Back to Journals » International Journal of General Medicine » Volume 15
Prevalence of Hyperuricemia and Associated Factors Among Type 2 Diabetic Patients in Jordan
Authors Abujbara M , Al Hourani HM , Al-Raoush RI, Khader YS , Ajlouni K
Received 2 June 2022
Accepted for publication 5 August 2022
Published 16 August 2022 Volume 2022:15 Pages 6611—6619
DOI https://doi.org/10.2147/IJGM.S376857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mousa Abujbara,1 Huda M Al Hourani,2 Reem Ibrahim Al-Raoush,1 Yousef S Khader,3 Kamel Ajlouni1
1The National Centre (Institute) for Diabetes, Endocrinology and Genetics, The University of Jordan, Amman, Jordan; 2Department of Clinical Nutrition and Dietetics, Faculty of Applied Health Sciences, The Hashemite University, Zarqa, Jordan; 3Jordan University of Science and Technology (JUST), Irbid, Jordan
Correspondence: Kamel Ajlouni, The National Centre (Institute) for Diabetes, Endocrinology and Genetics, The University of Jordan, P.O. Box 13165, Amman, 11942, Jordan, Tel +962 6 534 7810, Fax +962 6 535 6670, Email [email protected]
Background: Previous studies showed variable estimate of the prevalence of hyperuricemia in patients with type 2 diabetes mellitus (T2DM). The prevalence of hyperuricemia and associated risk factors in Jordanian patients with T2DM is largely unknown. Therefore, this study aimed to determine the prevalence of hyperuricemia and its associated factors in Jordanian patients with T2DM.
Methods: A cross-sectional study was conducted on 655 patients with T2DM. A structured questionnaire was used to collect socio-demographic data. In addition, records of the study subjects were reviewed to obtain other clinical data. Weight, height, and waist circumference were measured, and body mass index was calculated. Lipid profile, serum uric acid and glycated haemoglobin were analysed. The study was conducted in accordance with the Declaration of Helsinki. An informed written consent was obtained from each participant. The confidentiality of the information was assured and only used for scientific purposes.
Results: Overall, the prevalence of hyperuricemia was 28.1%. Female gender (OR: 2.37; 95%, CI: 1.63– 3.45), intake of angiotensin-converting enzyme (ACE) and angiotensin-II receptor blockers (ARBs) (OR: 1.68; 95%, CI: 1.12– 2.50), intake of β-blockers (OR: 2.20; 95%, CI: 1.51– 3.22), increased waist circumference (OR: 3.17; 95%, CI: 1.39– 7.22) and family history of hyperuricemia (OR: 2.56; 95%, CI: 1.57– 4.16) were associated with increased odds of hyperuricemia.
Conclusion: Hyperuricemia was high among type 2 diabetic patients, and screening test will be useful for those patients.
Keywords: uric acid, hyperuricemia, diabetes mellitus
Introduction
Uric acid is a product of the metabolic degradation of purine nucleotides and is excreted largely by the kidneys and has been associated with the incidence of gout and kidney stones.1,2 Hyperuricemia is defined as SUA concentrations of greater than 7.0 mg/dl in men, greater than 6.0 mg/dl in women and greater than 5.5 mg/dl in children.2,3
Causes of hyperuricemia can be classified into two functional types: increased production of uric acid and decreased excretion of uric acid. Causes of increased production include high levels of purine in the diet and increased purine metabolism. Causes of decreased excretion include kidney disease, certain drugs, and competition for excretion between uric acid and other molecules. Mixed causes include high levels of alcohol and starvation.3,4
Observation established that high levels of UA are predictive of diabetes,6 obesity and metabolic syndrome.2 Although much of the literature addresses the association of hyperuricemia with hypertension, and renal disease,5–7 the role of uric acid in diabetes risk remains controversial. Insulin resistance is a principal component that is affiliated to hyperuricemia and further development of metabolic syndrome. Hyperuricemia may advance metabolic syndrome by inducing endothelial dysfunction and also through activating pro-inflammatory pathways.2
Serum uric acid (SUA) level has been suggested to be associated with risk of type 2 diabetes. Biologically, uric acid (UA) plays an important role in worsening of insulin resistance in animal models by inhibiting the bioavailability of nitric oxide, which is essential for insulin-stimulated glucose uptake.6 Many studies were conducted to estimate the prevalence of hyperuricemia in patients with type 2 diabetes mellitus (T2DM). The prevalence was 32.6% in Chinese patients with T2DM,8 33.6% and 25.3% in Indian patients,9,10 33.8% in Ethiopian patients2 and 25.3% in Nigerian patients.11
Previous studies have also determined the risk factors of elevated SUA, including female gender,2 intake of certain medications such as antihypertensive drugs,12 diet,13 family history of hyperuricemia, obesity and central obesity.14,15
To the best of our knowledge, the prevalence of hyperuricemia and associated risk factors in Jordanian diabetic patients has not been investigated. Therefore, this study was conducted to determine the prevalence of hyperuricemia and its associated factors in Jordanian patients with T2DM.
Methods
A cross-sectional study was conducted from 1 October 2017 to 1 February 2018 on patients with T2DM attending the National Centre of Diabetes, Endocrinology and Genetics (NCDEG) in Jordan. The study included 655 patients with T2DM. This study was reviewed and approved by the research ethics committee at NCDEG. Informed consent was obtained from all participants. The privacy of participants was respected and identifying information was kept strictly confidential. Exclusion criteria included type 1 diabetes, chronic renal diseases, use of loop diuretic drugs, cancer, autoimmune diseases, gout, children and adolescents, hypothyroidism and pregnancy.
The data were collected from the patient’s medical files and structured questionnaire-interviews. Weight and height were measured during the clinic visit. Height was recorded to the nearest 0.5 cm using a stadiometer, with the subject in standing position and without shoes. Body weight was recorded to the nearest 0.1 kg, using a calibrated scale. Body mass index (BMI) was calculated as weight (kg) divided height squared (m)2: normal if MBI 18.5–24.99 kg/m2, overweight if BMI 25–29.9 kg/m2, obese if BMI ≥ 30 kg/m2. Waist circumference (WC) was measured and considered normal in women if less than 80 cm, and in men if less than 94 cm.16
According to the American Diabetes Association (ADA) 2016 guidelines, diabetes mellitus was diagnosed if the patient had an FPG ≥ 126 mg/dL (7.0 mmol/L) in two occasions or if the patient had a random plasma glucose ≥200 mg/dL (11.1 mmol/L) in the presence of classical symptoms of hyperglycemia, or if he or she had HbA1c ≥6.5%.17
Dyslipidaemia was defined according to the American Diabetes Association 2016 as follows17: total cholesterol level (TC) ≥200 mg/ dl, high-density lipoprotein cholesterol (HDL) <40 mg/dl for males, high-density lipoprotein cholesterol (HDL) <50 mg/dl for females. LDL cholesterol is considered elevated when the level ≥100 mg/dl. Triglyceride considered elevated when serum TG level is ≥150 mg/dl, or if the patient is on specific medication for any of them.
Blood pressure was measured by trained nursing staff using validated automated device OMRON® with an appropriate cuff size while patients sat quiet with both arms outstretched and supported. Hypertension is defined as systolic blood pressure (SBP) ≥140 mm of mercury and/or diastolic blood pressure (DBP) ≥90 mm of mercury.17
HbA1c was measured by Bio-Rad VARIANT II TURBO HbA1C Kit −2.0 which utilizes the principles of ion-exchange high performance liquid chromatography (HPLC).
Serum uric acid (SUA) was measured using an enzymatic colorimetric method by Roche/Hitachi cobas c 501 analysers (Indianapolis, Germany). High-density lipoprotein cholesterol (HDL), total cholesterol, triglycerides and low-density lipoprotein cholesterol (LDL) were measured using homogeneous enzymatic colorimetric assay by Roche/Hitachi cobas c 501/701 analysers (Indianapolis, Germany).
Analyses were carried out using the Statistical Package for Social Science (SPSS version 21, Chicago, Illinois). Categorical variables were described using percentages. Chi-square test was used to compare percentages. Multivariate analysis using binary logistic regression analysis was conducted to determine the association between hyperuricemia and studied variables. A p-value of <0.05 was considered statistically significant.
Results
A total of 655 subjects were enrolled in this study. The prevalence of hyperglycaemia was 28.1%. Table 1 shows the prevalence of hyperuricemia according to demographic, clinical and anthropometric characteristics of the study participants.
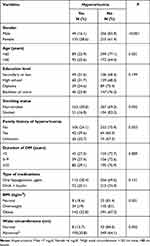 |
Table 1 The Prevalence of Hyperuricemia According to Demographic and Anthropometric Characteristics of the Study Participants |
The prevalence was significantly higher among females when compared to males (38.6% vs 16.1%; p<0.0001) and higher among patients 60 years old or older compared to those aged less than 60 years (35.6% vs 22.9%; p=0.001). Hyperuricemia was significantly higher among non-smokers compared to smokers (30.8% vs.16.8%; p=0.002). Moreover, the prevalence of hyperuricemia was significantly higher in obese subjects compared to overweight and normal subjects (32.8% vs 19.0% and 18.6%; p=0.001). The prevalence of hyperuricemia was also higher among study subjects with a family history of hyperuricemia as compared to study subjects with no family history of hyperuricemia (39.6% vs 42.1%; p=0.003). It was also significantly higher in subjects with an high waist circumference (35.8% vs 18.7%; p=0.002).
Table 2 shows the prevalence of hyperuricemia according to clinical characteristics of the study participants. The prevalence of hyperuricemia was significantly higher in patients with hypertension compared to normotensive patients (32.7% vs 14.8%; p=0.000). Patients with hypertriglyceridemia and reduced HDL cholesterol had higher prevalence of hyperuricemia (32.5% vs 23.1%; p=0.008 and 33.7% vs 24.3%; p=0.014, respectively).
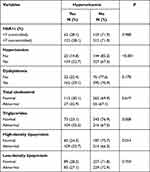 |
Table 2 The Prevalence of Hyperuricemia According to Clinical Characteristics of the Study Participants |
The prevalence rates of hyperuricemia according to the medications used are shown in Table 3. All studied medications except aspirin were associated with a higher prevalence of hyperuricemia. Table 4 shows the prevalence of hyperuricemia according to purine intake by the study participants. Diabetic patients who did not eat sheep liver were significantly more likely to have hyperuricemia than those who did (34.3% vs 25.2%; p=0.015). Patients who ate turkey or sausage, which is moderately high in purines (100–400mg of uric acid/100g), were more likely to be hyperuricemic.
 |
Table 3 Drugs Taken by the Study Participants |
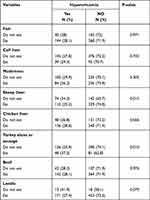 |
Table 4 The Prevalence of Hyperuricemia According to Purine Intake by the Study Participants |
Table 5 shows the factors associated with hyperuricemia among patients with type 2 diabetes in multivariate analysis. Female subjects were approximately 2.37 times more likely to have hyperuricemia than males (OR=2.37, 95% CI: 1.63–3.45, P=0.000). Taking anti-hypertension drugs like angiotensin-converting enzyme or angiotensin receptor blockers were significantly associated with an increased odds of hyperuricemia (OR=1.68, 95% CI: 1.12–2.50, p=0.011). In addition, subjects taking β blockers were significantly more likely to have hyperuricemia than subjects not taking β blockers (OR=2.20, 95% CI: 1.51–3.22, P=0.000). Family history of hyperuricemia was associated with a twofold higher odds of hyperuricemia (OR=2.56, 95% CI: 1.57–4.16, P=0.000). High waist circumference was associated with increased odds of hyperuricemia (OR=3.17, 95% CI: 1.39–7.22, p=0.006).
 |
Table 5 Factors Associated with Hyperuricemia Among Patients with Type 2 Diabetes in Multivariate Analysis |
Discussion
Hyperuricemia is a common biochemical abnormality that is frequently associated with other metabolic perturbation of great clinical importance such as obesity, HTN, hypertriglycerdemia, metabolic syndrome or T2 DM.6
Serum uric acid is known to act as anti-oxidant and may be one of the strongest determinants of plasma ant oxidative capacity; however, when SUA levels are elevated this previously, anti-oxidant paradoxically becomes prooxidant which may contribute to endothelial dysfunction and well increase the oxidative stress.6
This study was conducted to measure the prevalence of hyperuricemia and associated risk factors among type 2 Jordanian diabetic patients. The prevalence of hyperuricemia was identified as 28.1%. Numerous studies have estimated the prevalence of hyperuricemia among diabetic patients and the results of the current study were similar to those obtained from China,8 Nigeria,11 India and Italy.10,18 Contrary to the current finding, a lower prevalence of hyperuricemia was reported by Kim et al in Korea.19
Our study showed that females is one of the risk factors of hyperuricemia. On the other hand, previous studies which reported that males had a higher prevalence of hyperuricemia when compared with females.2,20 Consistent findings were reported by Wang et al, who demonstrated that diabetic females were 1.5 times more likely to develop hyperuricemia (OR: 1.576; 95% CI: 1.231–2.018).8 Our study showed a higher rate of hyperuricemia in females than that in males (38.6% vs 16.1%; p=0.000). This could be due to their age and menopausal status. This is supported by our finding that most of the hyperuricemic females were more than 60 years old, and therefore, changes in female sex hormones after the menopause could be linked to the increased urate level and the nonexistence of oestrogen in improving renal urate excretion.21
One of the associated risk factors was the intake of antihypertensive drugs, such as thiazide type diuretics, β blockers, ACE and ARBs, which were reported to increase SUA by different mechanisms of action, such as increasing the net renal reabsorption of urate22 and reducing glomerular filtration rate.23 In this study, patients who received ARBs and/or ACE were around 1.5 times more likely to have hyperuricemia (OR=1.68, CI: 1.12–2.50; P=0.011). Several studies have assessed the effect of ARBs drugs on SUA and of the known ARBs drugs, losartan showed a significant reduction in SUA levels, which may be due to the role of this drug in enhancing urinary excretion of uric acid by avoiding the reabsorption of uric acid. This drug was not among the choices for the current study participants, and around 90% of the study participants were taking other types of ARBs, such as valsartan and candesartan, which have been reported to increase SUA,24,25 which is consistent with the results of the current study. The ARBs have differences in chemical structure and also different effects on the uptake of uric acid by the urate transporter 1 (URAT1) receptor.26
The other type of antihypertensive drugs taken by the study participants were β blockers. Several studies have estimated the effect of β blockers on SUA, which are known to reduce glomerular filtration rate and the mean renal clearance of uric acid, thus increase SUA levels.23,27 The results of the current study showed that patients who were taking β blockers were 2 times more likely to develop hyperuricemia, which was consistent with other studies assessing the intake of β blockers.28
Genome-wide association studies (GWAS) have discovered genetic irregularities primarily involving renal urate transport, which may explain certain individuals’ susceptibility for developing hyperuricemia.29 Kuo et al30 reported that the risk of gout was significantly higher in individuals with affected first-degree relatives than in the general population with their relative risk being 1.91, which was consistent with the results of the current study.
In the present study, the prevalence of hyperuricemia increased with waist circumference (WC) suggesting that a relationship exists between hyperuricemia and increased visceral fat accumulation. Different Chinese studies have reported that waist circumference is positively associated with hyperuricemia.14,15 A Japanese study found that hyperuricemia was linked to increased abdominal obesity,31 which is consistent with the results of the current study. Abdominal obesity showed a stronger association with increased SUA, and the mechanisms underlying the obesity linked to an increase in SUA levels involve two factors: over-production of uric acid and poor uric acid excretion.32 An interesting finding in De Pergola study who showed that that weight loss, achieved by dietary intervention or bariatric surgery, is effective in reducing SUA levels.33
The main strength of this study is its relatively large numbers of participants. However, there are three main limitations. First, estimates of the dietary status by questions about the consumption of some foods through the year rather than 24-hour recalls will not give a clear idea about the quality and quantity of patients’ diet. Second, the study is clinic-based rather than population-based, and it may not be representative of diabetic patients in the community. Third, the results of this study cannot be generalized on all type 2 diabetic patients in Jordan.
In conclusion, our results indicate that hyperuricemia is prevalent in T2DM patients and significantly associated with women, high waist circumference, family history of hyperuricemia and intake of antihypertensive drugs such as ARBs, ACE and β blockers. Screening for hyperuricemia is needed for patients with T2DM.
Informed Consent
Informed consent was obtained from patients.
Disclosure
The authors have no financial relationship relevant to this article to disclose. The authors declare that they have no conflicts of interest in relation to this work.
References
1. Han Y, Zhang M, Lu J, et al. Hyperuricemia and overexcretion of uric acid increase the risk of simple renal cysts in type 2 diabetes. Sci Rep. 2017;7:3802. doi:10.1038/s41598-017-04036-6
2. Woyesa SB, Hirigo AT, Wube TB. Hyperuricemia and metabolic syndrome in type 2 diabetes mellitus patients at Hawassa university comprehensive specialized hospital, South West Ethiopia. BMC Endocr Disord. 2017;17:76. doi:10.1186/s12902-017-0226-y
3. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi:10.1016/j.ijcard.2015.08.109
4. Ruoff G, Edwards NL. Overview of serum uric acid treatment targets in gout: why less than 6 mg/dL? Postgrad Med. 2016;128:706–715. doi:10.1080/00325481.2016.1221732
5. Johnson RJ, Nakagawa T, Jalal D, Sanchez-Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28:2221–2228. doi:10.1093/ndt/gft029
6. Robinson PC. Gout - An update of aetiology, genetics, co-morbidities and management. Maturitas. 2018;118:67–73. doi:10.1016/j.maturitas.2018.10.012
7. Fenech G, Rajzbaum G, Mazighi M, Blacher J. Serum uric acid and cardiovascular risk: state of the art and perspectives. Joint Bone Spine. 2014;81:392–397. doi:10.1016/j.jbspin.2014.01.008
8. Wang J, Chen RP, Lei L, et al. Prevalence and determinants of hyperuricemia in type 2 diabetes mellitus patients with central obesity in Guangdong Province in China. Asia Pac J Clin Nutr. 2013;22:590–598. doi:10.6133/apjcn.2013.22.4.16
9. Billa G, Dargad R, Mehta A. Prevalence of hyperuricemia in Indian subjects attending hyperuricemia screening programs-a retrospective study. J Assoc Physicians India. 2018;66:43–46.
10. Mundhe S, Mhasde D. The study of prevalence of hyperuricemia and metabolic syndrome in type 2 diabetes mellitus. Int J Adv Med. 2016;3:241–249. doi:10.18203/2349-3933.ijam20160655
11. Ogbera AO, Azenabor AO. Hyperuricaemia and the metabolic syndrome in type 2 DM. Diabetol Metab Syndr. 2010;2:24. doi:10.1186/1758-5996-2-24
12. Hosoya T, Kuriyama S, Yoshizawa T, Kobayashi A, Otsuka Y, Ohno I. Effects of combined antihypertensive therapy with losartan/hydrochlorothiazide on uric acid metabolism. Intern Med. 2012;51:2509–2514. doi:10.2169/internalmedicine.51.7584
13. Zykova SN, Storhaug HM, Toft I, Chadban SJ, Jenssen TG, White SL. Cross-sectional analysis of nutrition and serum uric acid in two Caucasian cohorts: the AusDiab Study and the Tromso study. Nutr J. 2015;14:49. doi:10.1186/s12937-015-0032-1
14. You L, Liu A, Wuyun G, Wu H, Wang P. Prevalence of hyperuricemia and the relationship between serum uric acid and metabolic syndrome in the Asian Mongolian area. J Atheroscler Thromb. 2014;21:355–365. doi:10.5551/jat.20529
15. Villegas R, Xiang YB, Cai Q, et al. Prevalence and determinants of hyperuricemia in middle-aged, urban Chinese men. Metab Syndr Relat Disord. 2010;8:263–270. doi:10.1089/met.2009.0084
16. International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome; 2005. Available from: http://www.idf.org/webdata/dosc/idf-Metasyndrome-Definition.pdf.
17. American Diabetes Association. Glycemic targets. Diabetes Care. 2016;39(1):S39–S46. doi:10.2337/dc16-S008
18. Mantovani A, Rigolon R, Civettini A, et al. Hyperuricemia is associated with an increased prevalence of paroxysmal atrial fibrillation in patients with type 2 diabetes referred for clinically indicated 24-h Holter monitoring. J Endocrinol Invest. 2018;41:223–231. doi:10.1007/s40618-017-0729-4
19. Kim TH, Lee SS, Yoo JH, et al. The relationship between the regional abdominal adipose tissue distribution and the serum uric acid levels in people with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4:3. doi:10.1186/1758-5996-4-3
20. Liu R, Han C, Wu D, et al. Prevalence of hyperuricemia and gout in Mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:762820. doi:10.1155/2015/762820
21. MacFarlane LA, Kim SC. Gout: a review of nonmodifiable and modifiable risk factors. Rheum Dis Clin North Am. 2014;40:581–604. doi:10.1016/j.rdc.2014.07.002
22. Rubio-Guerra AF, Garro-Almendaro AK, Elizalde-Barrera CI, Suarez-Cuenca JA, Duran-Salgado MB. Effect of losartan combined with amlodipine or with a thiazide on uric acid levels in hypertensive patients. Ther Adv Cardiovasc Dis. 2017;11:57–62. doi:10.1177/1753944716678538
23. Ueno S, Hamada T, Taniguchi S, et al. Effect of antihypertensive drugs on uric acid metabolism in patients with hypertension: cross-sectional cohort study. Drug Res. 2016;66:628–632. doi:10.1055/s-0042-113183
24. Rayner BL, Trinder YA, Baines D, Isaacs S, Opie LH. Effect of losartan versus candesartan on uric acid, renal function, and fibrinogen in patients with hypertension and hyperuricemia associated with diuretics. Am J Hypertens. 2006;19:208–213. doi:10.1016/j.amjhyper.2005.08.005
25. Elliott WJ, Calhoun DA, DeLucca PT, Gazdick LP, Kerns DE, Zeldin RK. Losartan versus valsartan in the treatment of patients with mild to moderate essential hypertension: data from a multicenter, randomized, double-blind, 12-week trial. Clin Ther. 2001;23:1166–1179. doi:10.1016/S0149-2918(01)80099-0
26. Wolff ML, Cruz JL, Vanderman AJ, Brown JN. The effect of angiotensin II receptor blockers on hyperuricemia. Ther Adv Chronic Dis. 2015;6:339–346. doi:10.1177/2040622315596119
27. Choi HK, Soriano LC, Zhang Y, Rodriguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ. 2012;344:d8190. doi:10.1136/bmj.d8190
28. Juraschek SP, Appel LJ, Miller ER. Metoprolol increases uric acid and risk of gout in African Americans with chronic kidney disease attributed to hypertension. Am J Hypertens. 2017;30:871–875. doi:10.1093/ajh/hpx113
29. Reginato AM, Mount DB, Yang I, Choi HK. The genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2012;8:610–621. doi:10.1038/nrrheum.2012.144
30. Kuo CF, Grainge MJ, See LC, et al. Familial aggregation of gout and relative genetic and environmental contributions: a nationwide population study in Taiwan. Ann Rheum Dis. 2015;74:369–374. doi:10.1136/annrheumdis-2013-204067
31. Miyagami T, Yokokawa H, Fujibayashi K, et al. The waist circumference-adjusted associations between hyperuricemia and other lifestyle-related diseases. Diabetol Metab Syndr. 2017;9:11. doi:10.1186/s13098-017-0212-6
32. Matsuura F, Yamashita S, Nakamura T, et al. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism. 1998;47:929–933. doi:10.1016/S0026-0495(98)90346-8
33. De Pergola G, Zupo R, Lampignano L, et al. Higher body mass index, uric acid levels, and lower cholesterol levels are associated with greater weight loss. Endocr Metab Immune Disord Drug Targets. 2020;20(8):1268–1281. doi:10.2174/1871530320666200429235830
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
