Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
The Influence of Influenza Virus Infections in Patients with Chronic Obstructive Pulmonary Disease
Authors Liao KM , Chen YJ , Shen CW , Ou SK, Chen CY
Received 10 June 2022
Accepted for publication 22 August 2022
Published 14 September 2022 Volume 2022:17 Pages 2253—2261
DOI https://doi.org/10.2147/COPD.S378034
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Kuang-Ming Liao,1 Yi-Ju Chen,2 Chuan-Wei Shen,2 Shao-Kai Ou,2 Chung-Yu Chen2– 4
1Department of Internal Medicine, Chi Mei Medical Center, Chiali, Taiwan; 2School of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan; 3Department of Pharmacy, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan; 4Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
Correspondence: Chung-Yu Chen, School of Pharmacy, Kaohsiung Medical University, No. 100, Shihcyuan 1st Road, Sanmin District, Kaohsiung City, 80708, Taiwan, Tel +886-7-3121101 ext 2375, Fax +886-7-3210683, Email [email protected]
Introduction: Chronic obstructive pulmonary disease (COPD) is a common disease and is preventable and treatable. A previous study showed that influenza virus infections were also associated with the risk of acute exacerbation in patients with COPD, and other studies showed that the influenza virus might increase the risk of stroke. However, studies on the influence of influenza infection among COPD patients are limited. In this study, we review the role of influenza infection in contributing to mortality, pneumonia, respiratory failure, COPD acute exacerbation, and ischemic stroke among COPD patients.
Materials and Methods: We performed a population-based cohort study of COPD patients using data from Taiwan between January 1, 2011, and December 31, 2019. We excluded patients with lung cancer, lung transplantation and asthma. We also excluded patients who lacked COPD medication prescriptions and those treated with anti-influenza drugs without flu diagnosis records. Patients with missing or incomplete data were also excluded from the study cohort.
Results: After 1:1 matching by age, sex, COPD duration, diagnosed years and comorbidities, we enrolled 10,855 cases and controls for further analysis. The risks of pneumonia, respiratory failure, COPD acute exacerbation, and ischemic stroke were 1.770 (95% CI=1.638– 1.860; P< 0.0001), 1.097 (95% CI=1.008– 1.194; P=0.0319), 1.338 (95% CI=1.248– 1.435; P< 0.0001), and 1.134 (95% CI=1.039– 1.239, P=0.0051), respectively, in the influenza infection group compared with COPD patients without influenza infection.
Conclusion: Influenza infections are linked to an increased risk of ischemic stroke, pneumonia, respiratory failure, and COPD acute exacerbation among COPD patients. In conclusion, patients with COPD need to be closely monitored after having an influenza infection.
Keywords: acute exacerbation, chronic obstructive pulmonary disease, influenza virus, pneumonia, stroke, respiratory failure
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic and systemic inflammatory disease associated with airflow obstruction. Patients with COPD may experience an acute exacerbation, which is characterized by worsening symptoms of cough, sputum production, and dyspnea1 and results in lung function decline,2 increased hospital admission, morbidity, mortality,3 deteriorating quality of life,4 significant economic burden and medical expenditure.5 Most exacerbations are caused by infection with either bacteria or viruses,6 in which the primary pathogen is the rhinovirus, and one of the most common viruses is the influenza virus.7 Patients with superimposed bacterial infection or virus and bacteria coinfections may also occur after virus infections8 and progression in the disease process resulting in severe pneumonia or respiratory failure. A previous study also showed that influenza virus infections were also associated with the risk of stroke.9 However, studies on whether influenza infection is associated with an increased risk of stroke among COPD patients are limited. Thus, in this study, we examined the risk of ischemic stroke after influenza infection among patients with COPD in Taiwan. We also review the role of influenza infection in contributing to mortality, pneumonia, respiratory failure, and COPD acute exacerbation among COPD patients.
Methods
Study Design and Data Source
We performed a population-based cohort study of COPD patients using data from the Taiwan National Health Insurance Research Database (NHIRD) between January 1, 2011, and December 31, 2019. The NHIRD was retrieved from claims data of the National Health Insurance (NHI) program, which was launched in 1995 and contained comprehensive pharmacy and medical records. The NHI program covers up to 99.99% of Taiwan’s population, and the database can represent the health condition of the entire Taiwanese population. The study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Boards of Kaohsiung Medical University Hospital (Research Ethics Committee No. KMUHIRB-E(II)-20200044), which waived the requirement for written informed consent.
Definition of Study Cohort
The eligible subjects of the study included patients who were between 40 and 90 years of age and had more than three outpatient diagnoses or more than one inpatient diagnosis of COPD (ICD-9CM: 490–492, 496; ICD-10CM: J40-J44) from January 1, 2012, to December 31, 2018. The exclusion criteria were patients with lung cancer (ICD-9CM: 162; ICD-10CM: C33, C34, C78.00, C78.01, C78.02, C7A.090, Z85.118) or lung transplantation (ICD-9CM: 996.84, V42.6; ICD-10CM: T86.81, Z94.2) one year before cohort entry and patients with asthma (ICD-9CM: 493; ICD-10CM: J45) in the whole study period. We also excluded patients who lacked COPD medication prescriptions and those treated with anti-influenza drugs without a recorded flu diagnosis. Furthermore, patients with missing or incomplete data were also excluded from the study cohort.
Patients diagnosed with influenza (ICD-9CM: 487; ICD-10CM: J09-J11) after COPD from 2012 to 2018 were included in the influenza group, while others without an influenza diagnosis were included in the non-influenza group. We performed propensity-score matching of each influenza case matching to one randomly selected non-influenza case by age, gender, insurance premium, urbanization level, COPD duration, COPD severity, and comorbidities (including pneumonia, hypertension, respiratory failure, diabetes mellitus, hyperlipidemia, malignancy, chronic kidney disease, chronic liver disease, and anemia). The matching comorbidities were potential risk factors of influenza and related complications according to the Centers for Disease Control and Prevention. The index date was defined as the date of the first influenza diagnosis and was assigned to the non-influenza groups by matched grouping.
Outcome Measurement
The influenza and non-influenza groups were followed until the earliest outcomes of interest, including death, NHI program withdrawal, or one year after the index date. The primary outcome measurements included all-cause mortality, pneumonia (ICD-9CM: 480–486; ICD-10CM: J12-J18, B25, B44, A22.1, A37.91), respiratory failure (ICD-9CM: 518.81–518.84; ICD-10CM: J96), acute COPD exacerbation (ICD-9CM: 491.21; ICD-10CM: J44.1), and ischemic stroke (ICD-9CM: 433, 434; ICD-10CM: I63-I66).
Covariate Measurement
The demographic characteristics of COPD patients included age, age group (stratified by ten years), sex, urbanization level (urban, suburban, and rural), and monthly insurance premium rates. Comorbidities, concomitant medications, and COPD-related medications were measured one year before index date. The severity of COPD was divided into severe exacerbations and moderate exacerbations. Severe exacerbations were defined as exacerbations resulting in visiting the emergency room or hospitalization. In contrast, moderate exacerbations were defined as exacerbations not leading to hospital admission, but patients were treated with short-acting β2-agonists (SABA) plus antibiotics or oral corticosteroids. If patients had more than two moderate exacerbations or more than one severe exacerbation within one year, they were classified as having a high exacerbation risk. Instead, others with no exacerbation or one moderate exacerbation were considered low exacerbation risk.
Statistical Analysis
For the characteristics listed at baseline, continuous variables were presented as the means (standard deviation) and were analyzed by Student’s t-test. Categorical variables were shown as the number (percentage) and were analyzed by the chi-square test. We performed a time-to-event analysis by applying the Cox proportional hazard regression model with adjusted potential confounders to assess the risk of one-year outcomes of influenza and non-influenza groups. The potential confounders included age, sex, COPD severity, urbanization, monthly income, comedications, and comorbidities. Kaplan–Meier analysis was also used to compare the incidence of outcomes of interest. Data processing and analysis were conducted with Statistical Analysis Software (SAS), version 9.4 (SAS Institute Inc., Cary, NC). Statistical significance was defined as a P value of <0.05.
Results
Between January 1, 2012, and December 31, 2018, we identified 1,220,236 COPD patients who were followed until the end of 2019 or until death or withdrawal from the national health insurance system. The final cohort consisted of 117,125 COPD patients (Figure 1) after excluding exclusion criteria. There were 13,221 COPD patients with an influenza diagnosis and 103,904 COPD patients without an influenza diagnosis. After 1:1 matching by age, sex, COPD duration, diagnosed years and comorbidities, we enrolled 10,855 cases and controls for further analysis.
 |
Figure 1 Flow diagram of patient selection. |
Demographic characteristics and comorbidities in COPD patients with and without influenza after matching are presented in Table 1. Most patients’ ages were between 70 and 80 years, followed by between 60 and 70 years. There was a higher proportion of males in the study population, accounting for 73.53%. The three most common comorbidities in the COPD group were hypertension (42.86%), diabetes (24.04%), and hyperlipidemia (19.83%) in influenza group.
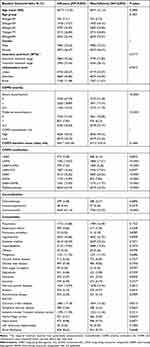 |
Table 1 Demographic Characteristics and Comorbidities in COPD Patients with and without Influenza After Matching |
In Figure 2, there were higher incident rate observed in pneumonia (p<0.001), respiratory failure (p<0.001) and ischemic stroke (p=0.0327). However, there was no significantly difference between case and control group, including all-cause mortality (p=0.0707) and acute exacerbation (p=0.0581). We compared the outcomes, including death, pneumonia, respiratory failure, COPD acute exacerbation, ischemic stroke between COPD patients with and without influenza infection 1 year after the index date. (Table 2). In addition to mortality, the risk of outcome in the influenza infection group cohort was significantly higher than that without influenza infection. The aHRs of pneumonia, respiratory failure, COPD acute exacerbation, and ischemic stroke were 1.770 (95% CI=1.638–1.860; P<0.0001), 1.097 (95% CI=1.008–1.194; P=0.0319), 1.338 (95% CI=1.248–1.435; P<0.0001), and 1.134 (95% CI=1.039–1.239, P=0.0051), respectively.
 |
Table 2 Comparison of Risks of One-Year Outcomes of Interest Between the Influenza and Non-Influenza Groups |
Discussion
This nationwide population-based study showed that compared without influenza infection, patients with COPD and influenza infection had a significantly higher risk of pneumonia, respiratory failure, COPD acute exacerbation, and ischemic stroke incidence after adjusting for age, sex, urbanization level, income, COPD severity, comorbidities, and co-medications listed in baseline characteristics.
Influenza Infection and COPD Acute Exacerbation
Studies have demonstrated that bacteria are responsible for most acute exacerbations in COPD; however, the prevalence of respiratory virus increased after using PCR testing.10 Serological and viral culture techniques can only detect viruses in approximately 30% of patients with COPD acute exacerbation.11 After applying PCR, it was found that viruses are associated with more than half of COPD exacerbations.12 There is growing evidence that host inflammation dysregulates the immune response against viral infection and increases plasma fibrinogen and serum IL-6 levels and associated lung functions.13 Respiratory virus infections are correlated with more severe and frequent exacerbations and may also lead to chronic infection in COPD.14 The mechanism of respiratory viral infections in COPD includes desquamation of epithelial cells in the airway, microvascular dilatation and edema, recruitment of inflammatory cell infiltrate, followed by decreased mucociliary clearance function and bacterial killing by macrophages, leading to bacterial infections and dysregulation of hyperinflammation that leads to disease progression.15 A systematic review combined nineteen studies with 1728 patients and evaluated the literature of the prevalence of respiratory viruses in patients with COPD acute exacerbation detected by PCR and found that rhino/enteroviruses (16.39%), respiratory syncytial virus (9.90%), and influenza (7.83%) were the most common viruses.16 These data present viruses in a meaningful proportion of COPD exacerbations; however, these studies do not demonstrate causality, and respiratory viruses appearing in stable COPD have also been shown.17,18 There are limited data to show the incidence of acute exacerbation after influenza virus infection. According to our findings, patients with COPD after influenza infection are 1.338 times more vulnerable to acute exacerbation.
Pneumonia and Respiratory Failure
There is a small number of clinical studies that delineate the association between influenza infection and COPD. Most studies focus on influenza vaccination reducing COPD exacerbations.19–22 Yap et al23 reported that influenza activity is associated with significant excess hospitalization among elderly individuals aged 65 or above with COPD in Hong Kong, comparable to Western countries. Influenza infection superimposed on secondary bacterial pneumonia was a common and severe complication. It is also the leading cause of morbidity and mortality during influenza pandemics.24–26 After an influenza infection, airway epithelial barrier disruption increases bacterial proliferation and enhances bacterial adherence. In addition, influenza downregulates the host immune response, induces neutrophil apoptosis and dysfunction, and suppresses macrophage phagocytosis to increase bacterial infection.27–30 The study showed that the influenza virus increased pneumonia risk but did not focus on the COPD population, and the risk of respiratory failure was also lacking. From our study, we found that COPD patients had a 1.770- and 1.097-fold increased risk of pneumonia and respiratory failure after influenza infection, respectively, compared with those without influenza infection.
Influenza Infection and Stroke
Influenza infections are linked to an increased risk of ischemic stroke and have been reported in previous research studies. Influenza virus may induce nonspecific proinflammatory and procoagulant changes and worsen underlying atherosclerotic disease.31 Toschke et al32 reported an association between influenza infections and subsequent first-ever stroke. Smeeth et al33 also reported that influenza infection is associated with a risk of stroke. Inflammation can induce atherosclerotic disease and result in a stroke. Kulick et al34 used the New York Statewide Planning and Research Cooperative System (SPARCS) to estimate the risk of ischemic stroke after an influenza-like illness from 2012 to 2014. They found that influenza-like illnesses in the 15 days prior increased the odds of ischemic stroke by 39% (95% CI 1.09–1.77) and increased the odds to approximately 70% when looking at influenza-like illness events over the last year. Our study found that influenza infection significantly increased the risk of ischemic stroke in COPD. COPD itself is a risk factor for ischemic stroke. Compared with patients with COPD without an influenza infection, patients with COPD with an influenza infection were more vulnerable to ischemic stroke. To sum up, due to relative poor outcomes in COPD patients with influenza, annual influenza vaccination is important for this population.
Strengths and Limitations
The strengths of our study include the use of nationwide, large amounts of data regarding the generalized COPD population for 8 years. We also controlled for multiple comorbidities, age, sex and COPD severity. In the cohort study, we showed outcomes after influenza infection, which is likely to indicate a causal relationship. The use of medication-confirmed measures improved COPD and influenza diagnostic specificity. Our population-based approach means that these results may be generalizable to COPD patients in similar conditions.
Our study nevertheless had some limitations. Although analyses used medication-confirmed measures to improve COPD and influenza diagnosis, pulmonary function test data were lacking in our database, and the COPD stage could not be obtained. It was not possible to investigate the dyspnea score of COPD patients. Despite indirect measurements of COPD severity in database analysis, COPD exacerbation frequency was considered because it was an alternative indicator of COPD disease severity.
We were also unable to investigate the pneumonia pathogen. The pneumonia pathogen also could not be identified in our patients. Therefore, we cannot discriminate between different causative organisms in this study. Moreover, nasopharyngeal swab or other reconfirmed tests were lack in database; therefore, influenza diagnosis was evaluated based on ICD codes. Last, smoking condition, personal lifestyle, or environmental effect could not be completely evaluated in NHIRD database. In hence, adjustments could not be made for these risk factors or potential confounders, which may influence the outcomes.
In conclusion, in our nationwide population-based cohort study, influenza infection was positively associated with the risk of pneumonia, respiratory failure, acute exacerbation and stroke. Patients with COPD need to be closely monitored after having an influenza infection. Influenza vaccination is also encouraged among this certain risk group.
Data Sharing Statement
The data supporting the present research findings were sourced from NHIRD in Taiwan. Owing to the legal restrictions imposed by the Government of Taiwan related to the Personal Information Protection Act, the database cannot be made publicly available.
Ethics Statement
This study was approved by the IRB of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20220055). Because patients’ data could not be identified, the requirement for informed consent was waived.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The research reported in this publication was supported by a grant from the Chi-Mei Medical Center and Kaohsiung Medical University Research Foundation (110-CM-KMU-011).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29(6):1224–1238. doi:10.1183/09031936.00109906
2. Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330. doi:10.1164/rccm.201605-1014OC
3. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi:10.1016/S0140-6736(07)61382-8
4. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi:10.1136/thorax.57.10.847
5. Liang L, Shang Y, Xie W, Shi J, Tong Z, Jalali MS. Trends in hospitalization expenditures for acute exacerbations of COPD in Beijing from 2009 to 2017. Int J Chron Obstruct Pulmon Dis. 2020;15:1165–1175. doi:10.2147/COPD.S243595
6. Miravitlles M, Anzueto A. Role of infection in exacerbations of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2015;21:278–283. doi:10.1097/MCP.0000000000000154
7. Mohan A, Chandra S, Agarwal D, et al. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology. 2010;15:536–542. doi:10.1111/j.1440-1843.2010.01722.x
8. Wang H, Anthony D, Selemidis S, Vlahos R, Bozinovski S. Resolving viral-induced secondary bacterial infection in COPD: a concise review. Front Immunol. 2018;9:2345. doi:10.3389/fimmu.2018.02345
9. Lanska DJ, Hoffmann RG. Seasonal variation in stroke mortality rates. Neurology. 1999;52:984–990. doi:10.1212/WNL.52.5.984
10. Beasley V, Joshi PV, Singanayagam A, et al. Lung microbiology and exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:555–569. doi:10.2147/COPD.S28286
11. Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest. 2000;117(Suppl 2):380S–385S. doi:10.1378/chest.117.5_suppl_2.380S
12. Singanayagam A, Joshi PV, Mallia P, Johnston SL. Viruses exacerbating chronic pulmonary disease: the role of immune modulation. BMC Med. 2012;10:27. doi:10.1186/1741-7015-10-27
13. Rohde G, Borg I, Wiethege A, et al. Inflammatory response in acute viral exacerbations of COPD. Infection. 2008;36(5):427–433. doi:10.1007/s15010-008-7327-5
14. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi:10.1164/ajrccm.164.9.2105011
15. Frickmann H, Jungblut S, Hirche TO, Groß U, Kuhns M, Zautner AE. The influence of virus infections on the course of COPD. Eur J Microbiol Immunol. 2012;2(3):176–185. doi:10.1556/EuJMI.2.2012.3.2
16. Zwaans WA, Mallia P, van Winden ME, Rohde GG. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—a systematic review. J Clin Virol. 2014;61(2):181–188. doi:10.1016/j.jcv.2014.06.025
17. McManus TE, Marley AM, Baxter N, et al. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102:1575–1580. doi:10.1016/j.rmed.2008.06.006
18. Rohde G, Wiethege A, Borg I, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case–control study. Thorax. 2003;58:37–42. doi:10.1136/thorax.58.1.37
19. Poole PJ, Chacko E, Wood-Baker RW, et al. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev;2006. CD002733. doi:10.1002/14651858.CD002733.pub2
20. Gorse GJ, Otto EE, Daughaday CC, et al. Influenza virus vaccination of patients with chronic lung disease. Chest. 1997;112:1221–1233. doi:10.1378/chest.112.5.1221
21. Nath KD, Burel JG, Shankar V, et al. Clinical factors associated with the humoral immune response to influenza vaccination in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:51–56. doi:10.2147/COPD.S53590
22. Wongsurakiat P, Maranetra KN, Wasi C, et al. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest. 2004;125(6):2011–2020. doi:10.1378/chest.125.6.2011
23. Yap FH, Ho PL, Lam KF, Chan PK, Cheng YH, Peiris JS. Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong Kong. J Med Virol. 2004;73(4):617–623. doi:10.1002/jmv.20135
24. McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014;12:252–262. doi:10.1038/nrmicro3231
25. Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309:275–282. doi:10.1001/jama.2012.194139
26. Smith AM, Adler FR, Ribeiro RM, et al. Kinetics of coinfection with influenza A virus and Streptococcus pneumoniae. PLoS Pathog. 2013;9:e1003238. doi:10.1371/journal.ppat.1003238
27. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi:10.1128/CMR.00058-05
28. Cheng YH, You SH, Lin YJ, et al. Mathematical modeling of post-coinfection with influenza A virus and Streptococcus pneumoniae, with implications for pneumonia and COPD-risk assessment. Int J Chron Obstruct Pulmon Dis. 2017;12:1973–1988. doi:10.2147/COPD.S138295
29. Bosch AA, Biesbroek G, Trzcinski K, et al. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9:e1003057. doi:10.1371/journal.ppat.1003057
30. McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun. 2006;74:6707–6721. doi:10.1128/IAI.00789-06
31. Singanayagam A, Singanayagam A, Elder DHJ, et al. Is community-acquired pneumonia an independent risk factor for cardiovascular disease? Eur Respir J. 2012;39:187–196. doi:10.1183/09031936.00049111
32. Toschke AM, Heuschmann PU, Wood O, Wolfe CD. Temporal relationship between influenza infections and subsequent first-ever stroke incidence. Age Ageing. 2009;38:100–103. doi:10.1093/ageing/afn232
33. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi:10.1056/NEJMoa041747
34. Kulick ER, Alvord T, Canning M, et al. Risk of stroke and myocardial infarction after influenza-like illness in New York State. BMC Public Health. 2021;21(1):864. doi:10.1186/s12889-021-10916-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

