Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Acute Exacerbation of Chronic Obstructive Pulmonary Disease Due to Carbapenem-Resistant Klebsiella pneumoniae-Induced Pneumonia: Clinical Features and Prognostic Factors
Authors Ouyang P, Zhou Z, Pan C, Tang P, Long S, Liao X, Liu Q, Xie L
Received 16 November 2023
Accepted for publication 21 February 2024
Published 7 March 2024 Volume 2024:19 Pages 683—693
DOI https://doi.org/10.2147/COPD.S447905
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Richard Russell
Pengwen Ouyang,1 Zhijie Zhou,2 Chanyuan Pan,3 Peijuan Tang,1 Sheng Long,1 Xiangjian Liao,1 Qiong Liu,1 Liangyi Xie1
1Department of Clinical Laboratory, Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, People’s Republic of China; 2Department of Respiratory Medicine, The Affiliated Zhuzhou Hospital of Xiangya School of Medicine, Central South University, Zhuzhou, People’s Republic of China; 3Department of Respiratory Medicine, Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, People’s Republic of China
Correspondence: Liangyi Xie, Email [email protected]
Purpose: Carbapenem-resistant Klebsiella pneumoniae (CRKP) is closely related to respiratory tract infection. The aim of this study was to investigate the clinical features and prognostic factors of CRKP-induced pneumonia in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients.
Methods: A single-centre, retrospective case-control study on COPD patients hospitalized for acute exacerbation and CRKP-induced pneumonia was conducted from January 1, 2016, to December 31, 2022. The mortality rate of acute exacerbation due to CRKP-induced pneumonia was investigated. The patients were divided into the CRKP-induced pneumonic acute exacerbation (CRKPpAE) group and the non-CRKP-induced pneumonic acute exacerbation (non-CRKPpAE) group, and the clinical characteristics and prognostic factors were compared using univariate analysis and multivariate analysis.
Results: A total of 65 AECOPD patients were included, composed of 26 patients with CRKPpAE and 39 patients with non-CRKPpAE. The mortality rate of CRKPpAE was 57.69%, while non-CRKPpAE was 7.69%. Compared with non-CRKPpAE, a history of acute exacerbation in the last year (OR=8.860, 95% CI: 1.360– 57.722, p=0.023), ICU admission (OR=11.736, 95% CI: 2.112– 65.207, p=0.005), higher NLR levels (OR=1.187, 95% CI: 1.037– 1.359, p=0.013) and higher D-dimer levels (OR=1.385, 95% CI: 1.006– 1.905, p=0.046) were independently related with CRKPpAE. CRKP isolates were all MDR strains (26/26, 100%), and MDR strains were also observed in non-CRKP isolates (5/39, 12.82%).
Conclusion: Compared with non-CRKPpAE, CRKPpAE affects the COPD patient’s condition more seriously and significantly increases the risk of death.
Keywords: chronic obstructive pulmonary disease, acute exacerbation, carbapenem-resistant K. pneumoniae, pneumonia
Introduction
Carbapenem-resistant Klebsiella pneumoniae (CRKP) is a notorious pathogen worldwide that can cause pneumonia, bloodstream infections, urinary tract infections or wound infections, which are often difficult or even impossible to treat.1 Carbapenems often serve as the final effective line of defense against infections caused by multidrug-resistant K. pneumoniae, and multiple mechanisms contribute to the development of drug-resistant strains.2 In China, the prevalence of CRKP has shown an alarming upward trend and has become a serious threat to public health due to high drug resistance, hypervirulence, and high fatality rates.3,4 Worryingly, this trend has been observed globally.5
Chronic obstructive pulmonary disease (COPD) is a complex and heterogeneous lung disease characterized by persistent, progressive airflow obstruction. Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is a state in which respiratory symptoms of COPD are rapidly exacerbated, with bacterial infection being one of the main causes.6 When AECOPD is complicated by bronchial infection or pneumonia, in-hospital mortality significantly increases, particularly in elderly patients.7 In 2015, 99.9 million Chinese adults suffered from COPD, making it the third most common chronic disease after hypertension and diabetes in China.8 Acute exacerbation increases the frequency of hospitalization in COPD patients, seriously affects the quality of life, and commonly accompanies a heavy economic burden.9
Previous studies have reported the association of Haemophilus influenzae or Streptococcus pneumoniae with AECOPD.10 However, pathogen types in COPD patients’ sputum appear to vary by population and geographic location. In China, some studies have identified K. pneumoniae as one of the most common pathogens (Pseudomonas aeruginosa, Acinetobacter baumannii and Staphylococcus aureus in addition) in the sputum of AECOPD patients.11,12 In recent years, there has been a rapid escalation in the drug resistance of CRKP, concurrent with a persistent surge in reported mortality rates associated with this formidable pathogen.13 At present, CRKP is mainly reported in medical institutions, and evidence indicating its proliferation beyond the confines of hospital settings has been found.14 COPD patients often visit healthcare institutions, potentially increasing the risk of future CRKP infection. In fact, CRKP is a pathogen closely associated with respiratory infection, and its impact tends to be more severe in elderly patients.15 Simultaneously, studies have shown a substantial increase in the risk of mortality following CRKP infection in individuals diagnosed with COPD.16,17
However, there is a paucity of current studies on CRKP-induced pneumonia in AECOPD patients. Here, we reported the prevalence of CRKP-induced pneumonia in AECOPD patients, and we retrospectively analyzed the clinical characteristics and prognostic factors of COPD patients with acute exacerbation due to CRKP-induced pneumonia. The results will help the diagnosis and treatment of CRKP-induced pneumonia in AECOPD patients.
Methods
Study Design
We conducted a retrospective case-control study in Hunan Provincial People’s Hospital (the First Affiliated Hospital of Hunan Normal University). The study period spanned from January 1, 2016 to December 31, 2022, and the subjects were AECOPD patients hospitalized for K. pneumoniae-induced pneumonia during this period. The diagnosis of AECOPD was made by the pulmonologist according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, the patient’s COPD history, and the clinical presentation of acute changes in symptoms.18 The diagnosis of K. pneumoniae-induced pneumonia was based on the patient’s clinical manifestations (characterized by intensified cough, increased sputum volume or purulent sputum, fever, shortness of breath, or a combination of these symptoms), findings from lung imaging (infiltrates detected on chest X-ray or CT scans), elevated peripheral white blood cell counts, and deep sputum or bronchoalveolar lavage fluid cultures positive for K. pneumoniae. All cases presented with either AECOPD as the primary diagnosis or a primary diagnosis of pulmonary infection, with AECOPD identified as a secondary diagnosis. Patients received at least one antibiotic with in vitro antimicrobial activity within 5 days of the pneumonia diagnosis. Patients with negative sputum or bronchoalveolar lavage cultures, non-K. pneumoniae cultured, respiratory tract colonization, incomplete hospitalization records, active pulmonary tuberculosis, or co-infection (defined as bacterial pathogens other than K. pneumoniae cultured within 48 hours),19 as well as those without AECOPD or pulmonary infection as the main diagnosis, were excluded. In cases where a patient had multiple hospitalizations for K. pneumoniae-induced pneumonia, only the initial hospitalization was documented for the study.
Data Collection
The patient’s hospitalization information was searched through medical records, including demographic data, smoking habits, admission routes, comorbidities, previous acute exacerbation (defined as at least one documented history of institutional visit due to acute exacerbation of respiratory symptoms in the past year), and prior use of carbapenems. Data collection also encompassed the results of blood cell tests, C-reactive protein tests, arterial blood gas tests, liver function tests, kidney function tests, serum ion tests, and coagulation tests conducted at the time of infection. Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) were calculated from blood cell analysis results. Stable-phase pulmonary function results were also recorded. Using the extracted data, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score for each patient was calculated based on the worst variable obtained at the time of infection to assess disease severity.20 Simultaneously, the treatment process and outcome of each patient were documented.
Microbiological Analysis
Deep sputum samples or bronchoalveolar lavage fluid samples were cultured in the clinical microbiology laboratory for identification and antibacterial susceptibility testing by VITEK MS (bioMérieux, Marcy-l’Étoile, France) or VITEK-2 compact system (bioMérieux, Marcy-l’Étoile, France). The interpretive criteria for tigecycline were based on the Food and Drug Administration (FDA) guidelines, while those for colistin were based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines. The other drugs were interpreted according to the M100 performance standards for antimicrobial susceptibility testing of the Clinical Laboratory Standards Institute (CLSI) 2015–2021 editions. CRKP was defined by K. pneumoniae with resistance to imipenem or meropenem. Multidrug-resistant (MDR) strains were defined according to the previous study.21 Patients were divided into the CRKP-induced pneumonic acute exacerbation (CRKPpAE) group and the non-CRKP-induced pneumonic acute exacerbation (non-CRKPpAE) group.
Statistical Analysis
Enumeration data were expressed as frequency (%), and the chi-squared test or Fisher’s exact probability method was used for comparison between groups. Normality of the distribution of numerical variables was checked by the Kolmogorov–Smirnov test. Normally distributed variables were described using mean ± standard deviation (SD) and compared using independent samples t-test. Non-normally distributed variables were described using median with 1st and 3rd quartiles and compared using the Mann–Whitney U-test. After checking for collinearity, variables associated with CRKP-induced pneumonia (p value <0.10) were allowed into a forward conditional binary logistic regression model to identify independently associated variables. The fitness of the multivariate model was performed using the Hosmer-Lemeshow goodness-of-fit test. All statistical analyses were conducted using IBM SPSS Statistics software, version 21.0 (SPSS Inc, Chicago, IL, USA). A two-tailed p value <0.05 was considered statistically significant.
Results
Patient Information and Clinical Characteristics
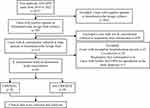
4227 non-duplicated AECOPD cases were investigated, and ultimately 65 cases hospitalized for acute exacerbation due to K. pneumoniae-induced pneumonia were analyzed from January 1, 2016 to December 31, 2022 (Figure 1). Most patients were male (63/65, 96.9%), the minimum age was 54 years old, and the average age was 74.65±9.52 years old. The CRKPpAE group comprised 26 patients, while the non-CRKPpAE group consisted of 39 patients. Patients in the CRKPpAE group were older than those in the non-CRKPpAE group (77.73±8.53 and 72.59±9.69 years, respectively, p=0.032). Patients in the CRKPpAE group were admitted more often through the emergency department (17/26, 65.38% and 7/39, 17.95%, respectively, p<0.001). A history of acute exacerbation in the past year (17/26, 65.38% and 15/39, 38.46%, respectively, p=0.033) and prior use of carbapenems (13/26, 50.00% and 3/39, 7.69%, respectively, p<0.001) were significantly associated with CRKPpAE. Patients in the CRKPpAE group generally required mechanical ventilation (18/26, 69.23% and 7/39, 17.95%, respectively, p<0.001) and ICU admission (19/26, 73.08% and 5/39, 12.82%, respectively, p<0.001), and showed more severe disease severity [The median of APACHE II scores with interquartile range were 25.25 (18.00, 29.60) and 15.00 (13.00, 18.00), respectively, p<0.001]. In addition, the risk of in-hospital death was significantly higher in the CRKPpAE group compared to the the non-CRKPpAE group (all-cause mortality 15/26, 57.69% and 3/39, 7.69%, respectively, p<0.001) (Table 1).
 |
Table 1 Clinical Features of CRKP-Induced Pneumonia in AECOPD Patients |
Univariate Analysis of Laboratory Test Results
By comparing the results of laboratory tests, the white blood cell counts [12.25(7.10, 16.89) and 6.75(4.94, 8.81) ×109/L, respectively, p<0.001], neutrophil counts [11.04(6.18, 14.79) and 5.09(3.52, 6.77) ×109/L, respectively, p<0.001], NLR levels [14.13(4.64, 22.39) and 5.06(3.72, 9.80), respectively, p<0.001], red blood cell distribution width coefficient of variation values [14.80(13.30, 16.05) and 13.70(12.80, 14.70) %, respectively, p=0.035] and mean platelet volume values [10.90(10.00, 11.65) and 10.00(9.10, 10.80) fL, respectively, p=0.026] of patients in the CRKPpAE group were significantly higher than those in the non-CRKPpAE group. However, hemoglobin values [104.50(84.50, 130.00) and 128.00(104.00, 144.00) g/L, respectively, p=0.033] and hematocrit values [32.80(26.53, 41.20) and 39.70(33.80, 44.40) %, respectively, p=0.043] in the CRKPpAE group were lower than those in the non-CRKPpAE group. C-Reactive protein values [47.85 (23.69, 133.00) and 9.33 (3.34, 40.40) mg/L, respectively, p<0.001] in the CRKPpAE group were significantly higher than those in the non-CRKPpAE group. For the CRKPpAE patients, there was a more serious liver function damage [direct bilirubin values: 14.16(5.10, 18.27) and 4.80(3.19, 7.40) μmol/L, respectively, p=0.004, alanine aminotransferase values: 38.48(19.15, 307.15) and 23.80(13.80, 28.20) U/L, respectively, p=0.022, aspartate transaminase values: 25.83(18.38, 45.74) and 19.00(13.51, 25.58) U/L, respectively, p=0.004], and a higher risk of hypoalbuminemia [albumin values: 32.68(30.07, 34.05) and 39.40(36.50, 41.11) g/L, respectively, p<0.001]. Blood urea nitrogen values [11.57(6.01, 19.14) and 6.00(4.52, 7.68) mmol/L, respectively, p=0.002] of patients in the CRKPpAE group were higher than those in the non-CRKPpAE group, and there was a significant difference in the serum potassium levels between the two groups [4.13(3.89, 4.69) and 3.85(3.59, 4.12) mmol/L, respectively, p=0.013]. Through coagulation analysis, the CRKPpAE group exhibited higher values in prothrombin time [12.06(11.18, 13.45) and 10.90(10.40, 12.40) s, respectively, p=0.011], international normalized ratio [1.01(0.97, 1.18) and 0.95(0.90, 1.09), p=0.013], activated partial thromboplastin time [32.65(28.38, 38.28) and 29.40(27.30, 31.80) s, respectively, p=0.030] and D-dimer [2.45(1.62, 6.36) and 0.46(0.35, 1.22) mg/L, respectively, p<0.001] compared to the non-CRKPpAE group (Table 2). Stable-phase pulmonary function results were accessible for a subset of just 13 patients, all of whom were categorized in the non-CRKPpAE group. Due to the limited sample size, a comparative analysis of stable-phase pulmonary function results was not conducted.
 |
Table 2 Comparison of Laboratory Test Results Between the CRKPpAE Group and the Non-CRKPpAE Group |
Multivariate Analysis of Risk Factors
The factors that significant at p <0.10 in univariate analysis were allowed into the logistic regression model to identify independent risk factors. We found that a history of acute exacerbation in the past year (OR=8.860, 95% CI: 1.360–57.722, p=0.023), ICU admission (OR=11.736, 95% CI: 2.112–65.207, p=0.005), higher NLR levels (OR=1.187, 95% CI: 1.037–1.359, p=0.013) and higher D-dimer levels (OR=1.385, 95% CI: 1.006–1.905, p=0.046) were independently associated with CRKPpAE (Figure 2).
 |
Figure 2 Multivariate analysis of clinical characteristics of patients with CRKPpAE. Abbreviations: ICU, intensive care unit; NLR, neutrophil-lymphocyte ratio. |
Comparison of Drug Resistance Between CRKP and Non-CRKP
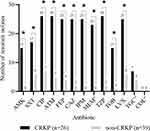
CRKP isolates exhibited significantly higher resistance to the majority of tested antimicrobials [including amikacin (57.69% and 0% respectively), trimethoprim/sulfamethoxazole (65.38% and 15.38% respectively), ciprofloxacin (100.00% and 17.95% respectively), aztreonam (100.00% and 12.82% respectively), cefepime (96.15% and 10.26% respectively), ceftazidime (96.15% and 5.13% respectively), imipenem (96.15% and 0% respectively), meropenem (95.83% and 0% respectively), piperacillin/tazobactam (100.00% and 5.13% respectively), tobramycin (57.69% and 2.56% respectively), and levofloxacin (96.15% and 15.38% respectively), all p<0.001] compared to non-CRKP isolates (Figure 3). Strains resistant to tigecycline were found (CRKP, 7/24, 29.17% and non-CRKP, 4/33, 12.12%, respectively), while all strains exhibited sensitivity to colistin. All CRKP isolates were MDR strains (26/26, 100.00%), while 5 non-CRKP isolates were MDR strains (5/39, 12.82%).
Discussion
CRKP infections are constantly being reported, and CRKP-induced pneumonia in AECOPD patients deserves more attention. Here, we conducted a retrospective study of acute exacerbation of COPD caused by CRKP-induced pneumonia in a tertiary hospital in China, reported the clinical features and mortality of CRKP-induced pneumonia, and analyzed factors related to prognosis.
Our results showed that the mortality rate of acute exacerbation of COPD caused by CRKP-induced pneumonia reached a staggering 57.69%. It is higher than the 42.1% in-hospital mortality rate reported in another study on CRKP infection in respiratory intensive care unit.22 Of the patients with CRKPpAE, 65.38% had at least one documented medical visit for acute exacerbation in the past year, a frequency higher than that observed in the non-CRKPpAE group. The CRKP-induced pneumonia could be attributed to healthcare-associated infections. Current research data on CRKPpAE are very limited. Previous studies have found exacerbation in the past year to be an independent risk factor for exacerbation readmission in patients with COPD.23 We speculate that a history of acute exacerbation led to a decline in lung health, providing an opportunity for CRKP infection. Studies have shown that CRKP carries more drug resistance and virulence determinants than non-CRKP, which make it more invasive.24 Together with the increased chances of contact with CRKP during frequent medical visit, CRKPpAE occurred. The existence of drug resistance and virulence determinants may be the reasons why patients with CRKPpAE were admitted to ICU. On the other hand, impairment of tracheal clearance in patients and wide distribution of CRKP in the ICU may also contribute to the above association.25,26 It is noteworthy that many studies have shown that prior use of carbapenems was an independent risk factor for CRKP infection.25,27 However, in our study, this association was not statistically significant through multivariate analysis, possibly owing to the relatively modest sample size in our study.
NLR and PLR are emerging markers closely associated with poor prognosis in AECOPD patients.28 In our study, higher NLR levels were independently associated with CRKPpAE by multivariate analysis, suggesting that CRKPpAE resulted in more severe inflammatory responses. Coagulation abnormalities often occur in AECOPD patients, with infection being one of the significant contributing factors.29 We found that higher D-dimer levels were independently associated with CRKPpAE, indicating that coagulation disorders were more severe when CRKPpAE occurred, and vigilance for thrombosis should be maintained.
In our study, CRKP not only resistant to carbapenems, but also had high resistance to many other types of antibacterial drugs, and all of them were MDR strains. Although isolates resistant to tigecycline were identified, they all remained sensitive to colistin. The emergence of drug resistance in these strains posed significant challenges to clinical treatment. Colistin and tigecycline could be regarded as potential treatment choices, and there was also the option to consider combination therapy.30 Notably, MDR strains were also found in non-carbapenem-resistant isolates. Proactively controlling the spread of resistant strains will aid in the successful recovery of patients undergoing treatment.
This was a single-center retrospective study and was limited by a relatively small sample size. Larger-scale research data will help increase the credibility of the conclusions. The incidence of CRKP-induced pneumonia in patients with AECOPD may be underestimated due to the limitations of sputum culture and co-infection. Unfortunately, we were unable to collect stable-phase pulmonary function data for all patients. Furthermore, we were not able to study the impact of early CRKP respiratory colonization on infection and outcomes. Given that patients with COPD often seek medical help, and CRKP is widespread in the hospital environment, analyzing the status of CRKP colonization in the respiratory tract of these patients would be of great value.
As a result, our report showed that CRKP-induced pneumonia significantly contributed to the acute exacerbation of COPD. The frequent exacerbations and medical visits might have contributed to the occurrence of CRKPpAE events. The salient clinical features of these patients were mainly manifested by more severe inflammatory response and coagulation disturbance, and the risk of ICU admission was significantly increased. CRKP showed high resistance to a variety of antibacterial drugs, which brought challenges to treatment. Our findings will contribute to clinical decision-making in CRKPpAE.
Conclusion
Our study reveals a significantly higher mortality rate in AECOPD with CRKP-induced pneumonia compared to non-CRKP-induced pneumonia. Additionally, a history of acute exacerbation in the last year, ICU admission, higher NLR levels, and elevated D-dimer levels are independently associated with AECOPD due to CRKP-induced pneumonia.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of Hunan Provincial People’s Hospital (the First Affiliated Hospital of Hunan Normal University) (approval number [2023]-160). Informed consent was waived due to the retrospective study and no intervention in patient treatment. This research was conducted in compliance with the tenets of the Helsinki Declaration.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang M, Earley M, Chen L, et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): a prospective, multicentre, cohort study. Lancet Infect Dis. 2022;22(3):401–412. doi:10.1016/S1473-3099(21)00399-6
2. Minerdi D, Loqui D, Sabbatini P. Monooxygenases and antibiotic resistance: a focus on carbapenems. Biology. 2023;12(10):1316. doi:10.3390/biology12101316
3. Hu F, Zhu D, Wang F, et al. Current status and trends of antibacterial resistance in China. Clin Infect Dis. 2018;67:S128–S134. doi:10.1093/cid/ciy657
4. Chen J, Ma H, Huang X, et al. Risk factors and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary-care hospital in China: an eight-year retrospective study. Antimicrob Resist Infect Control. 2022;11:161. doi:10.1186/s13756-022-01204-w
5. Lan P, Jiang Y, Zhou J, et al. A global perspective on the convergence of hypervirulence and carbapenem resistance in Klebsiella pneumoniae. J Glob Antimicrob Resist. 2021;25:26–34. doi:10.1016/j.jgar.2021.02.020
6. Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: gold executive summary. Eur Respir J. 2023;61:2300239. doi:10.1183/13993003.00239-2023
7. Song Y, Chen R, Zhan Q, et al. The optimum timing to wean invasive ventilation for patients with AECOPD or COPD with pulmonary infection. Int J Chron Obstruct Pulmon Dis. 2016;11:535–542. doi:10.2147/COPD.S96541
8. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–1717. doi:10.1016/S0140-6736(18)30841-9
9. Kunadharaju R, Sethi S. Treatment of acute exacerbations in chronic obstructive pulmonary disease. Clin Chest Med. 2020;41:439–451. doi:10.1016/j.ccm.2020.06.008
10. Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. doi:10.1016/S0140-6736(11)60968-9
11. Lin SH, Kuo PH, Hsueh PR, et al. Sputum bacteriology in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease in Taiwan with an emphasis on Klebsiella pneumoniae and Pseudomonas aeruginosa. Respirology. 2007;12(1):81–87. doi:10.1111/j.1440-1843.2006.00999.x
12. Ma X, Cui J, Wang J, et al. Multicentre investigation of pathogenic bacteria and antibiotic resistance genes in Chinese patients with acute exacerbation of chronic obstructive pulmonary disease. J Int Med Res. 2015;43(5):699–710. doi:10.1177/0300060515587577
13. Rodrigo-Troyano A, Sibila O. The respiratory threat posed by multidrug resistant Gram-negative bacteria. Respirology. 2017;22(7):1288–1299. doi:10.1111/resp.13115
14. Esposito F, Cardoso B, Sellera FP, et al. Expansion of healthcare-associated hypervirulent KPC-2-producing Klebsiella pneumoniae ST11/KL64 beyond hospital settings. One Health. 2023;17:100594. doi:10.1016/j.onehlt.2023.100594
15. Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2):e01882–17. doi:10.1128/AAC.01882-17
16. Qian Y, Bi Y, Liu S, et al. Predictors of mortality in patients with carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis and a systematic review. Ann Palliat Med. 2021;10(7):7340–7350. doi:10.21037/apm-21-338
17. Gonçalves Barbosa LC, Silva ESJA, Bordoni GP, et al. Elevated mortality risk from CRKp associated with comorbidities: systematic review and meta-analysis. Antibiotics. 2022;11(7):874. doi:10.3390/antibiotics11070874
18. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
19. Chen IR, Huang PH, Wu PF, et al. Clinical characteristics and outcomes of 56 patients with pneumonia caused by carbapenem-resistant Klebsiella pneumoniae. J Glob Antimicrob Resist. 2021;25:326–330. doi:10.1016/j.jgar.2021.03.028
20. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
21. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
22. Zhang H, Wang J, Zhou W, et al. Risk factors and prognosis of carbapenem-resistant Klebsiella pneumoniae infections in respiratory intensive care unit: a retrospective study. Infect Drug Resist. 2021;14:3297–3305. doi:10.2147/IDR.S317233
23. Wei X, Ma Z, Yu N, et al. Risk factors predict frequent hospitalization in patients with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:121–129. doi:10.2147/COPD.S152826
24. Yang X, Dong N, Chan EW, et al. Carbapenem resistance-encoding and virulence-encoding conjugative plasmids in Klebsiella pneumoniae. Trends Microbiol. 2021;29(1):65–83. doi:10.1016/j.tim.2020.04.012
25. Lou T, Du X, Zhang P, et al. Risk factors for infection and mortality caused by carbapenem-resistant Klebsiella pneumoniae: a large multicentre case-control and cohort study. J Infect. 2022;84(5):637–647. doi:10.1016/j.jinf.2022.03.010
26. Zhang Y, Yu S, Chen C, et al. Comprehensive surveillance and sampling reveal carbapenem-resistant organism spreading in tertiary hospitals in China. Infect Drug Resist. 2022;15:4563–4573. doi:10.2147/IDR.S367398
27. Li J, Li Y, Song N, Chen Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. J Glob Antimicrob Resist. 2020;21:306–313. doi:10.1016/j.jgar.2019.09.006
28. Zinellu A, Zinellu E, Mangoni AA, et al. Clinical significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute exacerbations of COPD: present and future. Eur Respir Rev. 2022;31:220095. doi:10.1183/16000617.0095-2022
29. Liu M, Hu R, Jiang X, et al. Coagulation dysfunction in patients with AECOPD and its relation to infection and hypercapnia. J Clin Lab Anal. 2021;35:e23733. doi:10.1002/jcla.23733
30. Agyeman AA, Bergen PJ, Rao GG, Nation RL, Landersdorfer CB. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int J Antimicrob Agents. 2020;55(1):105833. doi:10.1016/j.ijantimicag.2019.10.014
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.