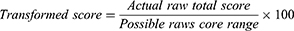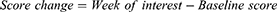Back to Journals » Patient Related Outcome Measures » Volume 13
Psychometric Validation of the Haemo-QOL-A in Participants with Hemophilia A Treated with Gene Therapy
Authors Quinn J, Delaney KA, Wong WY, Miesbach W , Bullinger M
Received 20 January 2022
Accepted for publication 7 July 2022
Published 18 July 2022 Volume 2022:13 Pages 169—180
DOI https://doi.org/10.2147/PROM.S357555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Howland
Jennifer Quinn,1 Kathleen A Delaney,2 Wing Yen Wong,2 Wolfgang Miesbach,3 Monika Bullinger4
1BioMarin Pharmaceuticals UK Ltd, London, UK; 2BioMarin Pharmaceutical Inc., Novato, CA, USA; 3Medical Clinic 2, Institute of Transfusion Medicine, University Hospital Frankfurt, Frankfurt, Germany; 4Department of Medical Psychology, University Medical Center Hamburg Eppendorf, Hamburg, Germany
Correspondence: Jennifer Quinn, BioMarin Pharmaceuticals UK Ltd, 10 Bloomsbury Way, London, WC1A 2SL, UK, Tel +44 7976 129 039, Email [email protected]
Purpose: The hemophilia-specific health-related quality of life (HRQOL) questionnaire (Haemo-QOL-A) is validated for detecting QOL changes following standard therapy for hemophilia A, but has not been rigorously evaluated after gene therapy. This post hoc analysis evaluated the psychometric properties of Haemo-QOL-A in adult people with severe hemophilia A (PWSHA) receiving valoctocogene roxaparvovec (AAV5-hFVIII-SQ) in 2 clinical trials (phase 1/2, NCT02576795; phase 3, NCT03370913).
Patients and Methods: Adult PWSHA (factor VIII levels ≤ 1 IU/dL) received 1 AAV5-hFVIII-SQ infusion (6× 1013 vg/kg). Participants were assessed using the Haemo-QOL-A and the EuroQOL (EQ)-5D-5L and visual analog scale (VAS) questionnaires pre- and post-infusion. Psychometric analyses included convergent and discriminant validity, internal consistency, and reliability. Clinically important difference (CID) was estimated using 3-point change in EQ-5D-5L VAS as anchor.
Results: Haemo-QOL-A data were analyzed from 7 (phase 1/2, 3-year follow-up) and 16 participants (phase 3, 26-week analysis). Change in Haemo-QOL-A Total Scores correlated with EQ-5D-5L VAS score change at 26 weeks (Pearson’s correlation 0.77). At 26 weeks, increased Haemo-QOL-A Physical Functioning was associated with decreased EQ-5D-5L Pain and Discomfort and decreased Anxiety and Depression (Spearman’s Rank correlations − 0.73 and − 0.62, respectively, P < 0.01). Internal consistency analysis showed good reliability for all domains (Cronbach’s alpha > 0.7) except Treatment Concern (Cronbach’s alpha = 0.31). Anchor-based CID estimates were met for Haemo-QOL-A Total Score (≥ 5.5) and domain scores (≥ 6) for Consequences of Bleeding, Physical Functioning, Role Functioning, and Worry.
Conclusion: Our preliminary results suggest that the Haemo‐QOL‐A is a valid, reliable instrument for HRQOL assessment in PWSHA undergoing gene therapy. Future research should be undertaken to confirm these findings in a larger number of participants.
Keywords: severe hemophilia A, gene therapy, quality of life, Haemo-QOL-A, psychometric testing, clinically important difference
Plain Language Summary
Hemophilia is a bleeding disorder where blood does not clot normally because of a genetic mutation that causes missing or defective clotting protein. People with severe hemophilia have painful spontaneous bleeding in their joints, which can lead to chronic pain and disability. Gene therapy, which introduces the instructions to make the missing/defective clotting protein and prevent spontaneous bleeding, is being investigated as a potential treatment for severe hemophilia. Gene therapy for severe hemophilia A is a new treatment that is much different from standard, intensive hemophilia treatments. The Haemo-QOL-A questionnaire is used to evaluate health-related quality of life in people with hemophilia on standard treatments. In this manuscript, we determine whether the Haemo-QOL-A questionnaire can be used to evaluate change in quality of life for people with severe hemophilia A after receiving gene therapy. Using data from previous clinical trials of the gene therapy valoctocogene roxaparvovec (AAV5-hFVIII-SQ), we show that the Haemo-QOL-A did measure a change in quality of life after gene therapy. We also estimate how much change in Haemo-QOL-A scores from before to after gene therapy represents a clinically meaningful improvement in quality of life. This threshold can be used in future research evaluating the effects of gene therapy on quality of life for people with hemophilia. While the results of this study are important, only 23 people were included in the analysis. To confirm our results, future analyses with larger numbers of participants are needed.
Introduction
Hemophilia is a genetic bleeding disorder caused by deficiency or inactivity of factor VIII (FVIII; hemophilia A) or factor IX (hemophilia B) protein. Hemophilia A accounts for approximately 80% of hemophilia cases worldwide.1 Severe hemophilia A (FVIII <1 IU/dL) is characterized by recurrent and spontaneous musculoskeletal bleeding episodes, resulting in joint damage, mobility issues, and early mortality.1 People with severe hemophilia A (PWSHA) experience significant health-related quality of life (HRQOL) impairment, including negative effects on emotional and cognitive health, joint pain, poor functioning in school, and difficulties securing and maintaining employment.2–7
Treatment options for hemophilia A are rapidly evolving. Standard of care for PWSHA is prophylactic factor replacement therapy using exogenous FVIII or bypassing agents such as emicizumab-kxwh.1,8 Factor replacement therapy must be administered frequently through intravenous infusions (1–4 infusions/week), resulting in substantial treatment burden and potential risk of breakthrough bleeding following poor treatment adherence.9 Despite prophylactic treatment, most PWSHA still require on-demand treatment with FVIII or emicizumab to treat bleeding episodes.10
Gene therapies using recombinant adeno-associated viral (AAV) vectors carrying human FVIII gene may offer a novel approach to hemophilia A treatment.11–13 In phase 1/2 and phase 3 studies, a 6×1013 vg/kg infusion of valoctocogene roxaparvovec, an investigational gene therapy utilizing a codon-optimized AAV serotype 5 vector encoding a B-domain–deleted human FVIII (AAV5-hFVIII-SQ), resulted in clinically relevant reductions in annualized treated bleed rate and exogenous FVIII replacement up to 5 and 1 years of follow-up, respectively.11,12,14,15 If long-term efficacy of viral vector gene transfer is established, it could represent a paradigm shift in hemophilia A treatment.
The general health questionnaire EuroQOL-5D-5L (EQ-5D-5L) is used to evaluate overall HRQOL.16 However, people with hemophilia completing the EQ-5D-5L reported higher health states than the general population, indicating the presence of a disability paradox in PWSHA.17 Thus, HRQOL assessment with the EQ-5D-5L scores may not accurately reflect the hemophilia-related burden they experience.18 To address the need for a reliable HRQOL assessment, the hemophilia-specific HRQOL questionnaire for adults (Haemo-QOL-A) was developed.19 It consistently performs well among adults relative to other hemophilia-specific questionnaires, demonstrating robust validity in people undergoing standard treatment regimens.20–23
Gene therapy is a novel, potentially one-time intervention. To date, HRQOL has been evaluated only in a small number of PWSHA receiving gene therapy.14 It is unclear how gene therapy will incrementally benefit the HRQOL for PWSHA compared with standard of care or whether the Haemo-QOL-A will be an appropriate measure to detect HRQOL changes after gene therapy.24 Thus, in addition to estimating Haemo-QOL-A clinically important differences (CID) specifically for gene therapy recipients, this post hoc analysis of phase 1/2 and phase 3 clinical trial data aimed to evaluate the content validity, construct validity, and reliability of the Haemo-QOL-A for measuring HRQOL in adult PWSHA treated with gene therapy.
Materials and Methods
Study Design and Treatments
A post hoc psychometric analysis to determine the content validity, construct validity and reliability of the Haemo-QOL-A was conducted using data from a phase 1/2 open-label dose-escalation study (Clinicaltrials.gov, NCT02576795; EudraCT, 2014-003880-38)12 and phase 3 open-label, single-arm study (NCT03370913, EudraCT, 2017-003215-19) to evaluate the efficacy and safety of valoctocogene roxaparvovec in adult PWSHA.15 Full study design details have been published previously.11,12,14,15 All protocols were reviewed and approved by local institutional review boards or ethics panels and conducted according to the Declaration of Helsinki; all participants provided informed consent.
All participants were male and ≥18 years old with severe hemophilia A (FVIII levels ≤1 IU/dL). The phase 1/2 dose-escalation study included participants on FVIII prophylaxis or on-demand therapy who had ≥12 bleeding episodes within 12 months prior to enrollment; participants received an infusion of AAV5-hFVIII-SQ at 6×1012 vg/kg, 2×1013 vg/kg, 4×1013 vg/kg, or 6×1013 vg/kg. The ongoing phase 3 study included participants from 48 sites worldwide who were on prophylactic FVIII replacement therapy for ≥12 months prior to enrollment; all participants received an infusion of 6×1013 vg/kg AAV5-hFVIII-SQ.
Analysis Populations
Data from up to 3 years of follow-up for participants in the phase 1/2 study who received 6×1013 vg/kg dose of AAV5-hFVIII-SQ were used. The phase 3 study intention-to-treat (ITT) population was defined as all enrolled and treated participants, and the modified ITT (mITT) population was defined as all treated participants who were human immunodeficiency virus (HIV)-negative and who completed the week 26 visit. Data up to 26 weeks from both populations were used in these analyses.
Assessments
Patient HRQOL was assessed using the Haemo-QOL-A questionnaire in both study populations, administered at baseline and at weeks 1, 2, 3, 4, 16, 28, 52, 78, 104, 130, and 156 in the phase 1/2 study population and at baseline and weeks 4, 12, and 26 in the phase 3 study population. The EQ-5D-5L was administered in the phase 3 study at baseline and weeks 4, 12, and 26.
Haemo-QOL-A
The 41 items on Haemo-QOL-A include the domains of Consequences of Bleeding (7 items), Emotional Impact (6 items), Physical Functioning (9 items), Role Functioning (11 items), Treatment Concern (3 items), and Worry (5 items). Items were scored on a 6-point Likert-type scale with higher scores indicating better HRQOL or less impairment. Subscale scores for each disease domain range from 0 to 5 and the Total Score ranges from 0 to 30. Both domain and total raw scores are transformed to a 0 to 100 scale using the formula:
Domain scores were imputed using mean domain scores if <50% of items were missing. Total Score was not calculated, and no imputations were performed if >50% of items were missing. Change in scores at specific time points were calculated using the formula:
EQ-5D-5L
EQ-5D-5L was used to assess general health status for the 5 domains of Mobility, Self-care, Usual Activities, Anxiety and Depression, and Pain and Discomfort. Items on EQ-5D-5L were scored on a scale of 1 to 5 with higher scores representing increased impairment. The EQ-5D-5L vertical visual analog scale (VAS) of current health status scores were assessed on a scale ranging from 0 to 100, with higher scores indicative of better HRQOL.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics 25 (IBM Corporation, Armonk, NY). Data normality was assessed graphically using frequency distribution histograms and statistically using skewness, kurtosis, and standard error. Categorical variables were described using frequencies and percentages. Continuous variables with normal distribution were assessed using descriptive statistics of mean ± standard deviation (SD) or range. Variables with non-normal distribution were assessed using mean ± SD and median (interquartile range [IQR]). Significance was assessed at α <0.05 (2-tailed) with 95% confidence intervals (CIs).
Item Facility
Item facility, which assesses the possible presence of floor or ceiling effects, was calculated using data from the phase 1/2 study and the phase 3 ITT study populations. An item was considered to have floor or ceiling effects and poor item facility if >50% of reported responses for items were either the minimum/maximum option.25
Validity
Convergent validity, which assesses the degree of correlation with existing measures for a condition, was measured by correlating Haemo-QOL-A scores with EQ-5D-5L scores in participants treated in the phase 3 mITT study population. Spearman’s Rank correlation coefficients were calculated between Haemo-QOL-A Total and domain scores vs EQ-5D-5L domain scores at baseline and week 26. Spearman’s Rank correlation coefficients were calculated to assess the relationship between Haemo-QOL-A Total and domain score change and score change vs EQ-5D-5L domain score changes from baseline to week 26 in the phase 3 study. Pearson’s Rank correlation coefficient was calculated between Haemo-QOL-A Total and domain score changes vs EQ-5D-5L VAS score changes. Regression analyses were also conducted to evaluate the relationship between the 2 instruments when assessing Haemo-QOL-A vs EQ-5D-5L score change from baseline to week 26 (“change on change”).
Discriminant validity is the ability of a scale to distinguish between different patient subgroups and was assessed by comparing groups of participants from the phase 3 mITT study population based on baseline EQ-5D-5L VAS scores. Independent sample t-tests were used to compare participants with baseline EQ-5D-5L VAS scores >87.15 vs <87.15 (the UK population norm for males aged 25–35).26 Data from baseline and week 26 were pooled in order to compare mean Haemo-QOL-A Total and domain scores for participants with baseline EQ-5D-5L VAS scores >87.15 vs those with baseline EQ-5D-5L VAS scores <87.15.
Item Discrimination and Internal Consistency
Item discrimination and internal consistency, which assess the ability of an item to discriminate against others on its subscale, were calculated using data from the phase 1/2 study population and the phase 3 ITT study population. Item discrimination was predicted using Spearman’s Rank correlation coefficient between individual item scores and Total Score. Cronbach’s alpha and squared multiple correlation were used to calculate internal consistency. Items with Cronbach’s alpha >0.7 were considered to have properties of internal consistency.
Determination of Clinically Important Difference
Distribution-Based Methods
For QOL evaluation in chronic disease, half SD of baseline mean is used to determine a distribution-based estimate of the CID threshold.27 We estimated the CID via distribution methods; the half SD rule (of baseline) was applied for Haemo-QOL-A Total and domain scores.
Anchor-Based Methods
Given the small sample size and large range of changes in EQ-5D-5L VAS score (ie, −25 to +25), different anchor methods were sequentially deployed to provide the most accurate CID estimate. Data from the phase 1/2 study population were not used since the EQ-5D-5L was not administered to study participants. The mean difference in Haemo-QOL-A Total Score change from baseline to week 26 was calculated between the following categories of participants based on their EQ-5D-5L VAS score change from baseline to week 26 in the phase 3 mITT study population:
a. Participants with VAS score change ≥+3.
b. Participants with a VAS score change between +3 and −3.
c. Participants with VAS score change ≤−3.
Next, results from regression analyses were extrapolated to predict change in Haemo-QOL-A Total Score (dependent variable) vs an EQ-5D-5L VAS score increase of 3. Then, the mean difference in Haemo-QOL-A Total and domain score changes from baseline to week 26 were calculated between categories of participants based on their EQ-5D-5L VAS score change from baseline to week 26 shown in Table 1. Between those categories, the relative magnitude of EQ-5D-5L VAS score change was mapped against relative magnitude of Haemo-QOL-A score change to derive the mean change in Haemo-QOL-A score vs EQ-5D-5L VAS score change of 1 and 3, respectively.
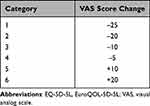 |
Table 1 EQ-5D-5L VAS Score Change Categories Used in Anchor-Based Estimation of Clinically Important Difference |
Results
Haemo-QOL-A data were analyzed from 7 participants in the phase 1/2 study through 156 weeks and 16 participants in the phase 3 study through 26 weeks (mITT = 16). Mean (SD) age was 30.4 (5.8) years and 29.7 (6.2) years for participants in the phase 1/2 and 3 trials, respectively.11,12,15
Haemo-QOL-A Score Changes with Treatment
Baseline Haemo-QOL-A scores were similar in the phase 1/2 and phase 3 studies (Table 2). In the phase 3 mITT study population, both EQ-5D-5L VAS and Haemo-QOL-A Total Scores at week 26 were higher than at baseline, suggesting an improvement in HRQOL of participants following AAV5-hFVIII-SQ infusion (Table 3; Supplemental Figure 1).
 |
Table 2 Mean Change from Baseline in Haemo-QOL-A Total and Domain Scores in the Phase 1/2 and 3 Studies |
 |
Table 3 Mean Transformed Haemo-QOL-A Total Score and EQ-5D-5L VAS Scores at Baseline and Week 26 by EQ-5D-5L VAS Score Change Subgroups |
Psychometric Validation of the Haemo-QOL-A
Item Facility
Items where >50% of responses were reported as the minimum/maximum response were considered to have poor items facility (ie, floor or ceiling effects). Out of 41 items, 13 had ceiling effects (Supplemental Table 1). The domains with the highest percentage of items with ceiling effects were Treatment Concern (2/3 items) and Emotional Impact (3/6 items). No floor effects were observed.
Convergent Validity
Our results indicate good convergent validity between the Haemo-QOL-A and EQ-5D-5L. Overall, Haemo-QOL-A Total and domain scores were inversely correlated with EQ-5D domain scores demonstrating that as degree of impairment in Mobility, Self-care, Usual Activities, Anxiety and Depression, and Pain and Discomfort decrease (demonstrating improvement), Haemo-QOL-A scores increase (demonstrating improvement), and vice versa.
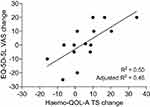
The strongest correlations were seen when comparing week 26 and “change vs change” scores. In both cases, the Pain and Discomfort domain of the EQ-5D-5L was strongly and significantly correlated with the Haemo-QOL-A. At week 26, the strongest correlations were seen between Role Functioning and Pain and Discomfort (−0.87, P <0.01), Emotional Impact and Pain and Discomfort (–0.85, P <0.01), Physical Functioning and Pain and Discomfort (–0.79, P <0.01), and Total Score and Pain and Discomfort (–0.87, P <0.01) (Supplemental Table 2). When comparing change in Haemo-QOL-A to EQ-5D-5L scores, the strongest correlations were seen between Emotional Impact and Anxiety and Depression (−0.88, P <0.01), Emotional Impact and Pain and Discomfort (–0.7, P <0.01), Physical Functioning and Pain and Discomfort (–0.73, P <0.01), Total Score and Anxiety and Depression (−0.82, P <0.01), and Total and VAS scores (0.77, P <0.01), implying that improvement in Physical Functioning and Emotional Impact are being driven by a decrease in pain (Table 4). At baseline, the strongest correlations were between Physical Functioning domain of the Haemo-QOL-A and the Usual Activities (–0.75, P <0.01) and Pain and Discomfort (–0.64, P <0.01) domains of EQ-5D-5L (Supplemental Table 3). Regression analyses showed a linear correlation between change in Haemo-QOL-A Total Scores and EQ-5D-5L VAS scores (Figure 1), with a predicted change of 0.63 in Haemo-QOL-A Total Score for every EQ-5D-5L VAS score change of 1 (R2 = 0.5, adjusted R2 = 0.46).
 |
Table 4 Correlations Between Change from Baseline to Week 26 in Haemo-QOL-A Transformed Scores and Corresponding Change in EQ-5D-5L Scores, Modified Intent-to-Treat Population, Phase 3 Study |
Discriminant Validity
There was a substantial difference between mean Haemo-QOL-A scores reported by participants with an EQ-5D-5L VAS score change of magnitude <3 vs those with an EQ-5D-5L score change of magnitude ≥3 (Table 3). Good discriminant validity was demonstrated by a mean difference between participants with EQ-5D-5L scores above and below the UK population norm ranging from 6.46−17.31. The Haemo-QOL-A Total Score (mean [CI] difference 11.81 [−20.2, −3.4], P = 0.01) and domains scores for Emotional Impact (17.3 [–26.4, –8.2], P = 0.001) and Role Functioning (15.4 [–24.6, –6.2], P = 0.002) were most sensitive to discriminate differences in impairment based on EQ-5D-5L scores (Figure 2).
Additionally, review of participant-level data indicated a relationship between clinical outcomes (eg, ongoing arthropathy, bleeds and comorbidities) and Haemo-QOL-A scores, suggesting that the tool can discriminate between different disease states. In the phase 3 study population, 3/15 participants reported HRQOL score decreases greater in magnitude than the instrument CID estimate. All 3 participants had degenerative joint damage alongside other comorbidities. Of the 4/15 participants who reported Haemo-QOL-A score changes lower in magnitude than the CID estimate, 3 had ongoing arthropathy. Of the 8 participants that reported score increase >CID, only 3 had arthropathy.
Item Discrimination and Internal Consistency
Of the 41 items in Haemo-QOL-A, 38 had corrected item-total correlation scores >0.4. Three items were identified as non-discriminatory in their domain (corrected item-total correlation scores <0.4). The item “I am able to complete household tasks” in the Physical Functioning domain had a corrected item-total correlation of –0.03. However, the domain showed good internal consistency overall, and although deletion of this item improved Cronbach’s alpha, it improved from an acceptable level regardless (from 0.71 to 0.76). In addition, 2 of 3 items in the Treatment Concern domain, “I worry about the safety of my treatment” and “I worry about the availability of hemophilia products”, had poor discrimination (item correlation-total scores 0.09 and 0.26, respectively). Overall, the domain showed poor internal consistency and deletion of each item only improved the Cronbach’s alpha for this domain from 0.31 to 0.42 and 0.317, respectively.
Clinically Important Difference in Haemo-QOL-A
Results of the distribution-based method to estimate CID in Haemo-QOL-A are shown in Supplemental Table 4. A variety of anchor-based methods were also tested. For participants with EQ-5D VAS score change categories of ≥+3, between +3 and −3, and ≤−3, the mean changes in Haemo-QOL-A Total Scores were 16.3, 1.8, and −2.8, respectively (Table 3). Comparison of groups indicated a score change ≥6.31 would represent a clinically meaningful change in the Haemo-QOL-A Total Score. However, as the mean VAS score change in the groups experiencing a score change ≥±3 (ie a clinically meaningful change in VAS28) is far greater than the CID threshold for the VAS (mean score change of 13), it is likely that the true CID is <6.31; thus, further analyses were conducted and detailed below. First, results from regression analyses were extrapolated to predicted change in Haemo-QOL-A Total Score (dependent variable) vs EQ-5D VAS score increase of 3. Analyses predicted that for each 1-point increase in VAS score, Haemo-QOL-A Total Score would increase by 0.63. Therefore, a 3-point VAS score change should equate to a Haemo-QOL-A Total Score change of 1.89. Next, given the distribution of the available data, mean change in Haemo-QOL-A Total and domain scores were mapped against reported VAS change categories shown in Table 1 that were greater in magnitude than the accepted CID of 3.28 An average of all domain CID estimates was calculated to give an overall estimate for what would constitute a CID score change for the domains of the Haemo-QOL-A (Supplemental Table 5). The resultant Haemo-QOL-A Total Score CID estimate (score change ≥5.5 in magnitude) was lower than the estimated domain score (score change ≥6.0 in magnitude) due to lack of relevance of 2/3 items in the Treatment Concern domain in patients treated with gene therapy. Given the distribution of the data and small sample size, the latter anchor analyses in Supplemental Table 5 were deemed most reliable. This is further corroborated by the fact that the Haemo-QOL-A Total Score CID estimate derived from this method matches others that use a distribution-based method.
Participants in the phase 1/2 and 3 studies had higher mean Haemo-QOL-A Total Score change from baseline than the CID estimate at weeks 28, 52, 104, and 156 post-infusion and at week 26 post-infusion, respectively (Table 2). Similarly, participants reported clinically meaningful improvements in the domain scores for Consequences of Bleeding, Physical Functioning, Role Functioning, and Worry at all time points (Table 2). Changes in mean domain scores for Emotional Impact and Treatment Concern were not consistently above the CID threshold in the phase 1/2 study population (Table 5).
 |
Table 5 Mean Change in Transformed Haemo-QOL-A Scores for Domains of Emotional Impact and Treatment Concern, Phase 1/2 Study |
Discussion
This is the first study to psychometrically validate the Haemo-QOL-A in PWSHA undergoing gene therapy. Validation of Haemo-QOL-A is important to inform clinical analysis of the effect of gene therapy on HRQOL. Our preliminary results demonstrate good psychometric validity of the Haemo-QOL-A when measured in participants undergoing gene therapy, consistent with the US Food and Drug Administration guidelines for patient-reported outcome instrument validation, though all results should be interpreted with caution and confirmed in a larger sample size.29
In this study, the Haemo-QOL-A had good preliminary construct validity, including convergent validity, as there was a high degree of correlation between the Haemo-QOL-A and EQ-5D-5L. Despite the limited sample size, mean Haemo-QOL-A Total and domain scores were capable of detecting differences in participant populations with vs without a high burden of disease, suggesting the instrument had good discriminant validity. The scale also showed good content validity with very few items displaying floor and ceiling effects, except in the Treatment Concern domain, where 2/3 questions are not relevant to an individual undergoing gene therapy in a clinical trial. Our results also establish CIDs in Haemo-QOL-A scores after gene therapy and demonstrate likely applicability in ongoing clinical studies.
Convergent validity preliminarily identified significant correlations between Haemo-QOL-A and EQ-5D-5L scores at both baseline and week 26. Correlations at baseline, although significant, were not as strong as at week 26, highlighting the disability paradox experienced by PWSHA.17 Improved outcomes on the Haemo-QOL-A Total Scores and domains of Emotional Impact, Physical Functioning, and Treatment Concern at week 26 post-infusion were inversely correlated with EQ-5D-5L domain scores for Usual Activities, Anxiety and Depression, and Pain and Discomfort. There were no significant correlations between the EQ-5D-5L domain of Mobility and the Haemo-QOL-A domain of Physical Functioning, suggesting that improvements in physical QOL of participants treated with gene therapy may be driven largely by a decrease in pain.
Mean Haemo-QOL-A Total Scores discriminated well among our limited sample of study participants with differing degrees of HRQOL impairment based on EQ-5D-5L VAS scores. Previous studies show similar discrimination between hemophilia A populations differing in disease severity, HIV status, and type of treatment (on-demand vs prophylaxis) using Haemo-QOL-A.19 The current analysis used general population scores based on UK norms.26 It will be interesting to evaluate the discriminant characteristics of Haemo-QOL-A in studies conducted in different geographic regions. Additionally, the tool discriminated between participants with and without ongoing arthropathy in our limited sample of study participants, suggesting that joint damage and subsequent pain heavily impact HRQOL in PWSHA; therefore, treatment should aim to prevent joint damage before it occurs.
Of the 6 Haemo-QOL-A domains, only Treatment Concern had poor internal consistency. Two items relating to treatment safety and availability lacked discrimination. The third item on concerns regarding clinician inexperience performed well. Thus, two-thirds of this domain was insensitive to the psychological HRQOL of participants pertaining to their treatment, which lowered the Haemo-QOL-A Total Score. Since a one-time infusion of gene therapy may circumvent the need for repeated factor infusions, participants may not be as concerned about treatment availability. Further, since gene therapy is provided through specialist treatment centers with thorough follow-up, participants may not be as concerned about the safety of their treatment. In the future, the Treatment Concern domain could be revised to be more appropriate for evaluating change post-gene therapy by, for example, using the remaining item “I worry about the safety of my treatment” to score the domain or adding additional questions that better reflect the current treatment landscape.
A total of 13 items showed ceiling effects. These were most prevalent in the Treatment Concern and Emotional Impact domains. Floor and ceiling effects diminish sensitivity of psychometric instruments and can result in underestimation of treatment effectiveness. Scores pertaining to these items must therefore be interpreted cautiously in future applications of Haemo-QOL-A in gene therapy.
It is important to benchmark HRQOL improvements in participants undergoing gene therapy for hemophilia A against CID estimates generated using both distribution- and anchor-based approaches. The current study used both methods to establish CID estimates that are in line with those previously published following FVIII prophylaxis, where the distribution-based CID estimate for Haemo-QOL-A Total Score ranged from 5 to 7 and that of the Physical Functioning domain ranged from 6 to 9.30 However, given the small number of participants receiving gene therapy in the current analysis and the vastly different nature of the HRQOL burden experienced by participants receiving gene therapy vs those receiving standard of care, clinicians should be cautious in comparing the magnitude of CID scores across these 2 studies.
Small sample size was a limitation in both populations, which precluded using several anchor-based estimation approaches.28 A low anchor magnitude of 3-point EQ-5D-5L VAS score change was adopted given the EQ-5D-5L’s limitation in determining differences in disease state in hemophilia.17 Of all participants evaluated across both populations, 4 reported baseline Total Score >85%, indicating possible ceiling effects. Here, some participants reported VAS scores higher than population norms; other research using the EQ-5D-5L in PWSHA found similar results, suggesting a disability paradox.17 People with chronic conditions often report higher or similar health state valuations than the general population,31,32 in part due to processes of adjustment and coping. A larger sample size may provide more meaningful anchor scores and allow more accurate estimation of CID.
Conclusion
Future availability of novel gene therapy interventions will likely shift the paradigm of hemophilia A treatment. This small study is the first to validate the Haemo-QOL-A for detecting clinically meaningful improvement in the HRQOL of PWSHA receiving gene therapy. Our analyses establish initial CID estimates and support the validity and reliability of the Haemo-QOL-A for measuring changes in HRQOL. These preliminary findings suggest that the Haemo-QOL-A is likely fit for evaluating HRQOL and provides a guide for future applications for measuring HRQOL outcomes in PWSHA following gene therapy. Prospective studies with larger sample sizes are needed to evaluate this instrument in a broader severe hemophilia A population.
Data Sharing Statement
De-identified individual participant data underlying these results (including text, tables, figures, and appendices) will be made available, together with the clinical protocol and data dictionaries, for non-commercial, academic purposes. Additional supporting documents may be available upon request. Investigators will be able to request access to these data and supporting documents via the Publication Data Request page at www.BioMarin.com beginning 6 months and ending 2 years after publication. Data associated with any ongoing development program will be made available within 6 months after approval of the relevant product. Requests must include a research proposal clarifying how the data will be used, including proposed analysis methodology. Research proposals will be evaluated relative to publicly available criteria available at www.BioMarin.com to determine if access will be given, contingent upon execution of a data access agreement with BioMarin Pharmaceutical Inc.
Ethics Statement
The protocol for the 201 trial was reviewed and approved by the Imperial College Research Ethics Committee at Imperial College London and the ethics committees of all other participating sites. The protocol for the 301 trial was reviewed and approved by the Research Ethics Committee at the State University of Campinas and the institutional review boards or ethics committees of all other participating sites. Both studies were conducted in accordance with the guidelines outlined in the Declaration of Helsinki. All participants in both trials provided written informed consent prior to participating in any protocols.
Acknowledgments
We thank the study participants, study site personnel, and investigators who were involved in these clinical trials. We thank Nina Mitchell of BioMarin Pharmaceutical Inc., for her contributions to this project. Project management support was provided by Sara Hawley of BioMarin Pharmaceutical Inc. Medical writing support was provided by Kathleen Pieper, PhD, and Atreju Lackey, PhD, of AlphaBioCom, LLC, and funded by BioMarin Pharmaceutical Inc.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding for this research was provided by BioMarin Pharmaceutical Inc.
Disclosure
Jennifer Quinn was an employee and stockholder of BioMarin Pharmaceutical Inc., London, UK at the time of the study. Wing Yen Wong and Kathleen A Delaney are employees of BioMarin Pharmaceutical Inc., Novato, CA, USA. Wolfgang Miesbach has received speaker honoraria and project grants from Bayer, BioMarin Pharmaceutical Inc., Biotest, CSL Behring, Chugai, Freeline, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, Takeda/Shire, and uniQure. Monika Bullinger has received speaker honoraria and project grants from Bayer, BioMarin Pharmaceutical Inc., Janssen Cilag, Otsuka Lundbeck, and Pfizer. The authors report no other conflicts of interest in this work.
References
1. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158. doi:10.1111/hae.14046
2. Barr RD, Saleh M, Furlong W, et al. Health status and health-related quality of life associated with hemophilia. Am J Hematol. 2002;71(3):152–160. doi:10.1002/ajh.10191
3. Forsyth AL, Witkop M, Lambing A, et al. Associations of quality of life, pain, and self-reported arthritis with age, employment, bleed rate, and utilization of hemophilia treatment center and health care provider services: results in adults with hemophilia in the HERO study. Patient Prefer Adherence. 2015;9:1549–1560. doi:10.2147/PPA.S87659
4. Limperg PF, Haverman L, Maurice-Stam H, et al. Health-related quality of life, developmental milestones, and self-esteem in young adults with bleeding disorders. Qual Life Res. 2018;27(1):159–171. doi:10.1007/s11136-017-1696-0
5. Witkop M, Neff A, Buckner TW, et al. Self-reported prevalence, description and management of pain in adults with haemophilia: methods, demographics and results from the pain, Functional Impairment, and Quality of life (P-FiQ) study. Haemophilia. 2017;23(4):556–565. doi:10.1111/hae.13214
6. Mercan A, Sarper N, Inanir M, et al. Hemophilia-specific quality of life index (Haemo-QoL and Haem-A-QoL questionnaires) of children and adults: result of a single center from Turkey. Pediatr Hematol Oncol. 2010;27(6):449–461. doi:10.3109/08880018.2010.489933
7. Ozelo M, Chowdary P, Regnault A, Busk A. Impact of severe haemophilia A on patients’ health status: results from The Guardian™ 1 clinical trial of turoctocog alfa (NovoEight®). Haemophilia. 2015;21(4):451–457. doi:10.1111/hae.12617
8. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–818. doi:10.1056/NEJMoa1703068
9. Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677–1686. doi:10.2147/PPA.S139851
10. Castaman G, Santoro C, Coppola A, et al. Emergency management in patients with haemophilia A and inhibitors on prophylaxis with emicizumab: AICE practical guidance in collaboration with SIBioC, SIMEU, SIMEUP, SIPMeL and SISET. Blood Transfus. 2020;18(2):143–151. doi:10.2450/2019.0186-19
11. Rangarajan S, Walsh L, Lester W, et al. AAV5-factor VIII gene transfer in severe Hemophilia A. N Engl J Med. 2017;377(26):2519–2530. doi:10.1056/NEJMoa1708483
12. Pasi KJ, Rangarajan S, Mitchell N, et al. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for Hemophilia A. N Engl J Med. 2020;382(1):29–40. doi:10.1056/NEJMoa1908490
13. George LA, Ragni MV, Samelson-Jones BJ, et al. Spk-8011: preliminary results from a phase 1/2 dose escalation trial of an investigational AAV-mediated gene therapy for hemophilia A. Blood. 2017;130(Suppl 1):604.
14. Pasi KJ, Laffan M, Rangarajan S, et al. Persistence of haemostatic response following gene therapy with valoctocogene roxaparvovec in severe haemophilia A. Haemophilia. 2021;27(6):947–956. doi:10.1111/hae.14391
15. Ozelo MC, Mahlangu J, Pasi KJ, et al. Valoctocogene roxaparvovec gene therapy for hemophilia A. N Engl J Med. 2022;386(11):1013–1025. doi:10.1056/NEJMoa2113708
16. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. doi:10.1007/s11136-011-9903-x
17. O’Hara J, Martin AP, Nugent D, et al. Evidence of a disability paradox in patient-reported outcomes in haemophilia. Haemophilia. 2021;27(2):245–252. doi:10.1111/hae.14278
18. O’Hara J, Hughes D, Camp C, Burke T, Carroll L, Diego DG. The cost of severe haemophilia in Europe: the CHESS study. Orphanet J Rare Dis. 2017;12(1):106. doi:10.1186/s13023-017-0660-y
19. Rentz A, Flood E, Altisent C, et al. Cross-cultural development and psychometric evaluation of a patient-reported health-related quality of life questionnaire for adults with haemophilia. Haemophilia. 2008;14(5):1023–1034. doi:10.1111/j.1365-2516.2008.01812.x
20. Wharfe G, Buchner-Daley L, Gibson T, et al. The Jamaican Haemophilia registry: describing the burden of disease. Haemophilia. 2018;24(4):e179–e186. doi:10.1111/hae.13517
21. Sun HL, McIntosh KA, Squire SJ, et al. Patient powered prophylaxis: a 12-month study of individualized prophylaxis in adults with severe haemophilia A. Haemophilia. 2017;23(6):877–883. doi:10.1111/hae.13319
22. Manco-Johnson MJ, Lundin B, Funk S, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15(11):2115–2124. doi:10.1111/jth.13811
23. Limperg P, Terwee C, Young N, et al. Health‐related quality of life questionnaires in individuals with haemophilia: a systematic review of their measurement properties. Haemophilia. 2017;23(4):497–510. doi:10.1111/hae.13197
24. Bullinger M, Gardner DL, Lewis HB, et al. The potential impact of gene therapy on health-related quality of life (HRQoL) domains in haemophilia. J Haemophilia Pract. 2021;8(1):56–68. doi:10.17225/jhp00176
25. Streiner D, Norman G. Health Measurement Scales: A Practical Guide to Their Development and Use.
26. Kind P, Hardman G, Macran S. UK population norms for EQ-5D. Discussion Paper 172. The University of York Centre for Health Economics; 1999.
27. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi:10.1097/01.MLR.0000062554.74615.4C
28. Kaplan RM. The minimally clinically important difference in generic utility-based measures. COPD. 2005;2(1):91–97. doi:10.1081/copd-200052090
29. US Department of Health and Human Services FDA Center for Drug Evaluation and Research, US Department of Health and Human Services FDA Center for Biologics Evaluation and Research, US Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:1–20. doi:10.1186/1477-7525-4-1
30. Valluri S, Flood E, Mink D, Bell J, Pocoski J, Sasane R. Determination of the minimal important difference (mid) of the hemophilia-specific quality of life questionnaire (Hemo-QOL-A) for adults with severe hemophilia A: PO-TU-233. Haemophilia. 2012;18(Suppl. 3):180–181.
31. Gandhi M, Tan RS, Ng R, et al. Comparison of health state values derived from patients and individuals from the general population. Qual Life Res. 2017;26(12):3353–3363. doi:10.1007/s11136-017-1683-5
32. Peeters Y, Stiggelbout AM. Health state valuations of patients and the general public analytically compared: a meta-analytical comparison of patient and population health state utilities. Value Health. 2010;13(2):306–309. doi:10.1111/j.1524-4733.2009.00610.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.