Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Risk Assessment of Acute Myocardial Infarction and Stroke Associated with Long-Acting Muscarinic Antagonists, Alone or in Combination, versus Long-Acting beta2-Agonists
Authors Rebordosa C, Plana E , Rubino A, Aguado J, Martinez D, Lei A, Daoud S, Saigi-Morgui N, Perez-Gutthann S , Rivero-Ferrer E
Received 9 March 2022
Accepted for publication 21 July 2022
Published 2 August 2022 Volume 2022:17 Pages 1715—1733
DOI https://doi.org/10.2147/COPD.S363997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Cristina Rebordosa,1 Estel Plana,2 Annalisa Rubino,3 Jaume Aguado,2 David Martinez,2 Alejhandra Lei,4 Sami Daoud,5 Nuria Saigi-Morgui,1 Susana Perez-Gutthann,1 Elena Rivero-Ferrer1
1Department of Epidemiology and Risk Management, RTI Health Solutions, Barcelona, Spain; 2Department of Biometrics, RTI Health Solutions, Barcelona, Spain; 3Epidemiology, Respiratory and Immunology, AstraZeneca, Cambridge, UK; 4Patient Safety Biopharma, AstraZeneca, Barcelona, Spain; 5BioPharmaceuticals Research and Development, Late-Stage Development Respiratory and Immunology, AstraZeneca, Gaithersburg, MD, USA
Correspondence: Cristina Rebordosa, RTI Health Solutions, Department of Epidemiology and Risk Management, Av. Diagonal, 605, 9-1, Barcelona, 08028, Spain, Tel +34.93.362.2807, Fax +34.93.760.8507, Email [email protected]
Background: The long-acting muscarinic antagonist (LAMA) aclidinium was approved in Europe in 2012 to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). A post-authorization safety study was initiated to assess potential cardiovascular risks associated with LAMAs versus long-acting beta2-agonists.
Purpose: To estimate incidence rates and adjusted incidence rate ratios (IRRs) for acute myocardial infarction (AMI), stroke, and major adverse cardiac events (MACE) in new users of aclidinium, aclidinium/formoterol, tiotropium, other LAMA, long-acting beta-agonists/inhaled corticosteroids (LABA/ICS), and LAMA/LABA compared with initiators of LABA.
Patients and Methods: This population-based cohort study included patients with COPD aged ≥ 40 years initiating COPD medications in the UK Clinical Practice Research Datalink (CPRD) Aurum database from 2012 to 2019. Poisson regression models were used to estimate the IRR for AMI, stroke, and MACE in users of COPD medications versus LABA, adjusting for clinically relevant covariables.
Results: The study included 11,121 new users of aclidinium, 4804 of aclidinium/formoterol, 56,198 of tiotropium, 23,856 of other LAMA, 17,450 of LAMA/LABA, 70,289 of LABA/ICS, and 13,716 of LABA. During periods of continuous medication use after initiation (current use), crude incidence rates per 1000 person-years for AMI ranged from 8.7 (aclidinium/formoterol) to 12.4 (LAMA/LABA), for stroke ranged from 4.8 (aclidinium/formoterol) to 7.2 (LAMA/LABA), and for MACE ranged from 13.5 (aclidinium/formoterol) to 19.3 (LAMA/LABA). Using LABA as reference, adjusted IRRs [95% confidence intervals] were close to 1 for all study drugs for AMI (lowest for aclidinium/formoterol, 0.95 [0.60– 1.52], and highest for LAMA/LABA, 1.23 [0.91– 1.67]), stroke (lowest for aclidinium/formoterol, 0.64 [0.39– 1.06], and highest for tiotropium, 1.02 [0.81– 1.27] for tiotropium) and for MACE (lowest for aclidinium, 0.93 [0.75– 1.16], and highest for LAMA/LABA, 1.24 [0.97– 1.59]).
Conclusion: Risks of AMI, stroke, and MACE in current users of aclidinium, aclidinium/formoterol, tiotropium, other LAMA, LAMA/LABA, or LABA/ICS were similar to the risks among current users of LABA.
Keywords: aclidinium, acute myocardial infarction, LAMA, stroke, United Kingdom
Introduction
Long-acting muscarinic antagonists (LAMAs) have been shown to be effective and safe in the treatment of chronic obstructive pulmonary disease (COPD) and are recommended in international clinical guidelines for symptom management for patients with COPD.1 While some concerns about the cardiovascular safety of the LAMAs tiotropium and ipratropium bromide emerged from prior studies,2–7 and from a further meta-analysis by Singh et al,8 this evidence was inconsistent and described as having potential biases. Disparate results of the UPLIFT trial, which included a large sample size and long follow-up and was described as methodologically strong,9 led the US Food and Drug Administration to conclude that the data did not support the conclusion that there is an increased risk of stroke, heart attack, or death associated with tiotropium bromide inhalation powder (HandiHaler).10 However, some authors have suggested that imbalance in rates of supraventricular tachyarrhythmias and strokes related to ischemia in the UPLIFT are suggestive of potential pro-ischemic and pro-arrhythmic effects.11
Aclidinium is a LAMA, and, as such, the evolution of its potential pro-ischemic and pro-arrhythmic effects leading to cardiovascular events is of interest and part of its risk management program.12,13 This post-authorization safety study (PASS) was conducted to assess the potential cardiovascular risks described in the European risk management plans of both aclidinium bromide as monotherapy (Eklira) and aclidinium bromide/formoterol fixed-dose combination (Duaklir; aclidinium bromide/formoterol fumarate dihydrate; marketing authorization holder: AstraZeneca AB, Södertälje, Sweden). A companion study program was conducted to evaluate utilization patterns for aclidinium.14 The aclidinium cardiovascular PASS and its protocol were registered at the EU PAS Register on 27 May 2016 with the registry identification number EUPAS13616 (http://www.encepp.eu/encepp/viewResource.htm?id=18823). Earlier substudies conducted as part of this PASS found no increased risk of all-cause mortality15 or hospitalization for congestive heart failure16 in new users of aclidinium, tiotropium, other LAMA, LAMA/LABA, or LABA/ICS compared with LABA.
Here, we report the results of the substudy in which we aimed to compare the risk of acute myocardial infarction (AMI), stroke, or major adverse cardiac events (MACE; a composite endpoint of first occurrence of AMI, stroke, or community coronary heart disease [CHD] or cerebrovascular disease [CeVD] death) in patients with COPD initiating treatment with aclidinium bromide and other selected COPD medications with the risk in patients with COPD initiating treatment with long-acting beta2-agonists (LABAs).
Materials and Methods
Study Design and Setting
This was a non-interventional, population-based cohort study of adult patients aged ≥40 years with a recorded diagnosis of COPD initiating treatment with aclidinium bromide monotherapy or other selected COPD medications identified in the Clinical Practice Research Datalink (CPRD) in the United Kingdom (UK). The Independent Scientific Advisory Committee (ISAC) of the Medicines and Healthcare products Regulatory Agency (MHRA) approved the protocol (protocol 19_200R) on 18 February 2020. The RTI International Institutional Review Board (IRB) reviewed the protocol and determined that the study did not constitute research involving human subjects. The IRB determined that because this study did not involve private, identifiable, human-subjects data nor interaction with any human subjects, informed consent was not needed. None of the members of the research team had access to identifying patient information when analyzing the data. The study design and eligibility criteria for the study cohorts are shown in Figure 1.
 |
Figure 1 Overview of study design and eligibility criteria for the study cohorts. Abbreviations: AMI, acute myocardial infarction; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; ICS, inhaled corticosteroid; IMD, Index of Multiple Deprivation; LABA, long-acting beta2-agonist; LAMA, long-acting anticholinergic; LTRA, leukotriene receptor antagonists; MACE, major adverse cardiovascular events; Rx, prescription; SABA, short-acting beta2-agonist; SAMA, short-acting muscarinic antagonist. Notes: aOther COPD medications: aclidinium/formoterol, tiotropium, other LAMA (glycopyrronium bromide, umeclidinium), LAMA/LABA (glycopyrronium/indacaterol, umeclidinium/vilanterol, and tiotropium/olodaterol), LABA (formoterol, salmeterol, indacaterol, olodaterol), LABA/ICS (formoterol/budesonide, formoterol/beclometasone, formoterol/fluticasone, salmeterol/fluticasone propionate, vilanterol/fluticasone). bCriterion A (1) patients with any of the following non-cardiovascular life-threatening conditions recorded in the database at any time before or on the start date: HIV (human immunodeficiency virus) infection or use of antiretroviral agents for HIV, organ transplant, or congenital cardiovascular anomalies; (2) patients with any of the following non-cardiovascular life-threatening conditions recorded in the database within 10 years before or on the start date: cancer (except nonmelanoma skin cancers), palliative care, respiratory failure or dependence on respiratory ventilation, end-stage renal disease, drug or alcohol abuse, or coma. cCriterion B patients with missing information on smoking history or body mass index. dAsthma, diabetes, hypertension, hyperlipidemia, CHD, AMI, unstable angina, chronic angina, aortocoronary bypass graft, cerebrovascular disease, stroke, transient ischemic attack, peripheral vascular disease, heart failure, outpatient diagnosis of heart failure, hospitalization for heart failure, arrhythmias, atrial fibrillation, severe ventricular arrhythmias, other arrhythmias, pulmonary embolisms, pneumonia, renal disease, liver disorders, and Charlson Comorbidity Index. eRespiratory medications (LAMA/LABA/ICS, SABA, oral glucocorticosteroids, mucolytics, antihistamines, ICS, SAMA, LTRA, omalizumab, cough and cold preparations) and non-respiratory medications (antibiotics, cardiovascular medications, lipid-lowering drugs, antihypertensive medications, antiarrhythmics, nitrates, digoxin, anticoagulants, platelet aggregation inhibitors, antidiabetics, and vaccines). fEarliest of endpoint of interest (AMI, stroke, MACE), death, disenrollment from the practice, or end of the study period. Source: Original design diagram template can be found at www.repeatinitiative.org/projects.html. |
The study was conducted in the UK using data from the CPRD Aurum database and includes routinely collected de-identified data from primary care practices.17 The validity of CPRD as a reliable data source for drug safety studies in numerous therapeutic areas is well established.18–23 The study also included information from the Office for National Statistics (ONS) and the Hospital Episode Statistics (HES) database. As of March 2020 (the time of data extraction for this study), CPRD Aurum contained information on symptoms, diagnoses, prescribing information, demographics, referrals, immunizations, lifestyle factors, tests, and results for approximately 10.3 million currently registered patients, representing 15% of the UK population and 12% of UK general practices.17,24 For a majority of practices in CPRD Aurum, these data are linkable with other health care data sets (eg, hospitalization records and national mortality data) via the patient’s National Health Service number, sex, date of birth, and postal code. Detailed information on prescriptions written by general practitioners, including prescribed dose and duration, is routinely recorded in the database and coded using the Dictionary of Medicines and Devices. Read 2, SNOMED CT (Systematized Nomenclature of Medicine Clinical Terms), and local codes from EMIS, a UK electronic health records system, were used for diagnoses. Identifying patients with both CPRD and HES data enabled access to hospital discharge diagnoses and procedural codes.
Cohort Selection
Patients included in the study were new users of aclidinium bromide (monotherapy or in non–fixed-dose combination with formoterol or other medications), aclidinium/formoterol, or another of the COPD medications of interest: tiotropium, other LAMA (glycopyrronium bromide, umeclidinium), LAMA/LABA (glycopyrronium/indacaterol, umeclidinium/vilanterol, and tiotropium/olodaterol), LABA (formoterol, salmeterol, indacaterol, olodaterol), and LABA/inhaled corticosteroid (ICS) (formoterol/budesonide, formoterol/beclometasone, formoterol/fluticasone, salmeterol/fluticasone propionate, and vilanterol/fluticasone).
New users of the study medications were selected if they fulfilled the eligibility criteria and the date of the first prescription (start date) occurred from 01 September 2012 through 30 June 2019. Patients were included in the study if they met all the following criteria: had been prescribed a study medication of interest during the study period, with no prescription of the same medication or medication group during the 6 months before the start date (new user); had ≥1 year of enrolment in CPRD prior to the start date; were aged 40 years or older at the start date; and had a recorded diagnosis of COPD, with or without asthma, at any time before the start date or up to 30 days after the start date (COPD diagnoses were identified through outpatient diagnoses recorded in the General Practitioner Online Database of CPRD [CPRD GOLD] or HES [International Classification of Diseases, Tenth Revision (ICD-10) codes J40-J44]). Patients were excluded if they met any of the following criteria: (criterion A) patients with any of the following non-cardiovascular, life-threatening conditions recorded in the database at any time before or on the start date—cancer, HIV (human immunodeficiency virus), respiratory failure, end-stage renal disease, organ transplant, drug or alcohol abuse, coma, or congenital cardiovascular anomalies; and (criterion B) patients with missing information on smoking status or body mass index (BMI).
New users were followed from the start date until the earliest of the following dates: occurrence of the endpoint, death, disenrollment from the practice, or end of the study period. The end of the study period was censored at the latest date of the period covered by both HES and ONS data, so that all person-time in CPRD Aurum was linkable to these databases. Practices in CPRD Aurum that were not linkable to HES or ONS were excluded.
Variables
Exposure
Exposure to the study medications was ascertained using the information recorded for prescribed medications in CPRD Aurum. Days’ supply was calculated from information on the prescription instructions, the daily dose, and the quantity prescribed. For patients with missing information, imputation was conducted using the mode of days’ supply for each product name.
The main exposure of interest was current use, defined as the sum of episodes of continuous use and further classified into single or multiple study medication use based on current use of one or more than one study medication group at index date. Continuous-use episodes were defined by consecutive prescriptions (ie, those with a gap of ≤7 days between the end of the days’ supply of one prescription and the start of the next prescription). An episode of continuous use ended at the earliest of the end of follow-up or 7 days after the end of the days’ supply of the earlier prescription when a gap of >7 days occurred.
Duration of current use of each study medication was calculated as the total time of continuous use of that medication. The effect of duration of use was estimated by categories of short duration of current use, if duration was <6 months, and long duration of current use, if duration was ≥6 months or longer. Supplementary Appendix A describes the duration of use categories in additional detail.
Recent and past uses were also considered. Recent use started at the end of the current use episode (which included the 7-day extension period) and ended at the earliest of (1) the beginning of a new episode of current use, (2) end of follow-up, or (3) 60 days after the end of the current use episode. Past use started after recent use ended (ie, 61 days after the end of the current use episode) and ended at the earliest of (1) the beginning of a new episode of current use or (2) the end of follow-up. Duration of recent/past use of each study medication was calculated as the total time of each category of use. Supplementary Appendix A describes the recent and past uses categories in additional detail.
Endpoints
With the use of previously validated algorithms, AMI was defined as hospitalization for AMI, either fatal or non-fatal (identified through specific ICD-10 codes recorded as primary discharge diagnoses in HES at any time after the start date), plus community CHD deaths (identified through ICD-10 codes recorded in ONS mortality data with CHD as an underlying cause of death).25–29 Because some patients suffering an AMI die suddenly before arriving at the hospital, community CHD deaths, including sudden cardiac death, were also included in the definition of AMI.30 Community deaths from CHD were defined as sudden cardiac death or fatal CHD events in persons outside a hospital setting.
Using previously validated algorithms, stroke was defined as hospitalization for or referral to a specialist for acute stroke, either fatal or non-fatal, plus patients with community CeVD deaths.26,29,31–33 Potential hospitalizations for stroke were identified using specific ICD-10 codes recorded as primary discharge diagnoses in HES at any time after the start date. In addition, stroke was ascertained in CPRD Aurum and identified through outpatient codes (SNOMED CT or local EMIS codes) suggestive of stroke that had an outpatient record for referral to a specialist within 1 day before or after the date of the stroke code. Because patients having a stroke may die before arriving at a hospital facility, community deaths from CeVD were also ascertained as part of this endpoint. Potential community deaths due to CeVD were identified through ICD-10 codes recorded in the ONS with CeVD as the underlying cause of death.
Finally, MACE was a composite endpoint of AMI, stroke, or community CHD or CeVD death. The date of the MACE endpoint was the first occurring date of either AMI, stroke, community CHD death, or CeVD death.
Covariables
Confounding factors were defined by diagnoses, procedures, and medication prescriptions recorded in either CPRD Aurum or HES data. Confounding factors (except concurrent use of study medications) were measured before the start date to account for confounding by indication and to avoid adjusting for variables that could be affected by the exposure of interest (intermediate variables). The following potential confounding factors were considered: age, sex, race/ethnicity, BMI, smoking history, history of alcohol use, socioeconomic status, health care utilization, comorbidities, comedications, and severity of COPD (determined by using the Global Initiative for Chronic Obstructive Lung Disease [GOLD] 2016 definition34).
Statistical Analyses
Cohort identification, attrition, and characteristics were summarized descriptively. Crude and adjusted incidence rates (IRs) and 95% confidence intervals (CIs) for AMI and stroke were estimated using the Poisson distribution. Crude and adjusted incidence rate ratios (IRRs) and 95% CIs for AMI and stroke for the effect of each study medication compared with LABA were estimated using Poisson regression models restricted to single current users, multiple current users, recent users, and past users, as well as by duration of use. Clinically relevant risk factors, including age, sex, COPD severity, prior history of CHD for the AMI outcome, and prior history of CeVD for the stroke outcome, were included in the final regression models. A forward stepwise regression approach was used to determine if additional potential confounding variables should be included in the final models. Each of the remaining potential confounding factors (variables associated with both the endpoint and being a new user of aclidinium bromide versus LABA) with a prevalence of ≥5% were included in the final regression model if it produced a change of ≥10% in the magnitude of the exposure coefficient estimated from the model comparing current use of aclidinium bromide versus current use of LABA.35 Variables already included in the definition of severity (eg, oral glucocorticosteroids, antibiotics) or correlated with variables already included (eg, history of CHD and AMI) were not subsequently considered for inclusion, allowing for inclusion of other variables that did not meet the 10% criterion but were known as potential risk factors (eg, use of lipid-lowering drugs).
As the direction and magnitude of the risk were similar across the individual components of MACE, the main analysis conducted for the AMI and stroke endpoints was performed with the combined endpoint. The individual AMI and stroke endpoints were evaluated, and their compatibility with the hypothesis that both endpoints show the same proportional elevation in risk was assessed, as measured by a P value for heterogeneity (Cochran’s Q Χ2 test).36
In subgroup analyses, crude and adjusted IRRs and 95% CIs for AMI and stroke for current use of each study medication versus LABA were stratified by the following patient characteristics of interest: COPD severity, age, history of asthma, and history of CHD for AMI and of CeVD for stroke. Sensitivity analyses were conducted to explore the following: (1) extending the duration of the gap between prescriptions to 30 days instead of 7 days (for identifying consecutive prescriptions for continuous-use episodes); (2) not considering as events the strokes identified from referrals to a specialist or emergency department visits; and (3) using stratification by propensity score,37 a measure to control for bias from confounding factors, ie, adjusting the Poisson model by propensity score deciles, before and after trimming at first percentile of aclidinium and 99th percentile of LABA.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
In accordance with the recommendations of the American Statistical Association and expert opinion, the research team avoided relying on statistical significance or its proxies to interpret study results.38–41
Results
Cohort Characteristics
The study included 11,121 new users of aclidinium, 4804 new users of aclidinium/formoterol, 56,198 new users of tiotropium, 23,856 new users of other LAMA, 17,450 new users of LAMA/LABA, 70,289 new users of LABA/ICS, and 13,716 new users of LABA (Figure 2). The distributions of age, sex, race/ethnicity, current smoking, BMI, and alcohol use were similar across the study medications (Table 1). Mean age was approximately 69 to 70 years for all the study medications. Approximately half of the users were women, 42% to 47% were current smokers, and one-third were obese.
 |  |  |  |
Table 1 Characteristics at the Start Date, by Study Medication |
The GOLD D severity category was more frequent in users of aclidinium bromide (33.4%) and users of other LAMA (29.5%) than in users of other study medications (Table 1). Users of LABA had the highest frequency of less severe COPD (GOLD A) (40.6%), followed by users of LABA/ICS (35.5%). Users of LABA/ICS had the highest frequency of asthma recorded in the 5 years before the start date (48.7%), while users of LAMA/LABA had the lowest frequency (23.5%). The frequency of cardiovascular diseases and other diseases was similar, as was the distribution of Charlson Comorbidity Index score, across the study medications. LABA/ICS and tiotropium were the study medications most frequently prescribed before the start date. In general, users of aclidinium bromide and of other LAMA had the highest frequency of previous use of other (non-study) respiratory medications, except ICS, the use of which was highest among users of LABA and of LABA/ICS. Vaccines, antibiotics, and cardiovascular medications were the most frequent non-respiratory medications prescribed. Users of LAMA medications had higher health care utilization than users of LABA or LABA/ICS.
The median duration of use of the study medications ranged from 6 months for aclidinium/formoterol to 14.5 months for LABA/ICS (Supplementary Appendix B). Approximately 50% of the users of aclidinium/formoterol, LAMA/LABA, and LABA; 58% of the users of aclidinium bromide; 60% of the users of other LAMA; 65% of the users of tiotropium; and 71% of the users of LABA/ICS had a median duration of use longer than 6 months.
Crude Incidence Rates of AMI, Stroke, and MACE
Crude incidence rates per 1000 person-years for AMI, during current use, ranged from 8.7 for aclidinium/formoterol to 12.4 for LAMA/LABA (Table 2). The crude IRs for stroke, during current use, ranged from 4.8 among users of aclidinium/formoterol to 7.2 among users of LAMA/LABA. Crude IRs per 1000 person-years for MACE, during current use, ranged from 13.5 for aclidinium/formoterol to 19.3 for LAMA/LABA.
 |
Table 2 Risk of AMI, Stroke, and MACE Associated with Current Use of the Study Medications versus Current Use of LABA |
Incidence Rate Ratios of AMI, Stroke, and MACE
Current Use
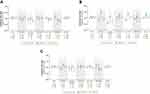
When the current overall use of each study medication was compared with LABA, the adjusted IRRs for AMI, stroke, and MACE were around 1 (Table 2; Figure 3A); therefore, no substantial differences were seen between medication groups. When current use of aclidinium bromide was compared with LABA, the adjusted IRRs (95% CI) were 1.00 (0.76–1.31) for AMI, 0.86 (0.62–1.18) for stroke, and 0.93 (0.75–1.16) for MACE. For current use of aclidinium/formoterol, the adjusted IRRs (95% CI) were 0.95 (0.60–1.52) for AMI, 0.64 (0.39–1.06) for stroke, and 0.94 (0.64–1.37) for MACE. Considering current single and current multiple uses of each study medication, the IRRs were similar to those observed for overall current use (that included single and multiple uses of the study medications) (Supplementary Appendix C).
Recent and Past Use
When each study medication was compared with LABA, adjusted IRRs for AMI and MACE for recent and past use and the adjusted IRRs for stroke for past use were similar to those observed for current use (Figure 3A and B; Supplementary Appendix D). The adjusted IRRs for stroke for recent use were generally higher than those observed for current use for all study medication groups compared with LABA. Compared with recent use of LABA, the adjusted IRR for stroke was 1.79 (95% CI, 1.06–3.00) for recent use of aclidinium bromide and 1.18 (95% CI, 0.50–2.74) for recent use of aclidinium/formoterol (Figure 3C).
Short versus Long Duration of Use
No meaningful increased risk of AMI, stroke, or MACE was observed for short or long duration of current use of any of the study medications compared with current short or long use of LABA, respectively, except for long duration of use of LAMA/LABA versus long duration of use of LABA, which showed an adjusted IRR for AMI of 1.68 (95% CI, 1.10–2.55) and an adjusted IRR for MACE of 1.68 (95% CI, 1.20–2.36) (Supplementary Appendix E).
Subgroup and Sensitivity Analyses
In subgroup analyses, IRRs for AMI and stroke for each study medication versus current use of LABA were estimated by categories of COPD severity, age, history of asthma, and history of CHD for AMI or history of CeVD for stroke (Figures 4 and 5). For all the study medications compared with use of LABA, the magnitude of the IRR estimates was generally higher among patients in GOLD 2016 COPD severity categories A and B than among patients in categories C and D, among younger than older patients, among patients without asthma than among patients with asthma, and among patients without prior history of CHD or CeVD than among patients with such a history. For several subgroups, the precision of the effect estimates was low, as it was based on a very low number of events.
Results were generally consistent across sensitivity analyses. When the carryover period for continuous-use episodes was extended from 7 days to 30 days, the IRRs for AMI and stroke were similar to those obtained in the main analysis for all study medications (Figures 3 and 4). When the definition of stroke was limited to hospital primary discharge diagnoses, the IRRs for stroke were similar to those obtained in the main analysis for all the study medications, although the magnitude of the point estimates was higher, and the 95% CIs were wider (Figure 4). The IRRs adjusted by propensity score deciles (as categories) for AMI and stroke among current users of aclidinium bromide and aclidinium/formoterol compared with current users of LABA were similar to those from the main analysis before and after trimming (Supplementary Appendix F).
Discussion
In this study conducted in patients with COPD aged 40 years or older selected from general practices in the UK, the crude IRs for AMI per 1000 persons-years among new users of study medications ranged from 8.7 for current use of aclidinium/formoterol to 12.4 for current use of LAMA/LABA, the IRs for stroke ranged from 4.8 for current use of aclidinium/formoterol to 7.2 for current use of LAMA/LABA, and the IRs for MACE ranged from 13.5 for current use of aclidinium/formoterol to 19.3 for current use of LAMA/LABA. The risks of AMI, stroke, and MACE observed for the current use of aclidinium bromide, aclidinium/formoterol, tiotropium, other LAMA, LAMA/LABA, or LABA/ICS were similar to the risks for the current use of LABA. This also applied to the risks observed for current single or current multiple use and for past use or for short duration of use of the study medications. A pattern of increased risk of stroke ranging from 12% to 79% was observed for recent use of all study medications compared with recent use of LABA. A trend toward a 20% increased risk of AMI and MACE was observed for the current use of LAMA/LABA compared to the current use of LABA, although results are compatible with a 10% reduced risk to around a 60% increased risk. Results indicating an increased risk of AMI when comparing long duration of use of the study medications with long duration of use of LABA suggest that patients that remained on LABA monotherapy for longer duration (>6 months) without switching to LAMA or to a fixed-dose combination of LAMA/LABA or LABA/ICS may have had different characteristics from those with short duration of use of LABA or those remaining on the other medications for longer duration. Given that baseline characteristics were measured at baseline and not after 6 months, these differences could not be completely addressed through adjustment of potential confounders and thus may explain the study results. In general, the results across categories of COPD severity, age, history of asthma, and history of CHD or CeVD were similar to those observed in the main analysis, but with less precision. Alternative definitions of duration of exposure and stroke did not affect the study results. The sensitivity analysis using propensity score stratification provided results similar to those observed in the main analysis for current use of aclidinium bromide and aclidinium/formoterol compared with current use of LABA.
Results from this study do not support a meaningful increase in risk of AMI, stroke, or MACE among patients with COPD treated with aclidinium bromide, aclidinium/formoterol, tiotropium, other LAMA, LAMA/LABA, or LABA/ICS when compared with patients with COPD treated with LABA, although long-term use of LAMA/LABA may deserve further evaluation of potential sources of bias and confounding. Overall, the study results were consistent across subgroups and sensitivity analyses. The analyses before and after trimming the patients in the extreme percentages of the distribution of the propensity scores showed that, before trimming, the adjusted IRRs did not differ from those in the main analysis, suggesting that the relevant variables were included in the Poisson regression models for current use. Differences in the risks observed in some of the analyses for specific medications and in the analyses stratified by subgroups of patients may be explained by random variability from the low number of events and potential unmeasured confounding.
Results of this non-interventional study are consistent with previous studies performed among users of tiotropium and aclidinium bromide. Although a few initial studies raised concerns about the cardiovascular safety of tiotropium, other studies did not confirm an existing increased risk.2–7,42–44 Discrepancies between results from clinical trials and those from observational studies have been attributed to the exclusion of patients with cardiovascular comorbidities and renal impairment in COPD clinical trials of LAMA therapy.45,46 However, the ASCENT COPD trial (a randomized placebo-controlled trial with up to 3 years of duration), which included patients with moderate to very severe COPD and cardiovascular risk factors, found no increased risk of MACE (hazard ratio [HR], 0.89; 1-sided 97.5% CI, 0–1.23), cardiovascular death (HR, 1.34; 1-sided 97.5% CI, 0–2.42), non-fatal AMI (HR, 0.72; 1-sided 97.5% CI, 0–1.18), non-fatal stroke (HR, 0.74; 1-sided 97.5% CI, 0–1.36), or MACE or other serious cardiovascular events of interest (eg, acute heart failure or life-threatening arrhythmias) (HR, 1.03; 1-sided 97.5% CI, 0–1.28) with aclidinium bromide versus placebo; the upper bound of the 95% CI excluded 1.8 for all endpoints.47
Several limitations should be considered when interpreting the results of this study. Although the precision of the study was sufficient to assess the risk of AMI and stroke associated with current use of the study medications, the precision of the IRRs for several of the subgroup analyses was low due to the low number of events; therefore, these IRRs should be interpreted with caution. Increased risk of stroke was higher for recent use of all study medication groups compared with recent use of LABA. This suggests that there may be additional reasons for stopping other medications, such as potential worsening of health status of the patients and related confounding that could not be controlled completely through adjustment for potential confounders measured at baseline. An increased risk of AMI was observed for long-term use of all study medications compared with LABA, and the risk was the highest for long-term use of LAMA/LABA compared with long-term use of LABA. This also suggests that patients that remained on LABA monotherapy for longer duration (>6 months) without switching to LAMA or to a fixed-dose combination of LAMA/LABA or LABA/ICS may have had different characteristics from those with short duration of use of LABA or those remaining on the other medications for longer duration. Given that baseline characteristics were measured only at the start date and not after 6 months, these differences could not be completely addressed by adjusting the models for potential confounders. Further, there are limitations to the analyses of past use, as users could have discontinued all medications or switched to an alternative medication. Our analyses did not evaluate time-varying confounders (eg, postindex healthcare resource utilization or postindex prescribing patterns).
There is the potential for selective prescribing of newly marketed drugs to patients with more severe or inadequately controlled COPD or comorbidities. To overcome the potential effect of selective prescribing and differences in the baseline characteristics of these patients, the analyses were adjusted at baseline using all captured variables in CPRD related to baseline COPD severity, comorbidities, and comedications, although some degree of channeling bias and residual confounding cannot be discarded. This study was conducted using health information recorded in automated, population-based databases. An advantage of these data sources is that the data are collected from routine health care without interfering with regular clinical practice. However, there are some limitations, including the potential for misclassification of exposure and outcome, gaps in duration of use, and limited granularity preventing full adjustment for potential confounders. Finally, confounding by indication could play a role in the observed differences between patients with and without a history of CHD.
Conclusion
The risks of AMI, stroke, and MACE in current users of aclidinium and aclidinium/formoterol were similar to the risks among current users of LABA. A lack of meaningful association was also observed during treatment with tiotropium, other LAMA, LAMA/LABA, and LABA/ICS versus LABA.
Abbreviations
AMI, acute myocardial infarction; BMI, body mass index; CeVD, cerebrovascular disease; CHD, coronary heart disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CPRD, Clinical Practice Research Datalink; EU, European Union; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, general practitioner; HES, Hospital Episode Statistics; HIV, human immunodeficiency virus; HR, hazard ratio; ICD-10, International Statistical Classification of Diseases, Tenth Revision; ICS, inhaled corticosteroid; IMD, Index of Multiple Deprivation; IR, incidence rate; IRR, incidence rate ratio; ISAC, Independent Scientific Advisory Committee; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonists; MACE, major adverse cardiovascular events; MHRA, Medicines and Healthcare Products Regulatory Agency; NR, not reported; ONS, Office for National Statistics; PASS, postauthorization safety study; PY, person-years; Q1, first quartile; Q3, third quartile; REF, reference category; Rx, prescription; SABA, short-acting beta2-agonist; SAMA, short-acting anticholinergic; SD, standard deviation; SNOMED CT, Systematized Nomenclature of Medicine Clinical Terms; SVA, supraventricular arrhythmia; UK, United Kingdom; US, United States.
Data Sharing Statement
This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. The same applies to the use of the Office of National Statistics and Health Episodes Statistics data, Copyright © 2021, re-used with the permission of The Health & Social Care Information Centre. All rights are reserved. The same applies to the use of the Office of Population Censuses and Surveys Classification of Interventions and Procedures, codes, terms and text, Crown copyright (2016) published by Health and Social Care Information Centre, also known as NHS Digital and licensed under the Open Government Licence, available at www.nationalarchives.gov.uk/doc/open-government-licence/open-government-licence.htm.
Ethics Approval and Informed Consent
The Independent Scientific Advisory Committee of the Medicines and Healthcare products Regulatory Agency approved the protocol (protocol 19_200R) on 18 February 2020.
Acknowledgments
The authors thank Jordi Castellsague (RTI-HS) and Cristina Varas (RTI-HS) for their scientific and clinical contributions to this work, Peter McMahon and Michael Stokes (AstraZeneca) for scientific review and contributions to this work, Christine Bui (RTI-HS) for project management support, Kate Lothman (RTI-HS) for medical writing support, John Forbes (RTI-HS) for manuscript editing, and Emily Gill (RTI-HS) for graphic design.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval for the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was performed under a research contract between RTI Health Solutions (RTI-HS) and AstraZeneca and was funded by AstraZeneca. The contract provides the research team independent publication rights. The sponsors had no role in the data collection or analysis; however, in line with the Guideline on Good Pharmacovigilance Practices: Module VIII: Post-authorisation Safety Studies of the European Medicines Agency, the sponsors had the opportunity to view the results and interpretations included in the manuscript and provide comments before submission of the manuscript for publication.
Disclosure
CR and EP are salaried employees of RTI Health Solutions, a non-profit research organization that conducts research with multiple pharmaceutical companies and has an independent right to publish the results of this study. AR is an employee of BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom. JA and DM are salaried employees of RTI Health Solutions, a non-profit research organization that conducts research with multiple pharmaceutical companies and has an independent right to publish the results of this study. AL is an employee of BioPharmaceuticals R&D, AstraZeneca, Barcelona, Spain. SD is an employee of BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, Maryland, United States. NSM, SPG, and ERF are salaried employees of RTI Health Solutions, a non-profit research organization that conducts research with multiple pharmaceutical companies and has an independent right to publish the results of this study.
References
1. GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report). Global initiative for chronic obstructive lung disease; 2021. Available from: https://goldcopd.org/2021-gold-reports/.
2. de Luise C, Lanes SF, Jacobsen J, et al. Cardiovascular and respiratory hospitalizations and mortality among users of tiotropium in Denmark. Eur J Epidemiol. 2007;22(4):267–272. doi:10.1007/s10654-007-9106-5
3. Jara M, Lanes SF, Wentworth C 3rd, et al. Comparative safety of long-acting inhaled bronchodilators: a cohort study using the UK THIN primary care database. Drug Saf. 2007;30(12):1151–1160. doi:10.2165/00002018-200730120-00007
4. Jara M, Wentworth C 3rd, Lanes S. A new user cohort study comparing the safety of long-acting inhaled bronchodilators in COPD. BMJ Open. 2012;2(3):e000841. doi:10.1136/bmjopen-2012-000841
5. Macie C, Wooldrage K, Manfreda J, et al. Cardiovascular morbidity and the use of inhaled bronchodilators. Int J Chron Obstruct Pulmon Dis. 2008;3(1):163–169. doi:10.2147/COPD.S1516
6. Ogale SS, Lee TA, Au DH, et al. Cardiovascular events associated with ipratropium bromide in COPD. Chest. 2010;137(1):13–19. doi:10.1378/chest.08-2367
7. Verhamme KM, Afonso AS, van Noord C, et al. Tiotropium handihaler and the risk of cardio- or cerebrovascular events and mortality in patients with COPD. Pulm Pharmacol Ther. 2012;25(1):19–26. doi:10.1016/j.pupt.2011.10.004
8. Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(12):1439–1450. doi:10.1001/jama.300.12.1439
9. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
10. FDA. Early communication about an ongoing safety review of tiotropium (marketed as Spiriva handihaler); 2010. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm070651.htm.
11. Singh S, Loke YK, Enright P, et al. Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Thorax. 2013;68(1):114–116. doi:10.1136/thoraxjnl-2011-201275
12. EMA. Summary of the risk management plan for Eklira genuair; 2021. Available from: https://www.ema.europa.eu/en/documents/rmp-summary /eklira-genuair-epar-risk-management-plan-summary_en.pdf.
13. EMA. Summary of the risk management plan for Duaklir genuair; 2021. Available from: https://www.ema.europa.eu/en/documents/rmp-summary /duaklir-genuair-epar-risk-management-plan-summary_en. pdf.
14. Rivero-Ferrer E, Olesen M, Plana E, et al. Characteristics of new users of aclidinium bromide, aclidinium/ formoterol, and other COPD medications in the United Kingdom, Denmark, and Germany. Clin Drug Investig. 2022;42(4):319–331. doi:10.1007/s40261-022-01120-2
15. Rebordosa C, Aguado J, Plana E, et al. Use of aclidinium did not increase the risk of death in a noninterventional cohort study in the clinical practice research datalink (CPRD), United Kingdom. Respir Med. 2019;152:37–43. doi:10.1016/j.rmed.2019.04.018
16. Rebordosa C, Plana E, Rubino A, et al. A cohort study to evaluate the risk of hospitalisation for congestive heart failure associated with the use of aclidinium and other chronic obstructive pulmonary disease medications in the UK clinical practice research datalink. Int J Chron Obstruct Pulmon Dis. 2021;16:1461–1475. doi:10.2147/COPD.S301624
17. Wolf A, Dedman D, Campbell J, et al. Data resource profile: clinical practice research datalink (CPRD) aurum. Int J Epidemiol. 2019;48(6):1740. doi:10.1093/ije/dyz034
18. Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302(6779):766–768. doi:10.1136/bmj.302.6779.766
19. Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. Validity of the general practice research database. Pharmacotherapy. 2003;23(5):686–689. doi:10.1592/phco.23.5.686.32205
20. Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi:10.1111/j.1365-2125.2009.03537.x
21. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi:10.1093/ije/dyv098
22. Jick SS, Hagberg KW, Persson R, et al. Quality and completeness of diagnoses recorded in the new CPRD Aurum database: evaluation of pulmonary embolism. Pharmacoepidemiol Drug Saf. 2020;29(9):1134–1140. doi:10.1002/pds.4996
23. Persson R, Vasilakis-Scaramozza C, Hagberg KW, et al. CPRD Aurum database: assessment of data quality and completeness of three important comorbidities. Pharmacoepidemiol Drug Saf. 2020;29(11):1456–1464. doi:10.1002/pds.5135
24. MHRA. Release notes: CPRD aurum March 2020. Medicines & Healthcare products Regulatory Agency; 2020.
25. García Rodríguez LA, Varas-Lorenzo C, Maguire A, et al. Nonsteroidal antiinflammatory drugs and the risk of myocardial infarction in the general population. Circulation. 2004;109(24):3000–3006. doi:10.1161/01.CIR.0000132491.96623.04
26. Ray WA, Varas-Lorenzo C, Chung CP, et al. Cardiovascular risks of nonsteroidal antiinflammatory drugs in patients after hospitalization for serious coronary heart disease. Circ Cardiovasc Qual Outcomes. 2009;2(3):155–163. doi:10.1161/CIRCOUTCOMES.108.805689
27. Varas-Lorenzo C, Castellsague J, Stang MR, et al. The use of selective cyclooxygenase-2 inhibitors and the risk of acute myocardial infarction in Saskatchewan, Canada. Pharmacoepidemiol Drug Saf. 2009;18(11):1016–1025. doi:10.1002/pds.1815
28. Hammad TA, McAdams MA, Feight A, et al. Determining the predictive value of Read/OXMIS codes to identify incident acute myocardial infarction in the General practice research database. Pharmacoepidemiol Drug Saf. 2008;17(12):1197–1201. doi:10.1002/pds.1672
29. Arana A, Margulis AV, Varas-Lorenzo C, et al. Validation of cardiovascular outcomes and risk factors in the clinical practice research datalink in the United Kingdom. Pharmacoepidemiol Drug Saf. 2021;30(2):237–247. doi:10.1002/pds.5150
30. Jangaard N, Sarkisian L, Saaby L, et al. Incidence, frequency, and clinical characteristics of type 3 myocardial infarction in clinical practice. Am J Med. 2017;130(7):
31. Andersohn F, Schade R, Suissa S, et al. Cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs and the risk of ischemic stroke: a nested case-control study. Stroke. 2006;37(7):1725–1730. doi:10.1161/01.STR.0000226642.55207.94
32. Arana A, Varas C, Gonzalez-Perez A, et al. Hormone therapy and cerebrovascular events: a population-based nested case-control study. Menopause. 2006;13(5):730–736. doi:10.1097/01.gme.0000233494.28335.71
33. Roumie CL, Mitchel E, Gideon PS, et al. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf. 2008;17(1):20–26. doi:10.1002/pds.1518
34. GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2016 report). Global initiative for chronic obstructive lung disease; 2016. Available from: http://goldcopd.org/gold-reports-2016/.
35. Rothman K, Greenland S, Lash TL. Modern Epidemiology.
36. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi:10.1002/14651858.ED000142
37. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. doi:10.1002/sim.5984
38. Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Stat. 2016;70(2):129–133. doi:10.1080/00031305.2016.1154108
39. Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31(4):337–350. doi:10.1007/s10654-016-0149-3
40. Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567(7748):305–307. doi:10.1038/d41586-019-00857-9
41. Rothman KJ, Lash TL. Precision and study size. In: Lash TL, VanderWeele TJ, Haneuse S, Rothman KJ, editors. Modern Epidemiology.
42. Barr RG, Bourbeau J, Camargo CA, et al. Tiotropium for stable chronic obstructive pulmonary disease: a meta-analysis. Thorax. 2006;61(10):854–862. doi:10.1136/thx.2006.063271
43. Celli B, Decramer M, Leimer I, et al. Cardiovascular safety of tiotropium in patients with COPD. Chest. 2010;137(1):20–30. doi:10.1378/chest.09-0011
44. Kesten S, Celli B, Decramer M, et al. Tiotropium handihaler in the treatment of COPD: a safety review. Int J Chron Obstruct Pulmon Dis. 2009;4:397–409. doi:10.2147/COPD.S4802
45. Schmiedl S, Fischer R, Ibanez L, et al. Tiotropium Respimat® vs. HandiHaler®: real-life usage and TIOSPIR trial generalizability. Br J Clin Pharmacol. 2016;81(2):379–388. doi:10.1111/bcp.12808
46. Walker S, Fingleton J, Weatherall M, et al. Limited generalisability of UPLIFT findings to clinical practice. Thorax. 2013;68(11):1066–1067. doi:10.1136/thoraxjnl-2013-203724
47. Wise RA, Chapman KR, Scirica BM, et al. Effect of aclidinium bromide on major cardiovascular events and exacerbations in high-risk patients with chronic obstructive pulmonary disease: the ASCENT-COPD randomized clinical trial. JAMA. 2019;321(17):1693–1701. doi:10.1001/jama.2019.4973
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.