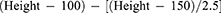Back to Journals » Journal of Inflammation Research » Volume 15
Prognostic Roles of Inflammation- and Nutrition-Based Indicators for Female Patients with Cancer
Authors Yang M, Zhang Q, Ge Y, Tang M, Hu C, Wang Z, Zhang X , Song M, Ruan G, Zhang X, Liu T, Xie H, Zhang H, Zhang K, Li Q, Li X, Liu X, Lin S, Shi H
Received 28 February 2022
Accepted for publication 8 June 2022
Published 17 June 2022 Volume 2022:15 Pages 3573—3586
DOI https://doi.org/10.2147/JIR.S361300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Ming Yang,1– 3 Qi Zhang,1– 3 Yizhong Ge,1– 4 Meng Tang,1– 3 Chunlei Hu,1– 3 Ziwen Wang,1– 3 Xi Zhang,1– 3 Mengmeng Song,1– 3 Guotian Ruan,1– 3 Xiaowei Zhang,1– 3 Tong Liu,1– 3 Hailun Xie,1– 3 Heyang Zhang,1– 3 Kangping Zhang,1– 3 Qinqin Li,1– 3 Xiangrui Li,1– 3 Xiaoyue Liu,1– 3 Shiqi Lin,1– 4 Hanping Shi1– 3
1Department of Gastrointestinal Surgery/Department of Clinical Nutrition, Beijing Shijitan Hospital, Capital Medical University, Beijing, 100038, People’s Republic of China; 2Beijing International Science and Technology Cooperation Base for Cancer Metabolism and Nutrition, Beijing, 100038, People’s Republic of China; 3Key Laboratory of Cancer FSMP for State Market Regulation, Beijing, 100038, People’s Republic of China; 4The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China
Correspondence: Hanping Shi, Department of Gastrointestinal Surgery/Department of Clinical Nutrition, Beijing Shijitan Hospital, Capital Medical University, Beijing, 100038, People’s Republic of China, Tel/Fax +86 10 6392 6983, Email [email protected]
Purpose: The incidence, progression, and prognosis of cancer could be affected by inflammation and nutrition. Female patients have different inflammatory and nutritional states depending on their age and tumor types. It is important to screen for suitable prognostic indicators in female patients with cancer of different ages and tumor types.
Patients and Methods: Baseline clinicopathologic and laboratory characteristics of 1502 female patients with cancer were obtained from a multicenter cohort study. Concordance indices (C-indices) were used to evaluate the prediction accuracy of following inflammation- and nutrition-based indicators: advanced lung cancer inflammation index (ALI), systemic immune inflammation index (SII), modified geriatric nutritional risk index (mGNRI), albumin-to-globulin ratio (AGR), prognostic nutritional index (PNI), lymphocyte-to-C-reactive protein ratio (LCR), controlling nutritional status score (CONUT), modified Glasgow prognostic score (mGPS), and lymphocyte-to-C-reactive protein score (LCS).
Results: The most suitable indicators in different female populations with cancer had C-indices as follows: LCR (0.668; 95% CI, 0.644– 0.693) for all females; AGR (0.681; 95% CI, 0.619– 0.743) for young females; LCR (0.667; 95% CI, 0.628– 0.706) for middle-aged females; ALI (0.597; 95% CI, 0.574– 0.620) for elderly females; LCR (0.684; 95% CI, 0.621– 0.747) for females with reproductive system cancer; and ALI (0.652; 95% CI, 0.624– 0.680) for females with non-reproductive system cancer.
Conclusion: The most suitable indicators for the different female populations with cancer are summarized as follows: LCR for all females, AGR for young females, LCR for middle-aged females, ALI for elderly females, LCR for females with reproductive system cancer, and ALI for females with non-reproductive system cancer.
Keywords: female, cancer, prognosis, inflammation, nutrition
Introduction
As the leading cause of morbidity and mortality worldwide, cancer continues to represent an enormous social and economic burden to society.1 The incidence, progression, and prognosis of cancer are affected by many factors, especially by inflammation and nutrition.2–5
Females vary in inflammatory and nutritional states at different ages. Elderly females have higher levels of inflammation than young females, but in younger females, malnutrition and iron deficiency anemia are more common.6 These conditions may be caused by physiological factors such as menstruation or external factors such as diet.7,8 It is worth noting that estrogen have impacts on female’s inflammation and nutrition statuses.9,10 Erratic estrogen levels have impacts on perimenopausal female’s inflammation and nutrition statuses, resulting in unstable inflammation and nutrition statuses.11 Compared with young and middle-aged females, most elderly females are post-menopausal. The lack of estrogen makes higher inflammatory levels and worse nutritional statuses in elderly females.9,10 The increment in age also has different degrees of impact on inflammatory and nutritional status.12,13
Hence, we believe that physiological differences among female at different ages would affect inflammation and nutrition statuses in females with cancer and play important roles in cancer prognosis. In past decades, increasing numbers of factors influencing inflammation and nutrition have been discovered and developed to evaluate and improve the prognosis of patients with cancer.14–23 Therefore, we would like to compare the prognostic values of inflammation- and nutrition-based indicators and assessed the most suitable indicators in different female populations with cancer (young females, middle-aged females, elderly females). Moreover, the inflammatory and nutritional loads of female with reproductive system and non-reproductive system cancers are quite different.24,25 The most suitable indicators for females with reproductive system cancer and females with non-reproductive system cancer would also be evaluated in this study.
Materials and Methods
Study Population
We enrolled 22,783 patients with cancer from 14 hospitals for a multicenter cohort study. The patients with cancer in this study were assessed between January 1, 2012 and October 31, 2020. All patients were enrolled based on the following inclusion criteria: ≥ 18 years of age, a pathological diagnosis of cancer, willing and able to provide written informed consent, and consciousness throughout treatment with no communication disorders.
A comprehensive list of clinicopathological symptoms were used as exclusion criteria. There were 17,562 patients who lacked data for one or more critical variables and were excluded from this study: 12 patients were missing age data; 1795 patients were missing height data; 35 patients were missing serum albumin (Alb) levels; 151 patients were missing serum globulin (Glb) levels; 13,583 patients were missing C-reactive protein (CRP) data; 111 patients were missing fasting blood glucose (FBG) levels; 1749 patients were missing serum total cholesterol (TC) levels; 83 patients were missing neutrocyte (Neu) counts; 8 patients were missing lymphocyte (Lym) counts; and 35 patients were missing platelet (Plt) data (Figure 1). Among the 5222 patients preliminarily screened, those who meet the criteria of any of the following groups would be included in the final analysis: non-menopausal female < 45 years of age (young female group; 243 patients); non-menopausal and menopausal female ≥ 45 and < 55 years of age (middle-aged female group; 629 patients); and post-menopausal female > 55 years of age (elderly female group; 630 patients) (Figure 1).
 |
Figure 1 Flow diagram for the selection of female patients with cancer from a multicenter clinical database. |
We obtained approval for the study from the Medical Ethical Review Committees and Institutional Review Boards of the participating registered hospitals. The study was conducted in accordance with the guidelines of the Declaration of Helsinki. The study was registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn) and assigned a registration number ChiCTR1800020329.
Patient Characteristics and Outcomes
The following demographic and clinicopathological data were collected within 24 hours of admission (Table 1): age; height; weight; type of cancer; smoking status; alcohol consumption; TNM stage; history of surgery; radiotherapy and chemotherapy; Karnofsky performance status score (KPS); Scored Patient-Generated Subjective Global Assessment (PG-SGA); Alb levels; Glb levels; CRP levels; FBG levels; TC levels; Neu counts; Plt counts; Lym counts; Neu-to-Lym ratio (NLR); Plt-to-Lym ratio (PLR); glucose-to-Lym ratio (GLR); advanced lung cancer inflammation index (ALI); systemic immune inflammation index (SII); CRP-to-Alb ratio (CAR); nutritional risk index (NRI); geriatric NRI (GNRI); modified GNRI (mGNRI); Alb-to-Glb ratio (AGR); prognostic nutritional index (PNI); Lym-to-CRP ratio (LCR); controlling nutritional status score (CONUT); modified Glasgow prognostic score (mGPS); and Lym-to-CRP score (LCS). The standard for smoking was smoking more than 20 cigarettes in a lifetime. The standard for drinking was regular drinking over the past year. TNM staging followed the guidelines of the American Joint Committee on Cancer.26 Laboratory tests consisted of standardized tests used throughout China using the same protocol and reference range used nationwide. Patient death due to cancer was defined as the primary endpoint.
 |
Table 1 Characteristics of All Females with Cancer |
The calculation methods and criteria of the inflammation- and nutrition-based indicators were as follows: body mass index (BMI) = weight/height2; NLR = Neu/Lym; PLR = Plt/Lym; GLR = FBG/Lym; ALI = BMI × Alb/NLR; SII = Neu × Plt/Lym; and CAR = CRP/Alb.14,16,18,19,21,27–31 Ideal weight (WLo) was determined using the Lorentz equation for women:20
Additional parameters included: NRI = 1.519 × Alb + 41.7 × Weight/WLo; GNRI = 1.489 × Alb + 41.7 × weight/WLo; mGNRI = 1.489/CRP + 41.7 × Weight/WLo; AGR = Alb/Glb; PNI = 10 × albumin + 0.005 × Lym; and LCR = Lym/CRP. The criteria for CONUT, mGPS, and LCS are presented in Supplemental Tables 1–3, respectively.
Statistical Analysis
Data were presented as simple percentages or medians and interquartile ranges (IQR). Chi-square tests or Fisher’s exact tests were used to assess baseline characteristics. Continuous variables with normal distributions were assessed using Student’s t-tests. Continuous variables with non-normal distributions were assessed using Mann‐Whitney tests.
Lasso regression analyses were used to remove unsuitable indicators. Receiver operating characteristic (ROC) curves were used to obtain cut-off values for the indicators. Hazard ratios (HR) and associated 95% confidence intervals (CI) from three analysis models used in this study were determined using univariate and multivariate Cox proportional hazard regression analyses. Model 1 was not adjusted for any covariates. Model 2 was adjusted for age, BMI, type of cancer, smoking status, alcohol consumption, and TNM stage. Model 3 was adjusted for age, BMI, type of cancer, smoking status, alcohol consumption, TNM stage, history of surgery, radiotherapy and chemotherapy, KPS, and PG-SGA. Concordance indices (C-indices) and Area Under the Curves (AUCs) were used to compare the prognostic values of the indicators. C-indices were used to the evaluate and select of prognostic models in terms of discrimination ability. AUC is a measure of the discriminative ability of prediction models. We further divided patients into 3 groups according to age (young, middle-aged, and elderly) and into 2 groups according to the type of cancer [reproductive system cancer (ovarian cancer, endometrial cancer, and cervical cancer) and non-reproductive system cancer (lung cancer, gastric cancer, hepatic cancer, breast cancer, esophageal cancer, bladder cancer, pancreatic cancer, nasopharyngeal cancer, colorectal cancer, and biliary tract cancer)] and repeated the above analyses using the three models.
The statistical significance threshold was set to 0.05. Analyses were performed using R (version 4.1.1) software.
Results
Patient Characteristics
The median age of patients in the study was 54 years (IQR, 47 to 63 years), the median BMI was 22.37 kg/m2 (IQR, 20.08 to 24.77 kg/m2), the median KPS was 90 (IQR, 80 to 90), and the median PG-SGA was 4 (IQR, 2 to 8). Among the cohort, 12.5% (187/1502) were stage I, 17.6% (265/1502) were stage II, 24.6% (370/1502) were stage III, and 45.3% (680/1502) were stage IV. The baseline characteristics of patients are summarized in Table 1.
Lasso Regression Analyses and Prognostic Values of Indicators for Females with Cancer
Based on Lasso regression analyses of all the indicators, NLR, PLR, GLR, CAR, NRI, and GNRI were removed from further consideration in subsequent group and subgroup analyses.
The trend between the risk of death and indicators in females with cancer and different subgroups is shown in Supplemental Figures 1–6. The cut-off values of indicators were obtained using ROC curves (Supplemental Figure 7, Supplemental Table 4). Patients with higher SII or CONUT or lower ALI, mGNRI, AGR, PNI, or LCR had lower overall survival than patients with the lower SII or CONUT or higher ALI, mGNRI, AGR, PNI, or LCR (Figure 2, Table 2).
 |
Table 2 Associations Between Indicators and OS in All Females with Cancer |
Univariate analyses indicated that ALI, SII, AGR, LCR, mGPS, LCS, PNI, mGNRI, and CONUT were prognostic factors for females with cancer (all P for trend < 0.001). Multivariate analyses found that ALI (HR, 0.80; 95% CI, 0.72–0.88; P for trend < 0.001), SII (HR, 1.18; 95% CI, 1.08–1.30; P < 0.001), AGR (HR, 0.81; 95% CI, 0.74–0.89; P for trend < 0.001), LCR (HR, 0.78; 95% CI, 0.71–0.86; P for trend < 0.001), mGPS (HR, 1.35; 95% CI, 1.18–1.54; P for trend < 0.001), LCS (HR, 1.53; 95% CI, 1.29–1.81; P for trend < 0.001), PNI (HR, 0.83; 95% CI, 0.75–0.90; P for trend < 0.001), mGNRI (HR, 0.73; 95% CI, 0.64–0.84; P for trend < 0.001), and CONUT (HR, 1.11; 95% CI, 1.06–1.17; P for trend < 0.001) were independent prognostic factors for females with cancer (Table 2). The associations between indicators and overall survival in different subgroups are shown in Figure 3.
Survival Prediction Values of Indicators and Inflammatory and Nutritional Status Evaluation
LCR showed the highest prognostic ability for females with cancer, followed by ALI, PNI, mGNRI, SII, LCS, AGR, CONUT, and mGPS (Figure 4).
The C-indices of LCR (0.668; 95% CI, 0.644–0.693), ALI (0.657; 95% CI, 0.632–0.683, P = 0.345), PNI (0.651; 95% CI, 0.625–0.676, P = 0.024), mGNRI (0.632; 95% CI, 0.606–0.657, P = 0.004), SII (0.629; 95% CI, 0.602–0.655, P = 0.002), LCS (0.624; 95% CI, 0.602–0.646, P < 0.001), AGR (0.622; 95% CI, 0.595–0.649, P = 0.001), CONUT (0.618; 95% CI, 0.591–0.645, P < 0.001), and mGPS (0.597; 95% CI, 0.574–0.620, P < 0.001) are presented in Table 3.
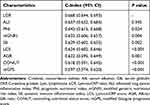 |
Table 3 C-Indices of Indicators in Females with Cancer |
LCR was used to evaluate the inflammatory levels of different subgroups, and PNI was used to evaluate the nutritional levels of different subgroups (Supplemental Figures 8 and 9).
Survival Prediction and Comparison Among Indicators for Young, Middle-Aged, or Elderly Female Patients
AGR showed the highest prognostic ability for young females with cancer, followed by mGNRI, LCR, PNI, LCS, ALI, SII, mGPS, and CONUT (Supplemental Figure 10). LCR showed the highest prognostic value for middle-aged females with cancer, followed by ALI, mGNRI, LCS, PNI, AGR, SII, mGPS, and CONUT (Supplemental Figure 11). For elderly female patients, ALI had the highest prognostic value, followed by LCR, SII, PNI, CONUT, mGPS, AGR, LCS, and mGNRI (Supplemental Figure 12). The C-indices of indicators are presented in Supplemental Table 5.
Survival Prediction and Comparison Among Indicators for Female Patients with or with Non-Reproductive System Cancer
SII showed the highest prognostic ability for female patients with reproductive system cancer, followed by LCR, ALI, LCS, AGR, PNI, CONUT, mGNRI, and mGPS (Supplemental Figure 13). ALI showed the highest prognostic ability for female patients with non-reproductive system cancer, followed by LCR, PNI, SII, mGNRI, AGR, LCS, mGPS, and CONUT (Supplemental Figure 14). The C-indices of indicators are presented in Supplemental Table 6.
Discussion
In this study, we investigated the correlation between 15 inflammation and nutrition-based indicators with cancer prognoses in females to determine the prognostic values of these indicators. Of the 15 indicators, 9 were found to be prognostic indicators for females with cancer by Lasso analysis. These indicators were also found to be independent prognostic factors for females with cancer by multivariate analysis. LCR was the most suitable prognostic indicator for all females with cancer, followed by ALI, PNI, mGNRI, SII, LCS, AGR, CONUT, and mGPS.
Patients were divided into 3 groups (young, middle-aged, and elderly) according to age or into 2 groups according to the type of cancer (reproductive system cancer and non-reproductive system cancer). In each subgroup, we compared the prognostic value of these indicators and determined the most suitable prognostic factors for different populations. We used LCR to evaluate the inflammatory levels of the different subgroups and PNI to evaluate the nutritional levels of the different subgroups. By comparing the prognostic values of these indicators with the LCR and PNI levels in different patient populations, we could determine the appropriate prognostic indicators for each subgroup.
Indicators are composed of nutrition-related indices (BMI, Lym, WLo, and TC), inflammation-related indices (CRP, Neu, Glb, and Plt), and Alb, which are related to both nutrition and inflammation.14,16,18,19,21,27–31 We divided the nine indicators into three categories: nutrition-related indicators (PNI, CONUT), inflammation-related indicators (LCR, SII, LCS), and indicators that take into account nutrition and inflammation (ALI, mGNRI, AGR, and mGPS).14,16,18,19,21,27–31
Inflammation affects the occurrence and development of cancer through different pathways.32,33 As evidence has accumulated linking inflammation with cancer development, additional indicators of cancer-related inflammation have been discovered. Cancer patients with higher SII or lower LCR or LCS have significantly worse prognoses than those with lower SII or higher LCR or LCS.18,23,28–31 Cancer patients are prone to malnutrition and an increased risk of death because inflammation influences the absorption and utilization of nutrients by cancer cells.34 Patients with higher PNI or lower CONUT have a lower risk of death than those with lower PNI or higher CONUT.20,21,27 Changes in inflammation and nutritional statuses have mutual effects on each other.13,35,36 The mutual interactions between inflammation and nutritional statuses accelerate cancer progression and lead to a worse cancer prognosis for patients.37
Young females have lower systemic inflammation compared to young males, which may be due to the protective effect of female hormones.9 Females have a higher incidence of malnutrition and iron-deficiency anemia than males, which may be due to the effects of menstruation and diet.7,8 Our results showed that compared with middle-aged and elderly females, young females have lower inflammatory levels and better nutritional statuses. We believe that although the nutritional statuses of young females are unstable, their status remains at a higher level compared with middle-aged and elderly women. Nutrition status still has a greater impact on the cancer prognosis of young females than inflammation status. In comparison with inflammation, the nutritional status of females with cancer is more apparent. Our results show that AGR is the most appropriate prognostic indicator in young females with cancer. Nutritional supplements could reverse this trend.38 We recommend using the nutrition-related prognostic index AGR to predict patients’ survival and to provide nutritional interventions.
The inflammation and nutritional statuses of middle-aged females differ from those of young females. Because most middle-aged females are in perimenopause, their hormone levels are in flux.11 The inhibitory effect of estrogen on inflammation is weakened, and anorexia caused by perimenopause affects the nutritional status of females with cancer.9,39,40 Cancers in patients with low estrogen levels progress faster than those with normal levels of estrogen, which help inhibit cancer progression.41 In addition, inflammatory levels in cancer patients increase with age as their nutritional statuses continue to deteriorate.12,42
We found that the most suitable prognostic indicator for middle-aged females with cancer was LCR. The level of inflammation in cancer patients had a greater impact on their prognosis than nutritional status. LCR may be a more suitable prognostic factor for middle-aged females with cancer and reducing the inflammatory state of these patients may bring greater clinical benefits.
Compared with young and middle-aged populations, elderly females are always in a state of low-level systemic inflammation.40 This is due to physiological changes and the loss of estrogen brought on by increased age. This reduces the inhibition of cancer growth.41 Due to the reduction of nutritional intake caused by a decreased appetite and the increase in nutritional consumption caused by systemic inflammation, the nutritional statuses of elderly females are worse than those of young and middle-aged females.16,43 We show that elderly females have the highest inflammatory load and the lowest nutritional status. Indicators of inflammatory and nutritional status were the most appropriate prognostic factors for elderly female patients. Our results showed that ALI was the most suitable prognostic indicator for elderly females with cancer. For elderly females with cancer, comprehensive interventions such as reducing inflammation, increasing nutrition, getting reasonable exercise, and enhancing immunity are desirable.
To provide more accurate prognostic indicators, we divided patients into 2 groups, one with reproductive system cancer and the other with non-reproductive system cancer. Inflammation is a fundamental cause of the occurrence and development of both reproductive system cancer and non-reproductive system cancer.44–47 However, patients with reproductive system cancer have higher levels of inflammation than those with non-reproductive system cancer.44 Nutritional consumption caused by high inflammatory levels is a critical source of malnutrition in patients with cancer.48 Our results show that patients with reproductive system cancers have higher levels of inflammation and worse nutritional status than those with non-reproductive system cancer. For female patients with reproductive system cancer, an index measuring inflammation is consistent with the most suitable prognostic index (LCR), and a treatment scheme should focus on reducing systemic inflammation. However, for patients with non-reproductive system cancer, an index that incorporates measures of both nutrition and inflammation provides the most suitable prognostic index (ALI). For these patients, a combination of anti-inflammatory and nutritional supplements is a desirable treatment strategy.
Our study had several limitations. The sample sizes were relatively small, which may have affected the statistical power of the study. In addition, we could not conduct a subgroup analysis based on each cancer type. Studies with larger sample sizes for each cancer type and more clinical factors may improve the prognostic prediction for females with cancer. Laboratory data were acquired using standard laboratory test protocols, which were limited compared to more advanced testing techniques. Although there was no measurement of hormone levels in our study, previous studies had shown that compared with males, females have more intense hormonal changes throughout their lives and are more vulnerable and more likely to be affected by age-related changes in hormone levels.49,50 Studies with more clinical factors (such as hormone should be carried out in the future.
Conclusion
In summary, LCR showed the highest prognostic ability for females with cancer, followed by ALI, PNI, mGNRI, SII, LCS, AGR, CONUT, and mGPS. For young females with cancer, we recommend using AGR to predict patient survival. For middle-aged females with cancer, inflammatory index (LCR) may be a more suitable prognostic factor. For elderly females with cancer, ALI was the most suitable prognostic indicator. We formulated individualized treatment plans for different populations according to their inflammatory and nutritional statuses to maximize the improvement of the populations’ prognoses.
Abbreviations
BMI, body mass index; KPS, Karnofsky performance status score; PG-SGA, Scored Patient-Generated Subjective Global Assessment; Alb, serum albumin; Glb, serum globulin; CRP, C-reactive protein; FBG, fasting blood glucose; TC, serum total cholesterol; Neu, neutrocyte; Lym, lymphocyte; Plt, platelet; NLR, Neu-to-Lym ratio; PLR, Plt-to-Lym ratio; GLR, FBG-to-Lym ratio; ALI, advanced lung cancer inflammation index; SII, systemic immune inflammation index; CAR, CRP-to-Alb ratio; GNRI, geriatric nutritional risk index; mGNRI, modified GNRI; AGR, Alb-to-Glb ratio; PNI, prognostic nutritional index; NRI, nutritional risk index; LCR, Lym-to-CRP ratio; CONUT, controlling nutritional status score; mGPS, modified Glasgow prognostic score; LCS, Lym-to-CRP score.
Data Sharing Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the corresponding author (Prof. Hanping Shi).
Ethics Approval and Informed Consent
We obtained approval for the study from the Medical Ethical Review Committees and Institutional Review Boards of the participating registered hospitals. The study was conducted in accordance with the guidelines of the Declaration of Helsinki. The study was registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn) and assigned a registration number ChiCTR1800020329.
Acknowledgments
We would like to thank TopEdit for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the National Key Research and Development Program [grant numbers 2017YFC1309200].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
2. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
3. Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168(4):657–669. doi:10.1016/j.cell.2016.12.039
4. Sapienza C, Issa JP. Diet, nutrition, and cancer epigenetics. Annu Rev Nutr. 2016;36:665–681. doi:10.1146/annurev-nutr-121415-112634
5. Muenst S, Laubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med. 2016;279(6):541–562. doi:10.1111/joim.12470
6. Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015;294(2):102–110. doi:10.1016/j.cellimm.2015.02.002
7. Boulos C, Salameh P, Barberger-Gateau P. The AMEL study, a cross sectional population-based survey on aging and malnutrition in 1200 elderly Lebanese living in rural settings: protocol and sample characteristics. BMC Public Health. 2013;13:573. doi:10.1186/1471-2458-13-573
8. Armstrong KL. Blood donation and anemia. Can Fam Physician. 2016;62(9):730–731.
9. Sahlan M, Devina A, Pratami DK, et al. Anti-inflammatory activity of Tetragronula species from Indonesia. Saudi J Biol Sci. 2019;26(7):1531–1538. doi:10.1016/j.sjbs.2018.12.008
10. Deng S, Ramos-Castaneda M, Velasco WV, et al. Interplay between estrogen and Stat3/NF-kappaB-driven immunomodulation in lung cancer. Carcinogenesis. 2020;41(11):1529–1542. doi:10.1093/carcin/bgaa064
11. Keller AC, Klawitter J, Hildreth KL, et al. Elevated plasma homocysteine and cysteine are associated with endothelial dysfunction across menopausal stages in healthy women. J Appl Physiol. 2019;126(6):1533–1540. doi:10.1152/japplphysiol.00819.2018
12. de Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25(7):875–881. doi:10.1093/bioinformatics/btp073
13. Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi:10.1093/ajcn/82.4.777
14. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.024
15. Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44(5):607–612. doi:10.1016/j.ejso.2018.02.003
16. Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3(12):e172319. doi:10.1001/jamaoncol.2017.2319
17. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–894. doi:10.21037/tlcr.2019.11.16
18. Kusunoki K, Toiyama Y, Okugawa Y, et al. Advanced lung cancer inflammation index predicts outcomes of patients with colorectal cancer after surgical resection. Dis Colon Rectum. 2020;63(9):1242–1250. doi:10.1097/DCR.0000000000001658
19. Luan CW, Yang HY, Tsai YT, Hsieh MC, Chou HH, Chen KS. Prognostic Value of C-reactive protein-to-albumin ratio in head and neck cancer: a meta-analysis. Diagnostics. 2021;11(3):403. doi:10.3390/diagnostics11030403
20. Kouzu K, Tsujimoto H, Sugasawa H, et al. Modified geriatric nutrition risk index as a prognostic predictor of esophageal cancer. Esophagus. 2021;18(2):278–287. doi:10.1007/s10388-020-00795-w
21. Mirili C, Yılmaz A, Demirkan S, Bilici M, Basol Tekin S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. 2019;24(10):1301–1310. doi:10.1007/s10147-019-01461-7
22. Hua X, Long ZQ, Huang X, et al. The value of Prognostic Nutritional Index (PNI) in predicting survival and guiding radiotherapy of patients with T1-2N1 breast cancer. Front Oncol. 2019;9:1562. doi:10.3389/fonc.2019.01562
23. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-C-reactive Protein Ratio as Promising New Marker for Predicting Surgical and Oncological Outcomes in Colorectal Cancer. Ann Surg. 2020;272(2):342–351. doi:10.1097/SLA.0000000000003239
24. Hu C, Zhang Q, Shi H. Nutrition status of patients with common cancer in China: gap, mission and challenge. Sci China Life Sci. 2021;64(11):1980–1983. doi:10.1007/s11427-021-1954-4
25. Xie H, Ruan G, Ge Y, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. 2022;41(6):1236–1243. doi:10.1016/j.clnu.2022.04.019
26. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi:10.3322/caac.21388
27. Ahiko Y, Shida D, Horie T, et al. Controlling nutritional status (CONUT) score as a preoperative risk assessment index for older patients with colorectal cancer. BMC Cancer. 2019;19(1):946. doi:10.1186/s12885-019-6218-8
28. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr. 2020;39(4):1209–1217. doi:10.1016/j.clnu.2019.05.009
29. Lv GY, An L, Sun XD, Hu YL, Sun DW. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta. 2018;476:81–91. doi:10.1016/j.cca.2017.11.019
30. Zhang H, Ren D, Jin X, Wu H. The prognostic value of modified Glasgow prognostic score in pancreatic cancer: a meta-analysis. Cancer Cell Int. 2020;20:462. doi:10.1186/s12935-020-01558-4
31. Albany C. Systemic immune-inflammation index in germ-cell tumours: search for a biological prognostic biomarker. Br J Cancer. 2018;118(6):761–762. doi:10.1038/bjc.2018.7
32. Wang Y, Liu S, Li B, et al. Staphylococcus aureus induces COX-2-dependent proliferation and malignant transformation in oral keratinocytes. J Oral Microbiol. 2019;11(1):1643205. doi:10.1080/20002297.2019.1643205
33. Shi N, Chen F, Zhang X, et al. Suppression of oxidative stress and NFkappaB/MAPK signaling by lyophilized black raspberries for esophageal cancer prevention in rats. Nutrients. 2017;9(4):413. doi:10.3390/nu9040413
34. Cheng TY, Lee PC, Chen YT, Chao Y, Hou MC, Huang YH. Pre-sarcopenia determines post-progression outcomes in advanced hepatocellular carcinoma after sorafenib failure. Sci Rep. 2020;10(1):18375. doi:10.1038/s41598-020-75198-z
35. Kim JS, Kim W, Park JY, et al. Effects of statin therapy on clinical outcomes after acute myocardial infarction in patients with advanced renal dysfunction: a propensity score-matched analysis. PLoS One. 2017;12(8):e0183059. doi:10.1371/journal.pone.0183059
36. Chiu H, Wu PY, Huang JC, et al. There is a U shaped association between non high density lipoprotein cholesterol with overall and cardiovascular mortality in chronic kidney disease stage 3-5. Sci Rep. 2020;10(1):12749. doi:10.1038/s41598-020-69794-2
37. Cuvelier C, Tintillier M, Migali G, Van Ende C, Pochet JM. Albumin losses during hemodiafiltration: all dialyzers are not created equal - a case report. BMC Nephrol. 2019;20(1):392. doi:10.1186/s12882-019-1567-8
38. Xiong J, Wang Y, Kang W, et al. Prognostic importance of the preoperative Naples prognostic score for patients with adenocarcinoma of the esophagogastric junction. Front Oncol. 2020;10:595793. doi:10.3389/fonc.2020.595793
39. Baker JH, Runfola CD. Eating disorders in midlife women: a perimenopausal eating disorder? Maturitas. 2016;85:112–116. doi:10.1016/j.maturitas.2015.12.017
40. Fujita K, Ito Y, Oguma T, Mio T, Niimi A, Hirai T. Association between Mycobacterium avium complex lung disease and serum vitamin D status, antimicrobial peptide levels, and bone mineral density. Medicine. 2018;97(38):e12463. doi:10.1097/MD.0000000000012463
41. Liang J, Shang Y. Estrogen and cancer. Annu Rev Physiol. 2013;75:225–240. doi:10.1146/annurev-physiol-030212-183708
42. Arima H, Nakano M, Koirala S, et al. Unique hemoglobin dynamics in female Tibetan highlanders. Trop Med Health. 2021;49(1):2. doi:10.1186/s41182-020-00289-6
43. Plotnikov G, Waizman E, Tzur I, Yusupov A, Shapira Y, Gorelik O. The prognostic role of functional dependency in older inpatients with COVID-19. BMC Geriatr. 2021;21(1):219. doi:10.1186/s12877-021-02158-1
44. Laniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol. 2020;17(4):232–250. doi:10.1038/s41585-020-0286-z
45. Lucas C, Barnich N, Nguyen HTT. Microbiota, inflammation and colorectal cancer. Int J Mol Sci. 2017;18(6):1310. doi:10.3390/ijms18061310
46. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196–202. doi:10.1016/j.canlet.2013.08.016
47. Conway EM, Pikor LA, Kung SHY, et al. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med. 2016;193(2):116–130. doi:10.1164/rccm.201508-1545CI
48. Maccio A, Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012;58(2):133–147. doi:10.1016/j.cyto.2012.01.015
49. Shepherd R, Cheung AS, Pang K, Saffery R, Novakovic B. Sexual dimorphism in innate immunity: the role of sex hormones and epigenetics. Front Immunol. 2020;11:604000. doi:10.3389/fimmu.2020.604000
50. Taneja V. Sexual dimorphism, aging and immunity. Vitam Horm. 2021;115:367–399.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.