Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Radiofrequency Ablation of Hepatocellular Carcinomas Adjacent to the Gallbladder Without Isolation Under Contrast-Enhanced Ultrasound Monitoring: A Comparative Study with Long Term Follow-Up
Authors Luo L, Yan R, Zeng Q, Long Y, He X , Li K, Xu E
Received 5 September 2022
Accepted for publication 20 March 2023
Published 13 April 2023 Volume 2023:10 Pages 631—642
DOI https://doi.org/10.2147/JHC.S388738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mohamed Shaker
Liping Luo,1,2,* Ronghua Yan,3,4,* Qingjing Zeng,2 Yinglin Long,2 Xuqi He,2 Kai Li,2 Erjiao Xu1
1Department of Medical Ultrasonics, The Eighth Affiliated Hospital of Sun Yat-sen University, Shenzhen, People’s Republic of China; 2Department of Medical Ultrasonics, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China; 3Department of Radiology, Peking University Shenzhen Hospital, Shenzhen, People’s Republic of China; 4Department of Radiology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Erjiao Xu, Department of Medical Ultrasonics, The Eighth Affiliated Hospital of Sun Yat-sen University, No. 3025, Shennanzhong Road, Shenzhen, 518033, People’s Republic of China, Email [email protected] Kai Li, Department of Ultrasound, The Third Affiliated Hospital of Sun Yat-sen University, 600 Tianhe Road, Guangzhou, 510630, People’s Republic of China, Email [email protected]
Objective: This study intends to compare the efficacy and safety between patients undergoing invasive isolation or monitoring measures and patients undergoing intra-operative contrast-enhanced ultrasound (CEUS) monitoring who underwent radiofrequency ablation (RFA) of hepatocellular carcinomas (HCC) adjacent to the gallbladder (GB).
Methods: We retrospectively assessed patients with HCC adjacent to the GB who underwent ultrasound-guided RFA. They were divided into two groups: group A was monitored under intra-operative CEUS, while group B was assisted by invasive auxiliary means. The efficacy, complications and survival were followed up and compared.
Results: Thirty-eight patients with 39 HCCs were enrolled into group A and 31 patients with 35 HCCs were enrolled into group B. The technique efficacy rates were both 100% in the two groups. There were no significant differences of the cumulative 1-, 3-, and 5-year local tumor progression, tumor-free survival and overall survival between the two groups (P = 0.851, 0.081 and 0.700, respectively). There were no significant differences of major and minor complications rates between the two groups (P = 1.000, 0.994, respectively). More importantly, no GB related complications occurred in group A.
Conclusion: Intra-operative CEUS monitoring without protective isolation of the GB might be also a potentially safe and effective method for the RFA of HCC adjacent to the GB, when compared with those assisted with invasive auxiliary means.
Keywords: hepatocellular carcinoma, gallbladder, contrast-enhanced ultrasound, thermal ablation, monitoring
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor in the world and the third cause of cancer-related mortality as estimated by the World Health Organization.1 Liver transplantation, hepatectomy and radiofrequency ablation (RFA) are considered as three radical modalities for early-stage HCC.2 As a safe, effective and minimally invasive therapeutic means, RFA has been recommended as a first-line treatment for HCC.2,3 However, RFA is considered as a relative contraindication for tumors adjacent to extrahepatic critical organs, such as the gallbladder (GB), extrahepatic bile duct and intestines.3–5 The collateral thermal damage caused by ablation cannot be ignored. For the GB, if thermal damage occurs, it can cause some serious major complications such as cholecystitis and perforation.6–10 Meanwhile, to avoid the thermal damage to the GB, the ablation range tends to be moderated. This leads to an increased incidence of residual and local tumor progression (LTP).11,12
For the RFA of the HCC adjacent to the GB, various auxiliary measures have been reported in the literature. Some of these studies reported GB isolation methods including artificial ascites or needle decompression.6,13,14 Some of these studies reported the use of a thermometric needle inserted in the GB fossa or laparoscopy monitoring to predict the potential thermal damage to the GB.15,16 Even cholecystectomy before or immediately after RFA was also reported to prevent the potential thermal damage.17 However, all these extra auxiliary measures were invasive which may increase the cost and operative trauma.
Previously, a few articles had reported that the RFA of HCC adjacent to the GB without extra auxiliary means was feasible and safe.12,18 However, due to lack of an effective monitoring technique, the incidences of LTP were as high as 14%.
Contrast-enhanced ultrasound (CEUS) was recommended for immediate evaluation of the treatment effect after ablation.19 At the same time, it was regarded as an effective evaluation method for the microvascular perfusion in non-liver organs,20 including the GB. So that we supposed thermal damage of the GB could be monitored by CEUS evaluation of the perfusion of the GB wall. We have successfully reported RFA of liver cancers adjacent to the GB without extra invasive measures under the monitoring of intra-operative CEUS.21 The preliminary results showed that the complete ablation rate was 100% (23/23), no LTP and no serious complications of the GB occurred in all patients with a median follow-up time of 17 months. However, this was a single arm and small sample study with a short follow-up period. The results of the previous study confirmed the feasibility of CEUS monitoring, but could not cover the long-term efficacy and late complications, such as long-term biliary complications. In addition, it needs to be compared with invasive gallbladder isolation, which was more frequently reported, to further verify the safety and effectiveness of the CEUS monitoring method for RFA of HCC adjacent to the GB.
Therefore, in this study, we intend to compare the long-term follow-up results between the patients undergoing invasive isolation or monitoring measures and the patients who employed CEUS monitoring who underwent RFA of the HCC adjacent to the GB.
Materials and Methods
Study Population
From January 2016 to December 2019, patients with HCC adjacent to the GB underwent ultrasound-guided RFA in the Third Affiliated Hospital of Sun Yat-sen University were consecutively included in this retrospective study. The inclusion criteria of this study were as follows: (1) patients diagnosed with HCC; (2) HCC within Milan criteria (one lesion up to 5 cm, or two to three lesions with the maximum diameter <3 cm); (3) the distance between the lesion and the GB less than 10 mm; (4) all parameters agreed with the indication for the RFA of HCC. The exclusion criteria were: (1) patients lost to follow up; (2) patients treated by RFA combined with ethanol injection; (3) patients allergic to ultrasound contrast agents (UCAs).
The diagnosis of HCC was based on the biopsy pathological result or was consistent with LI-RADS 5 criteria on contrast-enhance computed tomography/magnetic resonance imaging (CECT/CEMR).2,22
All the patients were divided into two groups. Patients in group A were ablated without additional auxiliary means under the monitoring of CEUS. Patients in group B were ablated with additional auxiliary means (ie: artificial ascites, or laparoscopy).
This study was in compliance with the Declaration of Helsinki. Informed consent of RFA and CEUS was obtained from each patient. The study was approved by the Medical Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University (the approval number is [2022]02-162-01).
Equipment
RFA was performed using the Cool-tip RFA system (Covidien, Mansfield, MA, USA) with a 17-gauge, internally cooled-tip electrode with a 3 cm tip. The RF generator was usually set in the impedance mode with maximum output. Four to twelve minutes were used for each insertion under the real-time ultrasound monitoring during RFA procedure.
MyLab Twice ultrasound machine (Esaote, Genoa, Italy) with the convex probe CA541 (frequency: 1–8 MHz) and GE Logiq E9 (General Electric, United States of America) with C1-6 VN (frequency: 1–6 MHz) were used for the ablation procedure.
Pre-Ablation Assessment of the GB
The clinical signs and imaging findings of the GB were assessed before the RFA procedure. Especially, cholecystitis and cholecystolithiasis were recorded. The thickness of the GB walls on the liver side and the shortest distance between the lesion and the GB were measured.
RFA Procedure
All patients underwent ultrasound-guided percutaneous RFA under general anesthesia by one of three experienced interventional physicians (X.E.J., Z.R.Q. and L.K.) with more than ten years of experience in liver ablation procedures. To avoid the difference of technical experience, the RFA procedure for group A was performed by a single experienced interventional physician (X.E.J.). Usually, the direction of the electrode insertion was parallel to the GB wall, and a minimum distance of 5 mm to the GB wall was maintained in group A. While in group B, the minimum distance was not restricted. According to the size of the lesion, single or overlapped multiple punctures were used to encompass the whole lesion and an additional 5–10 mm of the normal liver parenchyma around the tumor to achieve a sufficient ablative margin (AM), whenever possible (because the lesion adjacent to the GB fossa, the ablative margin of position in GB fossa was not always necessary. The ablation zone only needed to cover the lesion completely at this position).
Invasive Extra Auxiliary Measures in Group B
Artificial ascites were used to separate the HCC lesion adjacent to the GB from the GB by injecting normal saline (about 500–1000 mL) into the GB fossa through the indwelling drainage tube to avoid thermal damage.
For the patients undergoing laparoscopy or open surgery combined RFA, some isolation means were used: 1) the GB could be pulled away by surgical instruments, 2) the gauze was placed in the gallbladder fossa, 3) artificial ascites were produced.
Moreover, for some patients with cholecystolithiasis or cholecystitis, laparoscopic cholecystectomy (LC) was performed before or after the RFA procedure to avoid the occurrence of GB thermal damage.
CEUS Monitoring and Evaluation
SonoVue (Bracco, Milan, Italy) was used in this study which was injected as a rapid bolus of 1.0–2.0 mL via the antecubital vein with 5 mL normal saline for flushing. When necessary, SonoVue could be injected repeatedly.
Monitoring of the GB Wall
The thickness of the GB wall was measured on ultrasound images during and after the RFA procedure. Thickening of the GB wall was defined as an increase in the thickness of the GB wall of more than 2 mm. In group A, CEUS was employed to evaluate the perfusion of the GB wall after each RFA cycle when the electrode insertion came close to the GB wall (≤10 mm) during the RFA procedure. When the RFA cycles were away from the GB wall, CEUS was not applied routinely. After the RFA procedure was complete, CEUS was carried out to confirm the intact perfusion of the GB wall. Intact perfusion was considered when both the serosa and the mucosa were perfused continuously and uniformly. In contrast, if a perfusion defect was observed on the GB wall, the perfusion was considered to be not intact, and cholecystectomy or drainage would be suggested in order to avoid GB perforation.
Immediate Evaluation of Treatment Response
After the RFA completed the ablation plan, CEUS was used to evaluate the treatment response immediately. If no-perfusion zone covered the whole lesion and the surrounding had at least 5 mm normal liver parenchyma (except for the position in GB fossa), the procedure was considered to be technically successful. If not, supplementary ablation was performed immediately.
Follow-Up and Subsequent Treatment
To exclude early complications and assess the thickness and integrity of the GB wall, ultrasound examination and laboratory examination were carried out within 72 h after the RFA procedure both in group A and group B. Acute cholecystitis and perforation were diagnosed by the clinical manifestations and imaging finding. In group B, with patients who were assisted by artificial ascites, the peritoneal drainage tube was usually removed 1–3 days after the RFA process. CECT/CEMR was the standard reference for the evaluation of technique efficacy which was performed within one to three months after the RFA procedure. If technical efficacy was established, the patients conducted regular follow-ups every three months according to the standardization of tumor ablation.23 When residual, LTP or intrahepatic distant recurrence (IDR) appeared during subsequent follow-up, appropriate treatments were performed and percutaneous ablation was preferred. The follow-up of all patients ended before December 31, 2021 or the date of liver transplantation. The residual, LTP, intrahepatic distant recurrence (IDR), extrahepatic recurrence (ER), overall survival (OS), tumor free survival (TFS), complications and side effects (especially those related to the GB) were monitored and recorded during the follow-up period.
Data Analysis
All statistical analyses were accomplished by SPSS 22.0.0 (SPSS, Chicago, IL, USA). The measurement data were presented as the average ± standard deviation if they displayed a normal distribution (age, follow-up period) or as the median (range) if the data were not normally distributed (the thickness of GB wall, maximum diameter, distance to GB). Mann Whitney U-test was used for the comparison of non-parametric data (maximum diameter, distance to GB, and the thickness of GB wall before and after RFA between two groups et al). Comparison of the thickness of the GB wall both in group A and group B before and after RFA were performed by a t-test between two paired samples. Chi-square test was used for the comparison of percentages. The cumulative incidences of OS, TFS, LTP and IDR were displayed using a Kaplan-Meier curve and compared by the Log rank test. A P-value less than 0.05 indicated significant differences.
Results
Enrollment
Finally, 69 patients with 74 HCCs adjacent to the gallbladder were enrolled in this study. Sixty-four patients had one tumor and 5 patients had two tumors adjacent to the gallbladder. Among 69 patients, 38 patients with 39 HCCs who received RFA therapy without additional auxiliary means under the monitoring of CEUS were categorized into group A and other 31 patients with 35 HCCs were categorized into group B. The baseline characteristics of the patients and lesions are shown in Table 1. The baseline characteristics between the two group had no statistical significance (P > 0.05).
 |
Table 1 The Baseline Characteristics of the Two Groups |
RFA Procedure and Evaluation
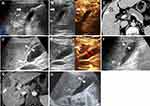
In group A, no invasive extra auxiliary measure was used in all patients. All the GBs were preserved after RFA. Under intra-operative CEUS monitoring during the RFA procedures, the perfusion of the GB wall was intact in all 38 patients (Figure 1). No further emergency cholecystectomy or drainage was needed in these patients.
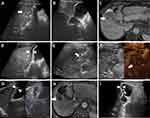
In group B, artificial ascites were utilized in 21 patients to isolate the GB and lesions (Figure 2). All the drainage tubes were successfully removed within 1–3 days after ablation, and no related complications occurred. Two patients underwent RFA combined with hepatectomy or laparoscopic colectomy. During the operation, surgical instruments were used to isolate the GB and lesions. Another eight patients underwent LC after the RFA procedure because of gallbladder polyps, gallstones or due to a history of severe cholecystitis. As a result, only 23 of the 31 patients in group B had their GB preserved.
According to the CEUS evaluation, immediately after the RFA procedure, all lesions in both groups were successfully ablated. The technical success rate for RFA for both groups was 100% (38/38) and 100% (31/31).
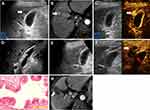
However, for one patient in group B with cholelithiasis who was meant to undergo LC after RFA (Figure 3), a perfusion defect of the GB wall was found by accident during the CEUS evaluation after the RFA procedure of the lesion adjacent to the GB (0 mm to the GB). The GB wall was considered to have had thermal damage. During the LC procedure, the GB wall was observed to be blackened by carbonization. The postoperative pathology confirmed the necrosis of the serosa and mucosa of the GB wall. In the other seven patients, a mild serosal edema was found in these GB walls by postoperative pathology.
Follow Up
Technique Efficacy Rate
No residual was detected in all lesions of both groups by CECT/MR one month after RFA, and the technical efficacy rate of group A and group B were both 100% (P = 1.00).
LTP, IDR and ER
During follow-up, there were two cases with LTP in group A and two cases in group B. And IDR occurred in 15 patients of group A and 18 patients of group B. Their subsequent treatments are shown in Table 2.
 |
Table 2 Therapeutic Effect and Re-Treatment |
The 1-, 3-, and 5-year cumulative LTP rates and cumulative IDR rates in groups A and B are shown in Table 3 and Figure 4. The cumulative LTP rates between group A and group B had no significant differences (P = 0.851, Figure 4A). While the cumulative IDR rate of group B was higher than that of group A (P = 0.039, Figure 4B).
 |
Table 3 Cumulative LTP, IDR, OS and TFS Rates of Group A and Group B |
 |
Figure 4 Comparison of the cumulative local tumor progression (A), intrahepatic distant recurrence (B), overall survival (C) and tumor free survival (D) curves between group A and group B. |
Among these patients, a total of 15 patients developed extrahepatic recurrences. There were no significant differences between these two groups (P = 0.878, Table 2).
Complications and Side Effects
The median thickness of the GB wall after RFA was 7 mm (range 2–12 mm) in group A and 4 mm (range 2–7 mm) in group B (Table 4). Thickening of the GB wall was diagnosed in 14 patients in group A. While, thickening of the GB wall was also diagnosed in 4 patients in group B.
 |
Table 4 Thickness of Gallbladder Wall |
The thicknesses of the GB wall, both in group A and group B, after RFA were thicker than those before RFA (P < 0.001 and P = 0.004). While the thickness of the GB wall after RFA in group A was thicker than that in group B (P = 0.001). However, there was no significant difference in the proportion of patients with thickening of the GB wall (thickening >2 mm) between group A and group B (P = 0.106) (Table 4).
No further treatment was required for the thickening of these GB walls. The thickness of the GB wall returned to the pre-ablation level in 7 patients (50.0%, 7/14) of group A and all 4 patients (100%, 4/4) of group B within a follow-up period of 1–5 months (Table 4). No apparent cholecystitis or perforation appeared in these gallbladder-preserving patients of both groups during the follow-up period. CECT/CEMR images one month after RFA confirmed integrity of the GBs in these gallbladder-preserving patients.
Three patients of group A and two patients of group B had major complications with an incidence rate of 7.9% (3/38) and 6.5% (2/31). Drainage was used to treat massive pleural effusions in three patients and a hemothorax in one patient. These thoracic complications were caused by the treatment of lesions adjacent to the diaphragm in patients with multiple lesions. One patient in Group B occurred peritonitis one week after RFA procedure which was treated by drainage combined with antibiotics. There was no definite manifestation of GB thermal damage in this case.
The minor complication rates were 28.9% (11/38) in group A and 29.0% (9/31) in group B (Table 4). They recovered spontaneously or after conservative treatment. There was no statistically significant difference in the incidence rate of major and minor complications between the two groups (P = 1.000 and P = 0.994).
Cumulative OS and TFS
During the follow-up period, one patient in group A died of liver failure, and one patient in group B died of severe intestinal obstruction. Another three patients in group A and three patients in group B died of HCC progression. The cumulative OS and cumulative TFS in group A and group B are shown in Table 3. There were no significant differences between group A and group B with these two results (P = 0.700, Figure 4C; P = 0.081, Figure 4D).
Discussion
HCC adjacent to the GB is considered as a high-risk site for thermal ablation in the literature, including guidelines of HCC management, which affects the efficacy and safety of thermal ablation.4,5 Several articles had reported a higher incidence of tumor residue and progression because of the limited ablation zone to reduce the thermal damage.12,24 For the thermal ablation of this site, most of the current clinical practices and literatures employ a variety of invasive methods to assist ablation.6,13,15,17
Previously, we reported the preliminary results of RFA of liver tumors without GB isolation under the monitoring of CEUS, suggesting that this strategy might be safe, feasible and effective.21 However, there are a lack of long-term follow-up results and comparative studies.
In this study, we compared the strategies of ultrasound guided RFA of HCC adjacent to the GB with or without extra invasive auxiliary methods in our hospital with long-term follow-up. The results showed that RFA of HCC without GB isolation under the monitoring of CEUS had similar efficacy and safety to RFA with different extra invasive auxiliary means (mainly artificial ascites isolation).6,13 There were no significant differences between group A and group B in terms of local tumor control rate (technical efficacy and LTP), survival rate (OS and TFS), or the incidence of complications (major and minor complications) (P > 0.05). On the basis of the preliminary study, it was further proved that RFA of HCC without GB isolation under the monitoring of CEUS was feasible, safe and effective. According to our practical experience, for the RFA of HCC located at the high-risk site close to the GB, we adopted the strategy of shortening the distance between the electrode and the GB wall (within 5–10 mm) and applying intraoperative CEUS monitoring to evaluate the perfusion of the GB wall and tumor necrosis after RFA, which improved the efficacy and ensured the safety. The results of local tumor control rate (technical efficacy and LTP) in group A (94.9%, 37/39) were better than those reported in the literature on thermal ablation of HCC adjacent to the GB (82.6–92.0%).12,13,15,25 At the same time, there was no increase in the incidence of major complications and thermal damage of the GB. Although the cumulative IDR rate in group B was higher than that in group A, the IDR was not only related to the ablation effect, but also related to the patient’s own conditions and tumor heterogeneity.3 To some extent, the lesion size in Group B was slightly larger than that in group A (but not statistically significant), which might also lead to the higher recurrence rate. But at least it showed that the RFA of HCC without GB isolation under the monitoring of CEUS had no adverse effect on IDR results.
Our results also showed that although there were no GB related complications in group A, the incidence of GB wall thickening in group A (36.8%) was higher than that in group B (17.4%) and in previous studies (6.1–27.8%),6,13 which was mainly related to the edema of the GB wall caused by heating due to no isolation of the GB.7,26 This judgment could also be confirmed by the specimen pathology of patients undergoing cholecystectomy in group B. When the intra-operative CEUS images of these patients were reviewed, no incomplete perfusion of gallbladder wall was found. In group A, the GB wall of 14 patients was significantly thicker than that before ablation (>2 mm), of which 50% recovered in the follow-up period. While in another 50% of these 14 patients, the GB wall thickness did not return to the pre-ablation level, but there were no symptoms of cholecystitis during the follow-up period. In group B, there were also four patients with GB wall thickening, but 100% recovered, indicating that GB isolation still had a certain protective effect on the GB wall.
In group B, a patient with HCC adjacent to the GB, complicated with cholelithiasis, planned to undergo LC after RFA. Because the radiofrequency electrode was too close to the GB (<5 mm) during RFA, the perfusion defect of the GB wall was observed during intraoperative CEUS in this patient. The thermal damage of the GB wall was confirmed by the post-operative pathology result, which verified the reliability of intra-operative CEUS monitoring of RFA strategy. Once the GB perfusion defect was detected on CEUS, the interventional therapy should be done in time, such as drainage or cholecystectomy, so as to avoid serious complications.
There were some limitations of this study. Firstly, this is a single center study. Secondly, the sample size of this study is still small. Thirdly, in group A, in order to ensure the consistency of ablation strategy, it was performed by a single experienced interventional doctor. Fourthly, this study is a non-randomized controlled comparative study. Therefore, it is necessary to carry out a multi-center, larger sample study with long-term follow-up with randomized control in the future to verify the effect of RFA without GB isolation under the monitoring of intra-operative CEUS.
In conclusion, intra-operative CEUS monitoring without protective isolation of GB might be a potentially safe and effective method for RFA of HCC adjacent to the GB, when compared with those assisted with invasive auxiliary means such as artificial ascites.
Abbreviations
HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; GB, gallbladder; LTP, local tumor progression; CEUS, contrast-enhanced ultrasound; UCAs, ultrasound contrast agents; CT, computed tomography; MR, magnetic resonance; AM, ablative margin; LC, laparoscopic cholecystectomy; IDR, intrahepatic distant recurrence; ER, extrahepatic recurrence; OS, overall survival; TFS, tumor free survival; MWA, microwave ablation; PEI, percutaneous ethanol injection; TACE, trans-arterial chemoembolization.
Acknowledgments
Liping Luo and Ronghua Yan should be considered co-first authors.
Funding
This work was supported by the National Natural Science Foundation of China (82272011), the Natural Science Foundation of Guangdong Province, China (2022A1515012155), the Shenzhen Science and Technology Program (JCYJ20220530160208018) and the Futian Healthcare Research Project (FTWS2020022, FTWS2020073, FTWS2020074, FTWS2020075, FTWS2021071).
Disclosure
No potential conflict of interest was reported by the authors.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. European Association for the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
3. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
4. Vivarelli M, Montalti R, Risaliti A. Multimodal treatment of hepatocellular carcinoma on cirrhosis: an update. World J Gastroenterol. 2013;19(42):7316–7326. doi:10.3748/wjg.v19.i42.7316
5. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
6. Chen M, Yang W, Yan K, et al. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33(4):428–436. doi:10.1007/s00261-007-9283-4
7. Lee J, Rhim H, Jeon YH, et al. Radiofrequency ablation of liver adjacent to body of gallbladder: histopathologic changes of gallbladder wall in a pig model. AJR Am J Roentgenol. 2008;190(2):418–425. doi:10.2214/AJR.07.2526
8. Shin KY, Heo J, Kim JY, et al. A case of hemocholecyst associated with hemobilia following radiofrequency ablation therapy for hepatocellular carcinoma. Korean j Hepatol. 2011;17(2):148–151. doi:10.3350/kjhep.2011.17.2.148
9. Akahane M, Koga H, Kato N, et al. Complications of percutaneous radiofrequency ablation for hepatocellular carcinoma: imaging spectrum and management. Radiographics. 2005;25(Suppl 1):S57–S68. doi:10.1148/rg.25si055505
10. Zeng J, Qin Z, Zhou L, et al. Comparison between cryoablation and irreversible electroporation of rabbit livers at a location close to the gallbladder. Radiol Oncol. 2016;51(1):40–46. doi:10.1515/raon-2017-0003
11. Chen MH, Wei Y, Yan K, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006;17(4):671–683. doi:10.1097/01.RVI.0000201985.61501.9E
12. Kim SW, Rhim H, Park M, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10(4):366–376. doi:10.3348/kjr.2009.10.4.366
13. Choi IY, Kim PN, Lee SG, Won HJ, Shin YM. Efficacy and safety of radiofrequency ablation for focal hepatic lesions adjacent to gallbladder: reconfiguration of the ablation zone through probe relocation and ablation time reduction. J Vasc Interv Radiol. 2017;28(10):1395–1399. doi:10.1016/j.jvir.2017.06.004
14. Fernandes DD, Shyn PB, Silverman SG. Gallbladder needle decompression during radiofrequency ablation of an adjacent liver tumour. Can Assoc Radiol J. 2012;63(3):S37–S40. doi:10.1016/j.carj.2011.05.003
15. Huang H, Liang P, Yu XL, et al. Safety assessment and therapeutic efficacy of percutaneous microwave ablation therapy combined with percutaneous ethanol injection for hepatocellular carcinoma adjacent to the gallbladder. Int J Hyperthermia. 2015;31(1):40–47. doi:10.3109/02656736.2014.999017
16. Jiang K, Su M, Zhao X, et al. ”One-off” complete radiofrequency ablation of hepatocellular carcinoma adjacent to the gallbladder by a novel laparoscopic technique without gallbladder isolation. Cell Biochem Biophys. 2014;68(3):547–554. doi:10.1007/s12013-013-9736-z
17. de la Serna S, Vilana R, Sánchez-Cabús S, et al. Results of laparoscopic radiofrequency ablation for HCC. Could the location of the tumour influence a complete response to treatment? A single European centre experience. HPB. 2015;17(5):387–393. doi:10.1111/hpb.12379
18. Chopra S, Dodd GR, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol. 2003;180(3):697–701. doi:10.2214/ajr.180.3.1800697
19. Dietrich CF, Nolsøe CP, Barr RG, et al. Guidelines and good clinical practice recommendations for Contrast-Enhanced Ultrasound (CEUS) in the liver-update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46(10):2579–2604. doi:10.1016/j.ultrasmedbio.2020.04.030
20. Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version). Ultraschall Med. 2018;39(2):e2–e44. doi:10.1055/a-0586-1107
21. Long YL, Yan RH, Li K, et al. Radiofrequency ablation of liver cancers adjacent to the gallbladder without gallbladder isolation under contrast-enhanced ultrasound monitoring: a preliminary study. Int J Hyperthermia. 2019;36(1):139–145. doi:10.1080/02656736.2018.1539776
22. Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289(3):816–830. doi:10.1148/radiol.2018181494
23. Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--A 10-year update. Radiology. 2014;273(1):241–260. doi:10.1148/radiol.14132958
24. Cai L, Li H, Guo J, et al. Drug-eluting beads-transarterial chemoembolization plus microwave ablation is an effective and safe treatment strategy in treating hepatocellular carcinoma adjacent to gallbladder. Am J Transl Res. 2021;13(7):7677–7686.
25. Li M, Yu XL, Liang P, Dong BW, Liu FY. Ultrasound-guided percutaneous microwave ablation for hepatic malignancy adjacent to the gallbladder. Int J Hyperthermia. 2015;31(6):579–587. doi:10.3109/02656736.2015.1014869
26. Hao Z, Wan C, Han Q, et al. Comparison of the gallbladder damage caused by microwave ablation and cryoablation in vivo porcine livers. Indian J Cancer. 2015;52(Suppl 2):e84–e90. doi:10.4103/0019-509X.172520
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.