Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Radiographic Phenotypes Affect the Risk of Inhaled Corticosteroid-Associated Pneumonia in Patients with COPD
Authors Lee HJ, Jin KN, Lee HW , Lee JK , Park TY, Heo EY, Kim DK
Received 10 May 2022
Accepted for publication 9 September 2022
Published 18 September 2022 Volume 2022:17 Pages 2301—2315
DOI https://doi.org/10.2147/COPD.S372735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Hyo Jin Lee,1 Kwang Nam Jin,2 Hyun Woo Lee,1 Jung-Kyu Lee,1 Tae Yeon Park,1 Eun Young Heo,1 Deog Kyeom Kim1,3
1Division of Respiratory and Critical Care, Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, South Korea; 2Department of Radiology, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, South Korea; 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, South Korea
Correspondence: Deog Kyeom Kim, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, 20, Boramae-ro 5-gil, Dongjak-gu, Seoul, 07061, South Korea, Tel +82-2-870-2130, Fax +82-2-831-0714, Email [email protected]
Purpose: Few studies have reported the association between the radiographic characteristics and the development of pneumonia in patients with chronic obstructive pulmonary disease (COPD) treated with inhaled corticosteroids (ICSs). Our study aimed to assess the effect of radiographic phenotypes on the risk of pneumonia in patients treated with ICSs.
Patients and Methods: This study retrospectively analysed all patients with COPD treated with ICSs in a subset of the Korea Chronic Obstructive Pulmonary Disorders Subgroup Study registry between January 2017 and December 2019. The association between radiographic phenotypes including the presence and severity of emphysema, airway wall thickening, or bronchiectasis on chest computed tomography were determined visually/qualitatively and the risk of pneumonia was analyzed using the Cox regression model.
Results: Among the 90 patients with COPD treated with ICSs, 41 experienced pneumonia more than once during the median follow-up of 29 (interquartile range, 8– 35) months. In univariate Cox regression analysis, older age, longer use of ICSs, use of fluticasone propionate or metered dose inhaler, and severe exacerbation events increased the risk of pneumonia. In multivariate analysis, the presence of emphysema (adjusted hazard ratio [aHR]=3.73, P=0.033), severity measured using the visual sum score (mild-to-moderate, aHR=8.58, P=0.016; severe, aHR=3.58, P=0.042), Goddard sum score (mild-to-moderate, aHR=3.31, P=0.058; severe, aHR=5.38, P=0.014), and the upper lobe distribution of emphysema (aHR=3.76, P=0.032) were associated with a higher risk of pneumonia. Subtypes of centrilobular and panlobular emphysema had a higher risk of pneumonia compared with paraseptal emphysema (aHR=3.98, P=0.033; HR=3.91, P=0.041 vs HR=2.74, P=0.304). The presence of bronchiectasis (aHR=2.41, P=0.02) and emphysema/bronchiectasis overlap phenotype (aHR=2.19, P=0.053) on chest CT was a risk factor for pneumonia in this population. However, severity of bronchiectasis and the presence or severity of bronchial wall thickening according to the visual sum score were not associated with the risk of pneumonia.
Conclusion: Among patients with COPD treated with ICSs, radiographic phenotypes including the presence of emphysema, bronchiectasis or emphysema/bronchiectasis overlap phenotype, severity with emphysema, subtypes of centrilobular or panlobular emphysema, and upper lobe distribution of emphysema may help predict the risk of pneumonia.
Keywords: chronic obstructive pulmonary disease, inhaled corticosteroid, pneumonia, chest computed tomography, CT
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by cough, sputum production, progressive dyspnea, and airflow obstruction.1 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends inhaled corticosteroids (ICSs) combined with bronchodilators to reduce exacerbation rates in patients in GOLD groups C and D, COPD patients with blood eosinophil levels of 300 cells/μL or higher, at least 2 moderate exacerbations per year or a hospitalization from AECOPD, history of asthma, or who remain breathless.1–3 However, ICSs increase the risk of pneumonia in patients with COPD4–7 according to their pharmacokinetic characteristics.8 Differences in the molecular structures of ICS formulations alter their relative potency ratios and durations of action.9,10 The local pharmacokinetic rate and degree of absorption in the airways and lungs depend on ICSs’ intrinsic physicochemical properties.8,11 For example, the prolonged presence of slowly dissolving particles of fluticasone propionate in the airway epithelial lining fluid compared to budesonide may cause protracted local immunosuppression.12,13 Moreover, phagocytosis of lipophilic ICS particles by airway/alveolar macrophages might lead to impaired macrophage function,12 possibly contributing to increased bacterial colonization in COPD.14 This can impair the clearance of airway pathogens, leading to airway/lung colonization by pathogens, which may further develop into pneumonia.
Considering the presence of anatomical abnormalities in the lungs or thorax of patients with COPD, the airway parenchyma is at risk of respiratory infections.15,16 Some studies have attempted to determine COPD phenotypes by investigating the morphological findings observed on chest computed tomography (CT) scans,17,18 including the presence of pulmonary emphysema,17 bronchial wall thickening,19 small airway disease,20 and bronchiectasis.21 Among these morphological findings, the degree of airflow limitation and presence of emphysema are independently associated with the development of severe pneumonia in patients with COPD.22
However, it remains unclear how a group of patients with a particular chest CT phenotype would be at greater risk of pneumonia in COPD. Emphysema causes significant changes in the central and peripheral airway structures, resulting in considerable airflow limitations or subsequent air trapping due to the loss of elastic recoil in the air sacs.23 The quotient between residual lung volume and total lung capacity is subject to an increase from 25% in healthy subjects to nearly 80% in patients with severe emphysema.24 This change in lung volume entails the development of asymmetric breathing cycles with considerably prolonged exhalation times.25 Inhaled aerosol boluses also undergo enhanced dispersion in patients with emphysema.26,27 Furthermore, lung deposition of aerosol particles in patients with varying degrees of airway obstruction and healthy individuals has also shown that the central to peripheral lung deposition ratio increases with disease severity.28,29
With regard to this physiology, we hypothesized that radiographic phenotypes, such as emphysema, bronchial wall thickening, or bronchiectasis, may act as risk factors for pneumonia in COPD patients treated with ICSs. Our study aimed to assess the effect of radiographic phenotypes on the risk of pneumonia in patients treated with ICSs.
Materials and Methods
This study followed the guidelines presented by the Strengthening the Reporting of Observational Studies in Epidemiology statement.30
Study Design and Participants
This retrospective study assessed all patients with COPD using ICSs in a subset of the Korea Chronic Obstructive Pulmonary Disorders Subgroup Study registry, which consisted of patients recruited from the Seoul Metropolitan Government–Seoul National University Boramae Medical Center and Seoul National University Hospital (SNUH) between January 2017 and December 2019.
We included the patients with a smoking history of more than 10 pack-years, who were treated with a single or combination of ICS therapy with bronchodilators for at least 6 months, and who underwent chest CT during the follow-up period. Indications for ICS treatment among COPD patients are those with moderate or severe/very severe COPD, blood eosinophil levels of 300 cells/μL or higher, at least 2 moderate exacerbations or a hospitalization per year from AECOPD, or who remain breathless.1–3 The ICSs were classified as follows: inhaled forms of fluticasone propionate, fluticasone furoate, budesonide, ciclesonide, and beclomethasone. The ICS used the longest during the follow-up period was designated as the representative ICS. Previous ICS was defined as exposure for at least 6 months immediately prior to representative ICS treatment. After the eligible patients were classified into two groups according to the presence or absence of pneumonia, the risk ratio for pneumonia was analyzed. Pneumonia was confirmed radiologically and clinically diagnosed. Radiological diagnostic criteria were defined as the presence of a new infiltrate on a chest radiography or reference to “pneumonia” or “bronchopneumonia” in the radiographic report. Clinical diagnostic criteria were defined as the presence of respiratory symptoms or signs with abnormal laboratory test results such as leukocytosis, elevated inflammatory markers, compatible chest imaging, or history of treatment with antibiotics.31
Baseline information, including age, sex, body mass index, smoking history, modified Medical Research Council dyspnea scale (mMRC), respiratory comorbidities, other underlying comorbidities, and spirometric examination, were obtained. COPD severity was assessed using the GOLD stage and ABCD classification. Clinical features, including adjuvant treatments, and annual acute exacerbation history were collected. Moderate and severe exacerbation were defined as an exacerbation leading to treatment with antibiotics or systemic glucocorticoids and an exacerbation resulting in hospitalization or death, respectively.7,32 The characteristics of ICSs, including treatment duration, dose, and formulation types, including metered dose inhalers (MDIs) or disc powder inhalers (DPIs), were obtained.
Chest Computed Tomography (CT) Phenotypes
COPD phenotypes were defined as emphysema, airway thickening, and bronchiectasis on CT, according to the Fleischner Society criteria.33 The absence or presence and involvement of these findings on CT were assessed using a pulmonary lobe-based visual grading system.34 Emphysema, airway wall thickening, and bronchiectasis were visually graded as absent (score=0) or present (score=1) for each of the following five lobes: right upper lobe (RUL), right middle lobe (RML), right lower lobe (RLL), left upper lobe (LUL), and left lower lobe (LLL). A total score ranging from 0 to 5 was calculated by summing the five separate scores for each lobe.34 Subsequently, three degrees of severity were obtained by categorizing each CT finding as follows: grade 0, absent (total score=0); grade 1, mild to moderate (total score=1–2); and grade 2, severe (total score=3–5). The lobe-based distribution of emphysema was divided into upper lobes containing RUL, RML, and LUL, or lower lobes containing RLL and LLL, based on a visual sum score of 3 or higher. Additionally, the presence and extent of emphysema were scored using a Goddard classification.35 Each involved lobe was visually scored for the percentage affected by emphysema (0=0%, 1=1–25%, 2=26–50%, 3=51–75%, 4≥75%). A total score ranging from 0 to 20 was calculated by summing the five separate scores for each lobe. The severity of emphysema was classified into four categories: grade 0, absent (total score=0); grade 1, mild (total score=1–3); grade 2, moderate (total score=4–6); and grade 3, severe (total score ≥7).34 The percentage ratio of low attenuation area to corresponding lung area (LAA%) on CT has been categorized into three categories: grade 1, low (<3%); grade 2, medium (3–10%); and grade 3, high (10%). In particular, high severity (>10%) of LAA% was defined as predominant emphysema, and the emphysema/bronchiectasis overlap was defined when the presence of predominant emphysema and bronchiectasis coexisted.36 The basic morphological types of COPD (centrilobular emphysema [CLE], paraseptal emphysema [PSE], and panlobular emphysema [PLE]) and all the above scoring assessments on CT were independently evaluated by a pulmonologist and a radiologist. The interobserver agreement of the CT assessment using the k-statistic was good (k statistic, 0.75).
Statistical Analyses
The primary endpoint was the comparison of the risk of pneumonia according to the CT phenotypes.
Data are presented as means with standard deviations or medians with standard errors and numbers with percentages for continuous and categorical variables, respectively. Student’s t-test was used to test independent samples of continuous, normally distributed data, whereas the Wilcoxon rank-sum test was used to examine continuous, skewed data. The chi-squared or Fisher’s exact test was used to analyze categorical data. Kaplan–Meier curves and Log rank tests were performed to compare the time to the first pneumonia event in the absence or presence of emphysema. We performed univariate and multivariate Cox regression analyses to compare the association between pneumonia and CT phenotypes in patients with COPD treated with ICSs. Clinically relevant factors were adjusted for in the multivariate analysis. Three models were constructed. Model 1 included demographic factors such as age, sex, BMI, smoking amount, mMRC grade and post-bronchodilator responsiveness (BDR) FEV1(L) as covariates. Model 2 included clinical characteristics of ICS such as use of fluticasone propionate, ICS dose, and ICS formulation and all covariates in Model 1. Model 3 added severe exacerbation event to all covariates in Model 2. We used SPSS Statistics (version 27.0; IBM Corp., Armonk, NY, USA) for the statistical analyses.
Results
Among the 222 patients with COPD aged ≥40 years, 132 (105, did not use ICSs; 13, had a smoking history of <10 pack-years; 14, did not undergo chest CT) were excluded. Finally, 90 eligible patients were assigned to the non-pneumonia (n=49) and pneumonia (n=41) groups (Figure S1). The median follow-up duration was 29 [interquartile range (IQR), 8–35] months in eligible patients. The median interval durations between ICS initiation and CT acquisition or ICS initiation and pulmonary function tests were 14.4 [IQR, 0–34] months or 1.9 [IQR, 0–14] months, respectively. Among the patients with pneumonia, the median interval duration between chest CT and pneumonia event was 4.6 [IQR, 0–31] months, and median frequency of pneumonia event was 1.9 [IQR, 1–5].
Baseline Characteristics and Clinical Features
The baseline characteristics of the included patients are presented in Table 1. There were significant differences in age and respiratory or underlying comorbid diseases between the pneumonia and non-pneumonia groups. Patients with pneumonia were more likely to be older than those without pneumonia. Regarding comorbidities, the pneumonia group comprised more patients with hypertension, whereas the non-pneumonia group comprised more patients with asthma, asthma and COPD overlap, rhinitis, or sinusitis. The pneumonia group had lower predicted FEV1 (%) and FEV1/FVC (%) than the non-pneumonia group. There was no significant difference in mMRC grade, GOLD stage, serum total IgE level, blood eosinophil level, or the proportion of blood eosinophil levels higher than 300 cells/μL between the two groups except the classification by the ABCD assessment tool. The pneumonia group contained more patients with groups C and D, whereas the non-pneumonia group contained more patients with groups A and B.
 |
Table 1 Baseline Characteristics of COPD Patients Treated with Inhaled Corticosteroid Containing Therapy |
The clinical features of the patients treated with ICS are presented in Table 2. The median duration of ICS treatment was significantly longer in the pneumonia group than in the non-pneumonia group (25.1 months vs 19.7 months). The single or combination therapy with fluticasone furoate (33%) was most commonly used in both groups, followed by those of budesonide (30%), and comparable distribution was found in both groups. However, the pneumonia group was more likely treated with single or combination therapy of fluticasone propionate than the non-pneumonia group (27% vs 8.2%, P=0.018). DPIs (64.4%) were more commonly used in most formulation types of ICSs than MDIs (35.6%). More than 73% of the patients in both groups had been exposed to ICS single or combination therapy prior to treatment with dominant ICS single or combination therapy. Oral or inhaled bronchodilators and systemic steroid were similarly used in both groups during the stable period. The pneumonia group had a significantly higher annual rate and number of moderate, severe, or moderate-to-severe exacerbations than the non-pneumonia group.
 |
Table 2 Clinical Features of COPD Patients Treated with Inhaled Corticosteroid Containing Therapy |
Risk Factors of Pneumonia
In the univariate Cox regression model for clinical factors, older age, history of lung cancer or hypertension, treatment with single or combination therapy of fluticasone propionate, use of MDIs or long-acting beta-agonist (LABA) and long-acting muscarinic antagonist (LAMA) combination therapy, history of previous ICS use, lower predicted and post-BDR FEV1 (L), lower DLCO (L) or (%), and greater annual number of severe exacerbation events were associated with an increased risk of pneumonia in patients with COPD (Table 3).
 |
Table 3 Hazard Ratio of Pneumonia in COPD Patients Treated with Inhaled Corticosteroid Therapy in Univariable Cox Regression Model |
Association of CT Phenotypes with Pneumonia
The univariate Cox regression model of chest CT phenotypes associated with pneumonia risk is shown in Table 4. Regarding emphysema, the presence of emphysema, mild-to-moderate grade in the visual sum score or severe grade in the Goddard sum score, higher LAA% score, subtypes of centrilobular or PLE, and distribution in the upper lobe were significantly associated with a higher risk of pneumonia compared to the absence of emphysema. However, the time to the first pneumonia event did not significantly differ between the absence and presence of emphysema (Figure S2). The presence of bronchiectasis was significantly associated with a higher risk of pneumonia compared to the absence of bronchiectasis, whereas the severity according to the visual sum score was not associated with an increased risk of pneumonia. Furthermore, the emphysema/bronchiectasis overlap phenotype was significantly associated with increased pneumonia risk. The presence or severity of bronchial wall thickening according to the visual sum score was not associated with the risk of pneumonia.
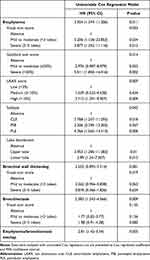 |
Table 4 Association of Pneumonia and CT Phenotype in COPD Patients Treated with Inhaled Corticosteroid Therapy in Univariable Cox Regression Model |
The multivariate Cox regression model of chest CT phenotypes associated with the risk of pneumonia is shown in Table 5, and Figure 1 depicts an overview of Model 1. The presence of emphysema showed significantly higher risk of pneumonia than the absence of emphysema in the adjusted models (Model 1, adjusted hazard ratio [aHR]=3.73 [95% confidence interval (CI)=1.11–12.53], P=0.033; Model 2, aHR=3.69 [95% CI=1.07–12.81], P=0.039; Model 3, aHR=3.72 [95% CI=1.07–12.97], P=0.039). In visual sum score in emphysema, the mild-to-moderate and severe grades had a higher risk of pneumonia even after adjusting clinically relevant factors in Models 1–3 compared to the absence of emphysema (mild-to-moderate, aHR=8.58 [95% CI=1.49–49.37], P=0.016; severe, aHR=3.58 [95% CI=1.05–12.25], P=0.042 in Model 1). The Goddard sum score with severe grade was associated with a higher risk of pneumonia compared to the absence of emphysema, whereas there was no significant association between mild-to-moderate grade and risk of pneumonia (mild-to-moderate, aHR=3.31 [95% CI=0.96–11.37], P=0.058; severe, aHR=5.38 [95% CI=1.41–20.44], P=0.014 in Model 1). Among the emphysema subtypes, CLE was significantly associated with increased pneumonia after adjusting for clinically relevant variables in Models 1–3, and PLE showed a significantly increased hazard ratio only in Model 1. The upper lobe distribution of emphysema was significantly associated with increased risk of pneumonia in Models 1–3, whereas the lower lobe distribution of emphysema was not. However, the severity of LAA% score for emphysema was not associated with an increased risk of pneumonia. The median frequency of the pneumonia was 1 (IQR, 1–2) in the pneumonia group. There were no significant differences in the clinical features of patients with pneumonia according to the absence or presence of emphysema, including pneumonia type, severity of pneumonia, involvement or pattern on chest CT, and mortality (Table S1).
 |
Table 5 Association of Pneumonia and CT Phenotype in COPD Patients Treated with Inhaled Corticosteroid Therapy in Multivariable Cox Regression Model |
There was significantly higher association with the risk of pneumonia in the presence of bronchiectasis on chest CT according to Models 1–3 in multivariate Cox regression analysis (Model 1, aHR=2.41 [95% CI=1.15–5.05], P=0.02; Model 2, aHR=2.55 [95% CI=1.19–5.46], P=0.016; Model 3, aHR=2.56 [95% CI=1.2–5.49), P=0.015). Furthermore, the emphysema/bronchiectasis overlap phenotype was significantly associated with increased pneumonia risk in Model 2 and 3 (Model 1, aHR=2.19 [95% CI=0.99–4.85], P=0.053; Model 2, aHR=3.34 [95% CI=1.44–7.72], P=0.005; Model 3, aHR=3.33 [95% CI=1.44–7.72], P=0.005). However, we found no association between severity according to the visual sum score in bronchiectasis on chest CT and the risk of pneumonia.
Discussion
Our study suggests that radiographic phenotypes including the presence of emphysema, its severity measured based on the visual and Goddard sum score affect ICS with the risk of pneumonia in patients with COPD. Additionally, subtypes of centrilobular and PLE had a higher risk of pneumonia compared with PSE, and emphysema distributed in the upper lobe had a higher risk of pneumonia compared with that in the lower lobe. Moreover, bronchiectasis and emphysema/bronchiectasis overlap phenotype were risk factors for pneumonia in this population.
Several medical studies have reported the enhanced dispersion of inhaled aerosol boluses in emphysema.26,27 Enhancement of aerosol bolus dispersion becomes trapped in the extent of alveolar destruction and peripheral air sacs for a prolonged duration, causing a considerable contribution of intra-alveolar particles and air to more spreading and alteration of the particle peak.37,38 From a physical point of view, in similar severity of emphysema, bolus dispersion continuously increases from centrilobular and PSE to PLE.25 With regard to this physiology, it was assumed that ICS dispersion also increases from CLE and PSE to PLE. However, in our study, PLE and CLE had a higher risk of pneumonia compared to PSE, considering the difficulty in distinguishing confluent or advanced destructive CLE from PLE on chest CT and the relatively low prevalence of PLE with alpha-1-proteinase inhibitor deficiency in Korea compared to Europe or America.39 Furthermore, as applied to ICS particles in previous deposition studies, the size of the alveolar structures has a significant effect on ICS particle deposition, as increased intra-alveolar distances and particulate substances consume additional time to reach the alveolar walls.40,41 Therefore, ICS particle deposition may increase according to the severity of emphysema, and these were associated with an increased risk of pneumonia.
There are several lung deposition data points for inhaled aerosols in subjects with explicitly defined emphysema and COPD. The cross-sectional area of the airways will be smaller; thus, an inhaled aerosol is more at risk to the effect in the more proximal parts of the lung in COPD.42 The central-to-peripheral ratios of aerosol deposition are slightly greater in patients with emphysema with COPD than in healthy subjects.43 Furthermore, pulmonary bioavailability following particle inhalation was modeled as a function of the regional lung deposition pattern and mucociliary clearance rate. Mucociliary clearance is reduced in patients with chronic obstructive lung disease44 and in smokers.45 Therefore, increased central lung deposition and reduced mucociliary clearance rate of ICS particles in emphysema in patients with COPD may lengthen the duration of settlement of ICSs in the lung, which may, in turn, be associated with pneumonia development.
COPD with concomitant bronchiectasis contributed to not only development of high severity of emphysema but also susceptibility to infection or pneumonia. The prevalence of bronchiectasis in patients with COPD ranges from 4% to 72%, and one consistent finding has been the association between the advanced stages or increased severity of emphysema-predominant COPD and the higher prevalence of bronchiectasis.36,46 Furthermore, the COPD and bronchiectasis overlap phenotype has clinical implications for more frequent severe exacerbations and chronic bronchial infections.47,48 It seems that the airway epithelial cells have dysfunction of regeneration and differentiation in patients with COPD and bronchiectasis, which results in reducing the airway barrier and makes it susceptible to infections.49,50
Typical radiological findings of combined pulmonary fibrosis and emphysema (CPFE) in chest CT are emphysema in the upper lobes, and fibrosis and honeycombing in the lower lobes of the lung.51,52 A retrospective study reported that CPFE might be classified as an emphysema-dominant or fibrosis-dominant phenotype according to the severity of LAA% or pulmonary fibrosis on chest CT.53 Although the incidence of interstitial lung disease was only 1.1% and CPFE was not quantitatively evaluated in our study, the result of the present study can be applied with caution, limited to patients with emphysema-dominant CPFE treated with inhaled bronchodilator.
Despite these interesting findings, this study has some limitations. First, our retrospective study included a small number of patients with COPD using ICSs, and it may not be free of selection bias, although the common risk factors for pneumonia in COPD are compatible with those of previous reports. Therefore, to generalize the potential pneumonia risk according to the radiological phenotypes of ICS therapy in COPD, more studies with larger numbers of patients are required. Second, our study did not include patients diagnosed with COPD who did not use ICS. However, as acknowledged by the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency,54 ICS treatment increases the risk of pneumonia in COPD.4–6 Third, the extent and severity of radiographic subtypes were measured using semi-quantitative methods, rather than recently updated quantitative computational methods, because of retrospective data collection. Moreover, the role of small airway dysfunction was not assessed. However, consistent findings through analyses using semi-quantitative methods may reflect the usefulness of relatively crude radiographic assessment. Fourth, the respiratory comorbidities that could affect radiographic phenotypes, including tuberculous destroyed lung, interstitial pneumonia, or lung cancer, were not excluded. However, quantitative evaluation studies on chest CT may be necessary to further clarify the association in the future.
Conclusion
Among patients with COPD treated with ICSs, radiographic phenotypes including the presence of emphysema, bronchiectasis or emphysema/bronchiectasis overlap phenotype, severity with emphysema, subtypes of CLE or PLE, and upper lobe distribution of emphysema measured on chest CT may help predict the risk of pneumonia. Therefore, when physicians consider the risk–benefit of ICSs in patients with COPD, radiographic characteristics and exacerbation history, eosinophil count, and combined asthma may be other determining factors.
Abbreviations
BDR, bronchodilator responsiveness; CLE, centrilobular emphysema; COPD, chronic obstructive pulmonary disease; CT, computed tomography; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroid; LAA%, low attenuation area; mMRC, modified Medical Research Council dyspnea scale; PLE, panlobular emphysema; PSE, paraseptal emphysema; SD, standard deviation; SE, standard error.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and informed consent
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki in 1975. This study was approved by the IRB Committee of Seoul National University Seoul Metropolitan Government (SNU-SMG) Boramae Medical Center and SNUH, and the requirement for informed consent from study subjects for access to electronic medical records was waived (IRB No. 10-2020-37).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-profit sectors.
Disclosure
All authors report no conflicts of interest in relation to this work.
References
1. (GOLD) GIfCOLD. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2021 report. Available from: www.goldcopd.org.
2. 2019 global strategy for prevention, diagnosis and management of COPD; 2019. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
3. 2022 global strategy for diagnosis, management and prevention of chronic obstructive pulmonary disease. Available from: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf.
4. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
5. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
6. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(3):Cd010115. doi:10.1002/14651858.CD010115.pub2
7. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
8. Edsbäcker S, Wollmer P, Selroos O, Borgström L, Olsson B, Ingelf J. Do airway clearance mechanisms influence the local and systemic effects of inhaled corticosteroids? Pulm Pharmacol Ther. 2008;21(2):247–258. doi:10.1016/j.pupt.2007.08.005
9. Rossios C, To Y, To M, et al. Long-acting fluticasone furoate has a superior pharmacological profile to fluticasone propionate in human respiratory cells. Eur J Pharmacol. 2011;670(1):244–251. doi:10.1016/j.ejphar.2011.08.022
10. Johnson M. Development of fluticasone propionate and comparison with other inhaled corticosteroids. J Allergy Clin Immunol. 1998;101(4 Pt 2):S434–9. doi:10.1016/S0091-6749(98)70155-1
11. Johnson M. Pharmacodynamics and pharmacokinetics of inhaled glucocorticoids. J Allergy Clin Immunol. 1996;97(1 Pt 2):169–176. doi:10.1016/S0091-6749(96)80217-X
12. Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch SV. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol. 2014;52(8):2813–2823. doi:10.1128/jcm.00035-14
13. Pragman AA, Kim HB, Reilly CS, Wendt C, Isaacson RE. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS One. 2012;7(10):e47305. doi:10.1371/journal.pone.0047305
14. Donnelly LE, Barnes PJ. Defective phagocytosis in airways disease. Chest. 2012;141(4):1055–1062. doi:10.1378/chest.11-2348
15. Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109(5):571–577. doi:10.1172/JCI0215217
16. Restrepo MI, Sibila O, Anzueto A. Pneumonia in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis. 2018;81(3):187–197. doi:10.4046/trd.2018.0030
17. Bafadhel M, Umar I, Gupta S, et al. The role of CT scanning in multidimensional phenotyping of COPD. Chest. 2011;140(3):634–642. doi:10.1378/chest.10-3007
18. Lynch DA, Austin JH, Hogg JC, et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner society. Radiology. 2015;277(1):192–205. doi:10.1148/radiol.2015141579
19. Mair G, Maclay J, Miller JJ, et al. Airway dimensions in COPD: relationships with clinical variables. Respir Med. 2010;104(11):1683–1690. doi:10.1016/j.rmed.2010.04.021
20. Singh D. Small airway disease in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis. 2017;80(4):317–324. doi:10.4046/trd.2017.0080
21. Martinez-Garcia MA, Miravitlles M. Bronchiectasis in COPD patients: more than a comorbidity? Int J Chron Obstruct Pulmon Dis. 2017;12:1401–1411. doi:10.2147/copd.S132961
22. DiSantostefano RL, Li H, Hinds D, Galkin DV, Rubin DB. Risk of pneumonia with inhaled corticosteroid/long-acting β2 agonist therapy in chronic obstructive pulmonary disease: a cluster analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:457–468. doi:10.2147/copd.S60498
23. Rennard SI. COPD: overview of definitions, epidemiology, and factors influencing its development. Chest. 1998;113(4Suppl):235s–241s. doi:10.1378/chest.113.4_supplement.235s
24. Luijendijk SC, van der Grinten CP. The ratio of the alveolar ventilations of SF6 and He in patients with lung emphysema and in healthy subjects. Respir Physiol Neurobiol. 2002;130(1):69–77. doi:10.1016/S0034-5687(01)00326-7
25. Sturm R. Theoretical diagnosis of emphysema by aerosol bolus inhalation. Ann Transl Med. 2017;5(7):154. doi:10.21037/atm.2017.03.28
26. Verbanck S, Schuermans D, Vincken W, Paiva M. Saline aerosol bolus dispersion. I. The effect of acinar airway alteration. J Appl Psysiol. 2001;90(5):1754–1762. doi:10.1152/jappl.2001.90.5.1754
27. Kohlhäufl M, Brand P, Rock C, et al. Noninvasive diagnosis of emphysema. Aerosol morphometry and aerosol bolus dispersion in comparison to HRCT. Am J Respir Crit Care Med. 1999;160(3):913–918. doi:10.1164/ajrccm.160.3.9811051
28. Saari SM, Vidgren MT, Koskinen MO, Turjanmaa VM, Waldrep JC, Nieminen MM. Regional lung deposition and clearance of 99mTc-labeled beclomethasone-DLPC liposomes in mild and severe asthma. Chest. 1998;113(6):1573–1579. doi:10.1378/chest.113.6.1573
29. Chung KF, Jeyasingh K, Snashall PD. Influence of airway calibre on the intrapulmonary dose and distribution of inhaled aerosol in normal and asthmatic subjects. Eur Respir J. 1988;1(10):890–895.
30. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X
31. Ferguson GT, Papi A, Anzueto A, et al. Budesonide/formoterol MDI with co-suspension delivery technology in COPD: the TELOS study. Eur Respir J. 2018;52(3):1801334. doi:10.1183/13993003.01334-2018
32. Pasteur MC, Bilton D, Hill AT. British thoracic society guideline for non-CF bronchiectasis. Thorax. 2010;65(Suppl 1):i1–58. doi:10.1136/thx.2010.136119
33. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi:10.1148/radiol.2462070712
34. Jairam PM, van der Graaf Y, Lammers JW, Mali WP, de Jong PA. Incidental findings on chest CT imaging are associated with increased COPD exacerbations and mortality. Thorax. 2015;70(8):725–731. doi:10.1136/thoraxjnl-2014-206160
35. Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in pulmonary emphysema. Clin Radiol. 1982;33(4):379–387. doi:10.1016/S0140-6736(07)61602-X
36. Dou S, Zheng C, Cui L, et al. High prevalence of bronchiectasis in emphysema-predominant COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:2041–2047. doi:10.2147/copd.S163243
37. Sturm R, Pawłak E, Hofmann W. Monte-Carlo-Modell der Aerosolbolusdispersion in der menschlichen Lunge--Teil 2: modellvorhersagen für die kranke Lunge [Monte-Carlo-Model for the aerosol bolus dispersion in the human lung--part 2: model predictions for the diseased lung]. Z Med Phys. 2007;17(2):136–143. doi:10.1016/j.zemedi.2006.10.010
38. Hofmann W, Pawlak E, Sturm R. Semi-empirical stochastic model of aerosol bolus dispersion in the human lung. Inhal Toxicol. 2008;20(12):1059–1073. doi:10.1080/08958370802115081
39. Luisetti M, Seersholm N. Alpha1-antitrypsin deficiency. 1: epidemiology of alpha1-antitrypsin deficiency. Thorax. 2004;59(2):164–169. doi:10.1136/thorax.2003.006494
40. Sturm R. Theoretical models for the simulation of particle deposition and tracheobronchial clearance in lungs of patients with chronic bronchitis. Ann Transl Med. 2013;1(1):3. doi:10.3978/j.issn.2305-5839.2012.11.02
41. Sturm R. A computer model for the simulation of nanoparticle deposition in the alveolar structures of the human lungs. Ann Transl Med. 2015;3(19):281. doi:10.3978/j.issn.2305-5839.2015.11.01
42. Thorsson L, Kenyon CJ, Newman S, Borgström L. Lung deposition of budesonide in asthmatics: a comparison of different formulations. Int J Pharmaceut. 1998;168:119–127.
43. Brown JS, Zeman KL, Bennett WD. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med. 2002;166(9):1240–1247. doi:10.1164/rccm.200205-399OC
44. Camner P, Mossberg B, Philipson K. Tracheobronchial clearance and chronic obstructive lung disease. Scand J Respir Dis. 1973;54(5):272–281.
45. Vastag E, Matthys H, Zsamboki G, Köhler D, Daikeler G. Mucociliary clearance in smokers. Eur J Respir Dis. 1986;68(2):107–113.
46. Gatheral T, Kumar N, Sansom B, et al. COPD-related bronchiectasis; independent impact on disease course and outcomes. Copd. 2014;11(6):605–614. doi:10.3109/15412555.2014.922174
47. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi:10.1164/rccm.200912-1843CC
48. Traversi L, Miravitlles M, Martinez-Garcia MA, et al. ROSE: radiology, obstruction, symptoms and exposure - a Delphi consensus definition of the association of COPD and bronchiectasis by the EMBARC Airways Working Group. ERJ Open Res. 2021;7(4):00399–2021. doi:10.1183/23120541.00399-2021
49. Crystal RG. Airway basal cells. The ”smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(12):1355–1362. doi:10.1164/rccm.201408-1492PP
50. Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc. 2014;11 Suppl 5(Suppl5):S252–8. doi:10.1513/AnnalsATS.201402-049AW
51. Sakai F, Tominaga J, Kaga A, et al. Imaging diagnosis of interstitial pneumonia with emphysema (combined pulmonary fibrosis and emphysema). Pulm Med. 2012;2012:816541. doi:10.1155/2012/816541
52. Alsumrain M, De Giacomi F, Nasim F, et al. Combined pulmonary fibrosis and emphysema as a clinicoradiologic entity: characterization of presenting lung fibrosis and implications for survival. Respir Med. 2019;146:106–112. doi:10.1016/j.rmed.2018.12.003
53. Kitaguchi Y, Fujimoto K, Hanaoka M, Honda T, Hotta J, Hirayama J. Pulmonary function impairment in patients with combined pulmonary fibrosis and emphysema with and without airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2014;9:805–811. doi:10.2147/copd.S65621
54. London U. Inhaled corticosteroids (ICS) containing medicinal products indicated in the treatment of chronic obstructive pulmonary disease (COPD). Eur Med Agency. 2016;2016:5.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

