, Wang J, He Z, et al. Mesenchymal stem cells reverse diabetic nephropathy disease via lipoxin A4 by targeting transforming growth factor β (TGF-β)/smad pathway and pro-inflammatory cytokines. Med Sci Monit. 2019;25:3069–3076.
35. Wang B, Jha JC, Hagiwara S, et al. Transforming growth factor-β1-mediated renal fibrosis is dependent on the regulation of transforming growth factor receptor 1 expression by let-7b. Kidney Int. 2014;85(2):352–361.
36. Zheng W, Qian C, Xu F, et al. Fuxin Granules ameliorate diabetic nephropathy in db/db mice through TGF-β1/Smad and VEGF/VEGFR2 signaling pathways. Biomed Pharmacother. 2021;141:111806.
37. Xu J, Xiang P, Liu L, et al. Metformin inhibits extracellular matrix accumulation, inflammation and proliferation of mesangial cells in diabetic nephropathy by regulating H19/miR-143-3p/TGF-β1 axis. J Pharm Pharmacol. 2020;72(8):1101–1109.
38. Sun Z, Ma Y, Chen F, et al. miR-133b and miR-199b knockdown attenuate TGF-β1-induced epithelial to mesenchymal transition and renal fibrosis by targeting SIRT1 in diabetic nephropathy. Eur J Pharmacol. 2018;837:96–104.
39. Pourghasem M, Shafi H, Babazadeh Z. Histological changes of kidney in diabetic nephropathy. Caspian J Intern Med. 2015;6(3):120–127.
40. Sean Eardley K, Cockwell P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005;68(2):437–455.
41. Poczatek MH, Hugo C, Darley-Usmar V, et al. Glucose stimulation of transforming growth factor-beta bioactivity in mesangial cells is mediated by thrombospondin-1. Am J Pathol. 2000;157(4):1353–1363.
42. Miao XJ, Bi TT, Tang JM, et al. Regulatory mechanism of TGF-β1/SGK1 pathway in tubulointerstitial fibrosis of diabetic nephropathy. Eur Rev Med Pharmacol Sci. 2019;23(23):10482–10488.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.