Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Uric Acid Metabolic Disorders in Pituitary-Target Gland Axis
Authors Li R, Wu B, Han M , Li M , Yang X, Zhang J, Zhang Y, Liu Y
Received 7 November 2023
Accepted for publication 18 January 2024
Published 7 February 2024 Volume 2024:17 Pages 661—673
DOI https://doi.org/10.2147/DMSO.S448547
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Ru Li,1,2,* Baofeng Wu,1,2,* Minmin Han,1,2 Mengnan Li,1,2 Xifeng Yang,1,2 Jian Zhang,1,2 Yi Zhang,3 Yunfeng Liu1
1Department of Endocrinology, The First Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 2The First Clinical Medical College, Shanxi Medical University, Taiyuan, People’s Republic of China; 3Department of Pharmacology, Shanxi Medical University, Taiyuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yunfeng Liu, Department of Endocrinology, The First Hospital of Shanxi Medical University, 85 Jiefang South Road, Taiyuan, People’s Republic of China, Tel +86 18703416196, Email [email protected] Yi Zhang, Department of Pharmacology, Shanxi Medical University, Taiyuan, People’s Republic of China, Email [email protected]
Abstract: Uric acid (UA) is the end product of purine metabolism in the human, and the imbalance between production and excretion results in the disturbance of serum uric acid (SUA). There is evidence suggesting that pituitary-target gland hormones can affect UA metabolism through regulating the activity of xanthine oxidase and UA transporters. Related endocrine diseases including thyroid dysfunction, polycystic ovary syndrome, acromegaly and Cushing’s syndrome are often accompanied by elevated UA levels. In addition to the direct influence of abnormal hormones, obesity and insulin resistant play a pivotal role. Diabetes insipidus and the syndrome of inappropriate antidiuretic hormone secretion also present with abnormal SUA levels due to the action of antidiuretic hormone. However, certain evidence within the population is disputed. This review summarized the effects of pituitary-target gland hormones on UA metabolism, and preliminarily described the related mechanisms, offering a theoretical foundation for assessing SUA in endocrine disorders as well as guiding its management.
Keywords: uric acid, hyperuricemia, pituitary-target gland, endocrine disease, insulin resistant
Introduction
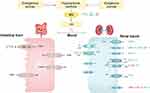
Uric acid (UA) is the end product of purine metabolism in the human. Hypoxanthine and xanthine finally form UA under the catalysis of xanthine oxidase (XO). The endogenous production of UA mainly derives from the liver, and small amounts can also be produced in other parts such as the intestine, muscles, kidneys, and vascular endothelium. About two-thirds of UA is excreted by the kidney and the remaining one-third is excreted by the intestines or absorbed by intestinal bacteria.1 The metabolic process of UA in the kidney includes four steps: filtration, reabsorption, secretion, and post-secretion reabsorption. Finally, about 10% of UA filtered by the glomerulus discharges from the body. This depends on the action of UA transporters (Table 1).2
 |
Table 1 The Location and Function of UA Transporters in Kidney |
The production and excretion of UA are physiologically maintained in a dynamic equilibrium. Hyperuricemia occurs when there is an increase in UA production or a decrease in its excretion, while conversely leading to hypouricemia. The presence of hyperuricemia is recognized to elevate the susceptibility to gout and exhibit a close correlation with hypertension, diabetes, metabolic syndrome, renal impairment and cardiovascular diseases.3 Recently, hypouricemia is also demonstrated to be associated with an elevated risk of acute and chronic kidney injury, urolithiasis, as well as certain neurodegenerative disorders.4
Endocrine disorders frequently exhibit abnormal UA metabolism. Relevant investigations have revealed that various pituitary-targeted hormones can modulate the activities of XO and UA transporter, thereby governing the synthesis and excretion of UA. Besides, the interaction between insulin resistance (IR) and hyperuricemia has been well documented.5,6 Given the crucial role of the pituitary-target gland axis in metabolism, the dysfunction within this axis may contribute to IR and subsequent complications related to metabolism. It is reasonable to speculate a potential correlation between pituitary-target gland hormones and UA metabolism. Our aim was to summarize population data and initially elucidate the mechanisms to enhance endocrinologists’ comprehension of this association.
Pituitary-Thyroid Axis
Several studies have demonstrated a higher incidence of hyperuricemia in patients with thyroid dysfunction, including those with subclinical thyroid dysfunction. On the basis of the high prevalence, more studies have attempted to explore the relationship between thyroid dysfunction and UA metabolism.
Hypothyroidism
Based on a limited number of cases, the prevalence of hyperuricemia in patients with hypothyroidism was 33.3%.7 Vandana et al demonstrated a positive correlation between thyroid-stimulating hormone (TSH) and UA, while free thyroxine (FT4) showed a negative correlation with UA levels.8 Similarly, in the Chinese population, there remained a positive association between TSH and UA levels.9 For individuals with subclinical hypothyroidism, the TH sensitivity index calculated using FT4 and TSH exhibited a statistically significant relationship with UA levels, suggesting that impaired TH sensitivity contributes to the development of hyperuricemia.10
Regarding the underlying mechanism, it is generally accepted that the decrease of renal UA excretion secondary to decreased renal blood flow (RBF) and glomerular filtration rate (GFR) is the main cause (Figure 1). In terms of renal function, decreased TH impairs the activities of tubular ion transporters such as Na+-K+-2Cl− cotransporters, Na+-H+ antiporter and Na+-K+-ATPase. Consequently, there is an increase in the concentrations of Na+ and Cl− in distal renal tubules, leading to enhanced tubule feedback and subsequent reductions in RBF and GFR.11 Additionally, decreased TH directly affects the synthesis and secretion of the renin-angiotensin-aldosterone system (RAAS), thereby diminishing self-regulation of renal perfusion.12 Furthermore, reduced TH levels exacerbate renal vasoconstriction through the indirect effects mediated by paracrine or endocrine factors like insulin-like growth factor 1 (IGF-1) and vascular endothelial cell growth factor (VEGF).13 It is worth noting that the binding of TH to nuclear receptors affects the development of renal structures. Hypothyroidism during development may affect GFR due to the reduction of renal capillaries and glomerular surface area.14 In terms of cardiac function, TH predominantly binds to nuclear TRα, exerting direct influence on cardiomyocyte activity or regulating the transcription rate of the β1 adrenoceptor gene. This leads to enhanced cardiac output through positive inotropic and chronotropic effects.15 The reduction of TH, accompanied by a decrease in cardiac output, induces a hypodynamic state within the circulatory system and diminishes renal perfusion. Following TH replacement therapy, there is an increase observed in both RBF and GFR, suggesting that its impact on kidney function is partially reversible.16
Hypothyroidism is also considered a risk factor for IR.17 Replacement therapy with levothyroxine sodium for 2 months in patients with subclinical hypothyroidism can improve homeostatic model assessment of insulin resistance (HOMA-IR) following with decrease in UA levels.18 This phenomenon suggests that thyroid hormones may lower UA levels by improving IR (Figure 2).
Hyperthyroidism
In 1995, Akira et al reported a significant increase in UA levels among patients with hyperthyroidism.19 Furthermore, the concentration of UA appeared to be closely correlated with FT3 and FT4 levels.20,21 However, no significant association between subclinical hyperthyroidism and elevated levels of UA or hyperuricemia was observed in the patient cohort.22
In contrast to hypothyroidism, hyperthyroidism alleviates systemic vascular resistance by increasing heart rate, cardiac output, as well as NO production. Furthermore, activation of the RAAS and reduced resistance of glomerular afferent arterioles contribute to enhanced RBF and GFR.13 At the same time, purine nucleotide metabolism in muscle is enhanced. Due to a poor supplement of ATP, type I fibers could be converted to type II fibers in skeletal muscles, so as to obtain acceleration of AMP deaminase activity.23 In the hyperthyroid state, increased XO activity also facilitates the production of UA.24 The investigator observed elevated UA in HepG2, Hep1-6 cell and mouse liver treated by T3, further making clear that T3 regulates nucleotide metabolizing enzyme partially through Pre2 expression mediated by TR-β.25 The hypermetabolic state associated with hyperthyroidism leads to an increased production of UA, surpassing its clearance capacity and resulting in the development of hyperuricemia (Figure 1).
Currently, the most direct evidence regarding the impact of TH on UA metabolism lies in its activation of the nuclear receptor THRβ, which subsequently induces the expression of postsynaptic density 95/disk-large/ZO-1 (PDZ) domain-containing protein 1 (PDZK1). PDZK1 interacts with solute carrier and ATP binding-cassette transporters, thereby regulating their function to facilitate urate processing (Figure 3).26 With the discovery of TSH receptor expression on the kidney, the mechanism of TSH affecting UA metabolism remains to be explored.27
Treatment of Hyperuricemia in Patients with Thyroid Dysfunction
Mild elevation of UA in thyroid dysfunction does not require treatment. A decrease of UA levels was observed after taking TH replacement therapy in hypothyroidism patients and methimazole treatment in hyperthyroidism patients.18,19 In cases of severe hyperuricemia, prescription of urate-lowering drugs may be necessary. In the clinical setting, a limited number of individuals have reported elevated TSH levels following treatment with febuxostat and allopurinol, therefore caution is advised when administering these medications to patients with hypothyroidism.28,29 However, it has been reported that allopurinol exhibits a protective effect against hyperthyroidism by reducing oxidative stress in a rat model.30
Pituitary-Gonadal Axis
Generally speaking, males have higher SUA levels compared to females, so the epidemiological definition of hyperuricemia is different. The upper limit of SUA is 420umol/L (7mg/dl) in males and 360 umol/L (6mg/dl) in females. This gender disparity begins to manifest during adolescence.31 And postmenopausal women are at high risk for hyperuricemia. These findings support that sex hormones may have an impact on UA metabolism.
Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH)
The available data on FSH, LH and UA is limited. Previous studies revealed that people with gout had lower levels of FSH and LH.32 In men with type 2 diabetes (T2DM), elevated UA was associated with decreased LH and FSH.33 In contrast, FSH and LH were positively correlated with UA in premenopausal and postmenopausal women with T2DM, respectively.34,35 Unfortunately, these studies were cross-sectional and could not establish a causal relationship between FSH, LH and UA. Different conclusions may be relevant to types of populations.
In a study investigating the correlation between SUA and erectile dysfunction, the previously observed negative correlation between FSH, LH and UA lost statistical significance after adjusting for age,36 suggesting that their relationship was not independent. With increasing research on downstream hormones, the argument that FSH and LH affect UA levels through target gland hormones has been more widely accepted.
Estrogen (E) and Progesterone (P)
The prevalence of hyperuricemia in women increases with age, which changes sharply from the age of 50 and peaks at the age of 70.37 Studies found that elevated SUA was associated with menopause, both naturally and surgically.38,39 A sharp decline in E after menopause, accompanied by an increase in UA, suggests a potential protective effect of E against hyperuricemia. According to the criteria of the Stages of Reproductive Aging Workshop, the menopause process can be categorized into pre-menopause, early- and late-menopausal transition, and post-menopause. In a subgroup analysis involving perimenopausal middle-aged women, the prevalence of hyperuricemia continued to increase as menopause progressed, with a significant increase beginning in the late-menopausal transition.40 This coincided with a rapid decline in estradiol (E2) levels.41 For premenopausal women, UA also varied throughout the menstrual cycle. It was highest during the follicular phase, decreased around ovulation, and further decreased during the luteal phase, which was correlated with elevated levels of E and P.34
Other studies focused on the impact of E hormonal therapy on SUA. Notably, both E therapy and E-P therapy demonstrated significant reductions in UA among postmenopausal women.42 Additionally, transgender men undergoing E treatment also exhibited decreased UA levels.43 Jae H et al studied the effect of different types of hormone therapy on SUA in postmenopausal women. Instead of E and tibolone, the administration of E-P reduced UA levels, suggesting that P rather than E was responsible for lowering SUA.44 Despite the controversy, these provides novel perspectives for the management of hyperuricemia in postmenopausal women.
Studies over the past decades have demonstrated that E and P influence the urate transport system within the kidney. Analysis of kidney samples obtained from ovariectomized mice with E replacement revealed that E inhibited protein levels of URAT1, GLUT9, and ABCG2, while P inhibited SMCT1 protein levels.45 In HK2 cells, it was observed that E2 bound to estrogen-binding receptor β and down-regulated GLUT9 through the PTEN/PI3K/AKT signaling pathway, which may be related to autophagy.46 Conflicting findings were reported for ABCG2 as some researchers identified an estrogen response element in the upstream promoter region of the ABCG2 gene. E therapy was found to increase mRNA levels of ABCG2 (Figure 3).47
In addition, E maintained lipid homeostasis via liver ERα,48 thereby attenuating the pentose phosphate pathway that supplies essential NADPH for purine synthesis and metabolism. In anoxic mouse models, E played an antioxidant role by modulating oxygen-induced XO activity at the post-transcriptional level through a non-receptor dependent mechanism.49 These alterations inevitably influenced UA production.
Androgen
Researches on androgen and UA are even more contradictory. It was previously thought that gender differences in UA were influenced by the protective effect of E against hyperuricemia. However, analysis of data from a large representative cohort revealed that gender disparities in SUA emerged during adolescence due to higher T levels rather than E2.50 A positive association between T and UA was also observed in both young healthy women and postmenopausal women with T2DM.35,51 Others put forward different opinions. According to data from the National Health and Nutrition Examination Survey, UA levels in American adult males showed an inverse association with T.52 In a 10-year prospective study, low T levels in men were linked to an increased risk of development hyperuricemia.53
In the study of hormone deficiency and exogenous hormone therapy, controversy remains. Prostate cancer patients who underwent androgen deprivation therapy exhibited significantly reduced levels of UA.54 In patients with female-to-male gender identity disorder undergoing T replacement therapy, elevated UA levels have been observed in a dose-dependent manner.55 Another prospective cohort study involving men with late-onset hypogonadism found that UA levels decreased in patients treated with combined T and atorvastatin compared to those treated with atorvastatin alone. It seems to be a consequence of improvement in insulin sensitivity.56
On the one hand, the elevation of UA induced by T replacement therapy can be partially attributed to the increase in muscle mass, which is considered to be the primary source of purines. In order to obtain more energy, increased muscle mass stimulates ATP consumption, leading to the release of purine intermediates within the muscle and elevated SUA.55 T also enhances UA levels by stimulating liver metabolism of purine nucleotides.57 In animal models, it has been shown to upregulate mRNA and protein expression of SMCT1, thereby affecting renal UA reabsorption system; however, it simultaneously downregulates mRNA and protein levels of GLUT9 (Figure 3).58
On the other hand, decreased T levels reduce protein and nucleic acid synthesis, while promoting endogenous purine production, which provides the raw material for UA production.33 Besides, diminished T levels contribute to IR through influencing body components such as visceral adipose tissue and muscle tissue, consequently impairing renal excretion of UA.59 It should be noted that excessive T also can impair insulin sensitivity. It is very well represented in polycystic ovary syndrome (PCOS).
Pcos
PCOS is not only a disorder of the reproductive system, but also a metabolic disorder. In PCOS patients, there is an elevation in UA levels and an increased prevalence of hyperuricemia, which is nearly three times higher than that observed in women without PCOS.60,61 The multi-platform metabolomics analysis of serum samples from PCOS patients revealed that purine metabolism was disturbed in PCOS, with UA emerging as a crucial indicator for identifying PCOS.62 In this study, PCOS patients were classified into four subtypes based on different phenotypes. Only individuals with hyperandrogenemia, anovulation, and polycystic ovaries exhibited higher levels of UA compared to the control group, suggesting a correlation between UA alterations and the severity of clinical symptoms.63
The elevation of UA in patients with PCOS is attributed to multiple factors; nevertheless, several clinical studies have endeavored to investigate these factors. A comprehensive single-center study involving 1183 patients with PCOS and 10,772 control subjects without PCOS demonstrated a robust correlation between elevated levels of T and increased UA as well as the prevalence of hyperuricemia.60 Another study performed UA tests on 40 patients with PCOS and 40 non-hyperandrogenemia women with matched weight and obesity index levels, finding no statistically significant differences of UA between the two groups. Notably, 24 weeks of treatment with Diane35, an antiandrogen contraceptive, improved androgen while reducing UA levels in these patients with PCOS.64 In addition, other studies observed a positive association between hyperuricemia and visceral adipose tissue mass or neck circumference in patients with PCOS61,65 Indeed, hyperandrogenemia and obesity are characteristic features in PCOS, which are closely associated with IR. These factors mutually reinforce each other, contributing to a metabolic vicious cycle. IR is widely acknowledged as a pivotal mediator of obesity and hyperuricemia,66 highlighting the significance of enhancing IR in ameliorating UA levels in PCOS (Figure 2).
Healthy lifestyle and weight management are the basis of PCOS treatment. But due to the diverse and complex clinical manifestations within the PCOS population, weight loss alone is insufficient. Metformin is commonly prescribed to to improve IR of PCOS. Theoretically, it can partly counteract the effects of IR and hyperandrogenemia, so that UA levels decrease. However, its clinical efficacy appears to be less significant.64 In both in vivo and vitro, metformin induced the expression and activation of AMPK as well as leptin to enhance adipose tissue function, resulting in a significant reduction of UA-induced FFA elevation and improvement of IR.67 Therefore, it is also beneficial for the treatment of metabolic disorders related to hyperuricemia. Furthermore, various anti-diabetic drugs such as GLP-1 receptor agonists and SGLT-2 inhibitors have demonstrated positive effects on metabolism improvement and reduction of androgen levels in PCOS. Surprisingly, they have been found to lower UA levels to varying degrees according to a Meta-analysis.68 Larger sample sizes and longer follow-up studies are needed, but at least they provide recommendations for the treatment of PCOS with hyperuricemia.
Pituitary-Adrenal Axis
Adrenocorticotropic Hormone (ACTH)
As early as the mid-19th century, it was discovered that the injection of ACTH in rabbits increased the excretion of UA.69 But it was not certain that the function was direct. This question was answered through a study conducted on a patient with isolated ACTH deficiency. In rapid ACTH tests, no significant changes were observed in plasma cortisol and UA levels. Conversely, in continuous ACTH loading test, ruling out the interference of other hormones, there was a decrease in plasma concentration of UA accompanied by an increase in cortisol.70 The conclusion can be drawn that the impact of ACTH on UA levels is mediated by downstream hormones.
Glucocorticoid (GC)
Recently, GC has been proven to have ability in regulating UA homeostasis via GR signaling pathway in animal models. Specifically, upon GR activation in the cremaster muscle of rats and bovine renal epithelial cells, there is an induction of XO expression.71,72 In the kidney, GR activation down-regulates mRNA and protein levels of URAT1 through the involvement of nuclear factor-kappa B and activated protein 1, enhancing UA excretion (Figure 3). Consequently, the SUA levels are reduced.73 Changes in SUA actually reflect the net role of GC in UA production and excretion. It should be noted that most mammals possess the enzyme uricase, which further converts UA into allantoin. Because of the loss of uricase activity during human evolution, UA remains as the end product of purine metabolism in humans. Additionally, variations in UA transporters between humans and other animals may contribute to inconsistent changes in SUA levels, highlighting the need for further population-based studies.
Currently, GC is widely used in the treatment of diseases. The well-recognized adverse reactions associated with GC therapy include hyperglycemia, hypertension, osteoporosis, and others. It is necessary to regularly monitor related indicators to avoid complications during long-term use of GC in clinical practice. But UA levels are seldom monitored in this context. A study demonstrated that high doses of methylprednisolone increased UA levels in multiple sclerosis patients.74 Because of its potent anti-inflammatory effects, GC is frequently employed during the acute phase of gout when NASIDs or colchicine can not be tolerated. In cases where gout is localized to a single joint, intra-articular hormone injections may be preferable to systemic hormone administration to avoid its adverse effects.75
Cushing’s Syndrome (CS)
Relevant literature on the association between CS and SUA levels remains limited. In a small-scale study, it was observed that 33% of patients diagnosed with CS exhibited elevated SUA levels, indicating an underlying disorder in UA metabolism.76 Among patients with ACTH-dependent and ACTH-independent CS, the former had higher ACTH level, while the latter had lower or even undetectable ACTH level, but no significant disparity in UA was found.77 Furthermore, individuals with CS are more susceptible to kidney stones due to chronically elevated urinary excretion of UA;78 nevertheless, this effect seems to be offset by the abnormal metabolism associated with the disease. Because in populations with cortisol-secreting adrenal tumors, non-secretory adrenal incident-tumors, and healthy controls matched for BMI, no significant differences in SUA levels were observed.79 This suggests that BMI may serve as an underlying determinant. Due to variations in body sensitivity to GC, CS usually presents with abdominal obesity. Moreover, chronic GC exposure also impairs insulin receptor signaling, leading to IR and perturbing of glucose metabolism. This information provides an explanation for hyperuricemia associated with CS (Figure 2).
Pituitary-Hepatic Axis
Growth Hormone (GH) and IGF-1
Both GH and IGF-1 have the potential to enhance GFR, subsequently influencing UA excretion. It is primarily IGF-1 that is believed to be responsible for this effect. Although GFR increased after exogenous GH application, there was a delay in the time compared to the immediate increase after IGF-1 application in animals and humans.80 And GFR was normalized after treatment with recombinant human insulin growth factor (rhIGF-1) for Laron syndrome, a disease characterized by defective GH due to mutations in GHR-related genes.81 In non-diabetic adult subjects, a significant correlation was observed between low circulating IGF-1 and the presence of hyperuricemia. However, this association lost its significance upon adjustment for eGFR, thereby confirming the initial hypothesis.82
Endogenous GH secretion is not stable, and daily determination may impact the accuracy of the results. But a clinical assessment of the relationship between GH and UA can be made based on long-term administration of exogenous GH. A study demonstrated that treatment with rhGH in children with GHD resulted in a significant increase in IGF-1 and UA levels after 6 months, compared to both the 3-month treatment period and the pre-treatment baseline.83 rhGH was also the most commonly utilized treatment for short stature associated with bone and cartilage disorders. Upon investigating the factors influencing its efficacy, researchers observed a statistically significant elevation in UA levels within the effective group compared to the ineffective group, thereby establishing UA as an influential factor affecting treatment outcomes.84 However, the levels of UA remain within the normal range, thus there is insufficient evidence to support the necessity of monitoring UA levels during GH treatment.
Few studies have explored the molecular mechanism of GH and IGF-1 underlying the impact of UA metabolism. In mouse livers and kidneys, it was previously suggested that pulsed GH down-regulated GLUT9 expression through the JAK2/STAT5b pathway.85 However, differences in the function and localization of GLUT9 in mouse and human livers must be taken into account.86 With respect to IGF-1, a recent study proposed that it stimulates UA transport mediated by GLUT9, OAT1, OAT3, ABCG2, and ABCC4 via Akt and ERK pathways while inhibiting insulin’s effect on GLUT9a (Figure 3).87
Acromegaly
In a retrospective study, 76 patients with pituitary growth hormone tumor were included, of which 8 (10.5%) were diagnosed with hyperuricemia. The present study identified a positive correlation between SUA and HOMA-IR, indicating that the elevation of SUA was associated with IR resulting from increased GH levels (Figure 2).88
In the physiological situation, the effects of the GH and IGF-1 on IR counteract each other. Acromegaly is characterized by IR in the liver and peripheral tissues, highlighting the dominant role of GH in glucose metabolism. Due to a certain degree of resistance at the IGF receptor level, elevated levels of IGF-1 are unable to prevent or effectively regulate this process.89 Consequently, under conditions of GH-induced IR and hyperinsulinemia, the impact of IGF-1 on UA excretion appears to be minimal.
Targets of acromegaly treatment are to normalize GH and IGF-I. Transsphenoidal surgery remains the preferred treatment for the majority patients with acromegaly. Somatostatin receptor ligands such as octreotide and lanreotide have been proven to maintain normal IGF-1 levels and shrink tumors. Compared to other medical therapy, pegvisomant is the most likely to improve glucose tolerance and insulin sensitivity,90 making it a favorable choice for acromegaly patients with hyperuricemia. Regrettably, the potential positive effects of this therapy have not received sufficient attention.
Antidiuretic Hormone (ADH)
Clinically, the role of ADH in water and salt metabolism has been well known. However, the effect on UA metabolism has not received widespread attention. At the end of the last century, G.Decaux et al induced hyponatremia in healthy individuals with 1-desamino-8-D-arginine vasopressin (dDAVP), a selective V2 receptor agonist, which resulted in no significant increase in UA excretion. In a patient diagnosed with central diabetes insipidus (DI), the administration of a potent V1 receptor agonist (triglycyl-lysine vasopressin) produced a rapid increase of urate clearance.91 It is V1 receptor, rather than the V2 receptor, plays a key role in the regulation of UA excretion. Taniguchi et al confirmed through rat experiments that V1a receptor stimulation down-regulated GLUT9 and up-regulated ABCG2 and NPT1, further clarifying the underlying mechanism (Figure 3).92
Siadh
It was estimated that 70% of SIADH was associated with hypouricemia.93 Increased renal clearance is the primary cause.4 Interestingly, despite persistent inappropriate secretion of ADH, water restriction normalized UA clearance, indicating a potential link with an effective expansion of vascular volume.94 Attempts to demonstrate this phenomenon in healthy individuals with acute or chronic vascular volume dilation revealed that while there was an increase in UA clearance, it was not as pronounced as observed in patients with SIADH. Further investigation explained that chronic hyponatremia appeared to independently enhance UA clearance.95 Urate reabsorption in the proximal tubule was indirectly coupled to sodium reabsorption in several ways.96 In case of chronic hyponatremia, renal tubular epithelial cells adapted to a hypotonic state by reducing intracellular solutes (including various anions), which consequently led to a decrease in urate and anion exchange.95 In SIADH patients, pyrazinamide and sulfinpyrazone were administered to specifically inhibit the secretion and post-secretion reabsorption of UA in renal tubules, respectively. The results confirmed that the increase of UA clearance in SIADH patients resulted from a reduction in post-secretion reabsorption.97
Restricting fluid intake is the most economical and safe method for the treatment of mild SIADH. ADH receptor antagonists are considered to be promising therapy due to their ability to counteract ADH effects. Tolvaptan, an oral selective V2 receptor antagonist, is particularly suitable for SIADH patients with severely hyponatremia. In autosomal dominant polycystic kidney disease, tolvaptan has been reported to reduce the renal clearance of UA, which was linked to increased proximal reabsorption of sodium.98 But attention should also be paid to the risk of hyperuricemia with long-term application of tolvaptan.
Diabetes Insipidus (DI)
In contrast to SIADH, CDI usually typically manifests with reduced UA clearance and hyperuricemia. According to a retrospective analysis, the prevalence of hyperuricemia was 46%.99 This characteristic can serve as a differentiating factor between CDI and primary polydipsia, as the latter often exhibits decreased UA levels due to volume expansion.100
It is generally accepted that increased urine flow simultaneously carries more solute out. However, in a rat model of lithium-induced DI, despite a more than 3-fold increase in urine flow compared to controls, UA clearance was similar.101 Therefore, the relationship between UA clearance and urine flow is weak. The hyperuricemia in such patients could be attributed to volume contraction resulting from the discharge of large amounts of hypotonic urine and the absence of V1 receptor stimulation.102
Desmopressin, a specific V2 receptor agonist, plays a pivotal role in the treatment of CDI. However, despite normal blood volume, treatment with dDAVP did not restore UA clearance and hyperuricemia in patients with CDI.100 Zhang et al reported a case of an adolescent patient with CDI complicated by hyperuricemia. Due to lack of timely UA lowering treatment, complications such as renal insufficiency, gouty stone, and joint deformity occurred, which seriously affected the quality of life. In light of these findings, the authors propose considering terlipressin therapy as a potential intervention if deemed necessary.102
Conclusion
Available data suggests that various pituitary-target hormones can influence the levels of SUA by modulating purine metabolism and the activity of UA transporters. In diseases associated with metabolic disorders, such as obesity and IR, individuals are more likely to exhibit elevated levels of SUA and hyperuricemia. Considering the potential systemic harm associated with UA disorders, it is crucial to monitor and manage abnormal UA levels in these populations. However, due to the limited or even conflicting studies on uric acid metabolism in certain diseases, conducting prospective studies with larger sample sizes within specific populations is imperative for accurately elucidating this relationship.
Acknowledgments
The coauthors thank the National Natural Science Foundation of China (81973378, 82073909), the Shanxi Provincial Central Leading Local Science and Technology Development Fund Project (YDZJSX2022A059, YDZJSX20231A059), Four ”Batches” Innovation Project of Invigorating Medical through Science and Technology of Shanxi Province (2023XM022), Special Project for Transformation and Guidance of Scientific and Technological Achievements in Shanxi Province (201804D131044) for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi:10.1016/j.ijcard.2015.08.109
2. Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet. 2021;397(10287):1843–1855. doi:10.1016/S0140-6736(21)00569-9
3. Sharaf El Din U, Salem MM, Abdulazim DO. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: a review. J Adv Res. 2017;8(5):537–548. doi:10.1016/j.jare.2016.11.004
4. Otani N, Ouchi M, Misawa K, Hisatome I, Anzai N. Hypouricemia and Urate Transporters. Biomedicines. 2022;10(3):652. doi:10.3390/biomedicines10030652
5. Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol. 2013;25(2):210–216. doi:10.1097/BOR.0b013e32835d951e
6. Dong M, Chen H, Wen S, et al. The mechanism of sodium-glucose cotransporter-2 inhibitors in reducing uric acid in type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2023;16:437–445. doi:10.2147/DMSO.S399343
7. Giordano N, Santacroce C, Mattii G, Geraci S, Amendola A, Gennari C. Hyperuricemia and gout in thyroid endocrine disorders. Clin Exp Rheumatol. 2001;19(6):661–665.
8. Saini V, Yadav A, Arora MK, Arora S, Singh R, Bhattacharjee J. Correlation of creatinine with TSH levels in overt hypothyroidism - a requirement for monitoring of renal function in hypothyroid patients. Clin Biochem. 2012;45(3):212–214. doi:10.1016/j.clinbiochem.2011.10.012
9. Yang M, Cao S. Gender and age-specific differences in the association of thyroid function and hyperuricemia in Chinese: a cross-sectional study. Int J Endocrinol. 2022;2022:2168039. doi:10.1155/2022/2168039
10. Sun Y, Teng D, Zhao L, et al. Impaired sensitivity to thyroid hormones is associated with hyperuricemia, obesity, and cardiovascular disease risk in subjects with subclinical hypothyroidism. Thyroid. 2022;32(4):376–384. doi:10.1089/thy.2021.0500
11. Echterdiek F, Ranke MB, Schwenger V, Heemann U, Latus J. Kidney disease and thyroid dysfunction: the chicken or egg problem. Pediatr Nephrol. 2022;37(12):3031–3042. doi:10.1007/s00467-022-05640-z
12. Vargas F, Rodríguez-Gómez I, Vargas-Tendero P, Jimenez E, Montiel M. The renin-angiotensin system in thyroid disorders and its role in cardiovascular and renal manifestations. J Endocrinol. 2012;213(1):25–36. doi:10.1530/JOE-11-0349
13. Iglesias P, Bajo MA, Selgas R, Díez JJ. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord. 2017;18(1):131–144. doi:10.1007/s11154-016-9395-7
14. van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: a review. Gen Comp Endocrinol. 2009;160(3):205–215. doi:10.1016/j.ygcen.2008.12.008
15. Brtko J. Thyroid hormone and thyroid hormone nuclear receptors: history and present state of art. Endocr Regul. 2021;55(2):103–119. doi:10.2478/enr-2021-0012
16. Eşme M, Bulur O, Atak MC, et al. Treatment of hypothyroidism improves glomerular filtration rate (GFR) in geriatric patients. Turk J Med Sci. 2021;51(3):1267–1272. doi:10.3906/sag-2011-257
17. Gierach M, Gierach J, Junik R. Insulin resistance and thyroid disorders. Endokrynol Pol. 2014;65(1):70–76. doi:10.5603/EP.2014.0010
18. Desideri G, Bocale R, D’Amore AM, et al. Thyroid hormones modulate uric acid metabolism in patients with recent onset subclinical hypothyroidism by improving insulin sensitivity. Intern Emerg Med. 2020;15(1):67–71. doi:10.1007/s11739-019-02065-9
19. Sato A, Shirota T, Shinoda T, et al. Hyperuricemia in patients with hyperthyroidism due to Graves’ disease. Metabolism. 1995;44(2):207–211. doi:10.1016/0026-0495(95)90266-X
20. Liu X, Zhang J, Meng Z, et al. Gender impact on the correlations between Graves’ hyperthyroidism and hyperuricemia in Chinese. Ir J Med Sci. 2019;188(3):843–848. doi:10.1007/s11845-018-1939-2
21. Yazar A, Döven O, Atis S, et al. Systolic pulmonary artery pressure and serum uric acid levels in patients with hyperthyroidism. Arch Med Res. 2003;34(1):35–40. doi:10.1016/S0188-4409(02)00457-5
22. Xing Y, Yang L, Liu J, Ma H. The association with subclinical thyroid dysfunction and uric acid. Int J Endocrinol. 2021;2021:9720618. doi:10.1155/2021/9720618
23. Fukui H, Taniguchi S, Ueta Y, et al. Enhanced activity of the purine nucleotide cycle of the exercising muscle in patients with hyperthyroidism. J Clin Endocrinol Metab. 2001;86(5):2205–2210. doi:10.1210/jcem.86.5.7516
24. Huh K, Kwon TH, Kim JS, Park JM. Role of the hepatic xanthine oxidase in thyroid dysfunction: effect of thyroid hormones in oxidative stress in rat liver. Arch Pharm Res. 1998;21(3):236–240. doi:10.1007/BF02975281
25. Chen X, Wu M, Liang N, Lu J, Qu S, Chen H. Thyroid hormone-regulated expression of period2 promotes liver urate production. Front Cell Dev Biol. 2021;9:636802. doi:10.3389/fcell.2021.636802
26. Ferreira C, Prestin K, Hussner J, Zimmermann U. Meyer Zu Schwabedissen HE. PDZ domain containing protein 1 (PDZK1), a modulator of membrane proteins, is regulated by the nuclear receptor THRβ. Mol Cell Endocrinol. 2018;461:215–225. doi:10.1016/j.mce.2017.09.017
27. Vieira IH, Rodrigues D, Paiva I. The mysterious universe of the TSH receptor. Front Endocrinol. 2022;13:944715. doi:10.3389/fendo.2022.944715
28. Choi W, Yang YS, Chang DJ, et al. Association between the use of allopurinol and risk of increased thyroid-stimulating hormone level. Sci Rep. 2021;11(1):20305. doi:10.1038/s41598-021-98954-1
29. Perez-Ruiz F, Chinchilla SP, Atxotegi J, Urionagüena I, Herrero-Beites AM, Aniel-Quiroga MA. Increase in thyroid stimulating hormone levels in patients with gout treated with inhibitors of xanthine oxidoreductase. Rheumatol Int. 2015;35(11):1857–1861. doi:10.1007/s00296-015-3355-5
30. Makay O, Yenisey C, Icoz G, et al. The role of allopurinol on oxidative stress in experimental hyperthyroidism. J Endocrinol Invest. 2009;32(8):641–646. doi:10.1007/BF03345734
31. Rao J, Ye P, Lu J, et al. Prevalence and related factors of hyperuricaemia in Chinese children and adolescents: a pooled analysis of 11 population-based studies. Ann Med. 2022;54(1):1608–1615. doi:10.1080/07853890.2022.2083670
32. Marinello E, Riario-Sforza G, Marcolongo R. Plasma follicle-stimulating hormone, luteinizing hormone, and sex hormones in patients with gout. Arthritis Rheum. 1985;28(2):127–131. doi:10.1002/art.1780280203
33. Cao W, Zheng RD, Xu SH, Fan YF, Sun HP, Liu C. Association between sex hormone and blood uric acid in male patients with type 2 diabetes. Int J Endocrinol. 2017;2017:4375253. doi:10.1155/2017/4375253
34. Mumford SL, Dasharathy SS, Pollack AZ, et al. Serum uric acid in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. Hum Reprod. 2013;28(7):1853–1862. doi:10.1093/humrep/det085
35. Wan H, Zhang K, Wang Y, et al. The associations between gonadal hormones and serum uric acid levels in men and postmenopausal women with diabetes. Front Endocrinol. 2020;11:55. doi:10.3389/fendo.2020.00055
36. Gao F, Jiang B, Cang Z, et al. Serum uric acid is associated with erectile dysfunction: a population-based cross-sectional study in Chinese men. Sci Rep. 2017;7(1):2087. doi:10.1038/s41598-017-02392-x
37. Li Y, Shen Z, Zhu B, Zhang H, Zhang X, Ding X. Demographic, regional and temporal trends of hyperuricemia epidemics in mainland China from 2000 to 2019: a systematic review and meta-analysis. Glob Health Action. 2021;14(1):1874652. doi:10.1080/16549716.2021.1874652
38. Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women--the third national health and nutrition examination survey. Arthritis Res Ther. 2008;10(5):R116. doi:10.1186/ar2519
39. Stöckl D, Döring A, Thorand B, Heier M, Belcredi P, Meisinger C. Reproductive factors and serum uric acid levels in females from the general population: the KORA F4 study. PLoS One. 2012;7(3):e32668. doi:10.1371/journal.pone.0032668
40. Cho SK, Winkler CA, Lee SJ, Chang Y, Ryu S. The prevalence of hyperuricemia sharply increases from the late menopausal transition stage in middle-aged women. J Clin Med. 2019;8(3):296. doi:10.3390/jcm8030296
41. Su HI, Freeman EW. Hormone changes associated with the menopausal transition. Minerva Ginecol. 2009;61(6):483–489.
42. Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999;354(9179):650. doi:10.1016/S0140-6736(99)92381-4
43. Yahyaoui R, Esteva I, Haro-Mora JJ, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. 2008;93(6):2230–2233. doi:10.1210/jc.2007-2467
44. Jung JH, Song GG, Lee YH, Kim JH, Hyun MH, Choi SJ. Serum uric acid levels and hormone therapy type: a retrospective cohort study of postmenopausal women. Menopause. 2018;25(1):77–81. doi:10.1097/GME.0000000000000953
45. Takiue Y, Hosoyamada M, Kimura M, Saito H. The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids. 2011;30(2):113–119. doi:10.1080/15257770.2010.551645
46. Zeng M, Chen B, Qing Y, et al. Estrogen receptor β signaling induces autophagy and downregulates Glut9 expression. Nucleosides Nucleotides Nucleic Acids. 2014;33(7):455–465. doi:10.1080/15257770.2014.885045
47. Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT. Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res. 2004;64(4):1247–1251. doi:10.1158/0008-5472.CAN-03-3583
48. Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol. 2017;1043:227–256.
49. Budhiraja R, Kayyali US, Karamsetty M, et al. Estrogen modulates xanthine dehydrogenase/xanthine oxidase activity by a receptor-independent mechanism. Antioxid Redox Signal. 2003;5(6):705–711. doi:10.1089/152308603770380007
50. Wang Y, Charchar FJ. Establishment of sex difference in circulating uric acid is associated with higher testosterone and lower sex hormone-binding globulin in adolescent boys. Sci Rep. 2021;11(1):17323. doi:10.1038/s41598-021-96959-4
51. Mantzoros CS, Georgiadis EI, Young R, et al. Relative androgenicity, blood pressure levels, and cardiovascular risk factors in young healthy women. Am J Hypertens. 1995;8(6):606–614. doi:10.1016/0895-7061(95)00051-P
52. Han Y, Zhang Y, Cao Y, et al. Exploration of the association between serum uric acid and testosterone in adult males: NHANES 2011-2016. Transl Androl Urol. 2021;10(1):272–282. doi:10.21037/tau-20-1114
53. Tsai MK, Hung KC, Liao CC, Pan LF, Hung CL, Yang DH. The association between serum testosterone and hyperuricemia in males. J Clin Med. 2022;11(10):2743. doi:10.3390/jcm11102743
54. Park JW, Lee JH, Cho HJ, et al. Influence of androgen deprivation therapy on serum urate levels in patients with prostate cancer: a retrospective observational study. PLoS One. 2018;13(12):e0209049. doi:10.1371/journal.pone.0209049
55. Kurahashi H, Watanabe M, Sugimoto M, et al. Testosterone replacement elevates the serum uric acid levels in patients with female to male gender identity disorder. Endocr J. 2013;60(12):1321–1327. doi:10.1507/endocrj.EJ13-0203
56. Krysiak R, Gilowski W, Okopień B. The effect of testosterone on cardiometabolic risk factors in atorvastatin-treated men with late-onset hypogonadism. Pharmacol Rep. 2016;68(1):196–200. doi:10.1016/j.pharep.2015.08.009
57. Marinello E, Leoncini R, Terzuoli L, Vannoni D, Porcelli B, Resconi G. Effect of testosterone on purine nucleotide metabolism in rat liver. Horm Metab Res. 2004;36(9):614–619. doi:10.1055/s-2004-825923
58. Hosoyamada M, Takiue Y, Shibasaki T, Saito H. The effect of testosterone upon the urate reabsorptive transport system in mouse kidney. Nucleosides Nucleotides Nucleic Acids. 2010;29(7):574–579. doi:10.1080/15257770.2010.494651
59. Dimopoulou C, Goulis DG, Corona G, Maggi M. The complex association between metabolic syndrome and male hypogonadism. Metabolism. 2018;86:61–68. doi:10.1016/j.metabol.2018.03.024
60. Mu L, Pan J, Yang L, et al. Association between the prevalence of hyperuricemia and reproductive hormones in polycystic ovary syndrome. Reprod Biol Endocrinol. 2018;16(1):104. doi:10.1186/s12958-018-0419-x
61. Zhang Y, Cai M, Dilimulati D, et al. Correlation between serum uric acid and body fat distribution in patients with polycystic ovary syndrome. Front Endocrinol. 2021;12:782808. doi:10.3389/fendo.2021.782808
62. Buszewska-Forajta M, Rachoń D, Stefaniak A, et al. Identification of the metabolic fingerprints in women with polycystic ovary syndrome using the multiplatform metabolomics technique. J Steroid Biochem Mol Biol. 2019;186:176–184. doi:10.1016/j.jsbmb.2018.10.012
63. Zhao Y, Fu L, Li R, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. 2012;10:153. doi:10.1186/1741-7015-10-153
64. Luque-Ramírez M, Alvarez-Blasco F, Uriol Rivera MG, Escobar-Morreale HF. Serum uric acid concentration as non-classic cardiovascular risk factor in women with polycystic ovary syndrome: effect of treatment with ethinyl-estradiol plus cyproterone acetate versus metformin. Hum Reprod. 2008;23(7):1594–1601. doi:10.1093/humrep/den095
65. Yang H, Liu C, Jin C, Yu R, Ding L, Mu L. Neck circumference is associated with hyperuricemia in women with polycystic ovary syndrome. Front Endocrinol. 2021;12:712855. doi:10.3389/fendo.2021.712855
66. Bae J, Park KY, Son S, Huh Y, Nam GE. Associations between obesity parameters and hyperuricemia by sex, age, and diabetes mellitus: a nationwide study in Korea. Obes Res Clin Pract. 2023;17(5):405–410. doi:10.1016/j.orcp.2023.09.007
67. Su M, Sun L, Li W, et al. Metformin alleviates hyperuricaemia-induced serum FFA elevation and insulin resistance by inhibiting adipocyte hypertrophy and reversing suppressed white adipose tissue beiging. Clin Sci. 2020;134(12):1537–1553. doi:10.1042/CS20200580
68. Najafi S, Bahrami M, Butler AE, Sahebkar A. The effect of glucagon-like peptide-1 receptor agonists on serum uric acid concentration: a systematic review and meta-analysis. Br J Clin Pharmacol. 2022;88(8):3627–3637. doi:10.1111/bcp.15344
69. Aonuma S, Inoue K. The effects of ACTH and dehydroascorbic acid on the serum and urinary uric acid level in rabbits. Endocrinol Jpn. 1956;3(2):125–129. doi:10.1507/endocrj1954.3.125
70. Shibutani Y, Ueo T, Takahashi S, Moriwaki Y, Yamamoto T. Effect of ACTH on renal excretion of purine bases in a patient with isolated ACTH deficiency. Clin Chim Acta. 2000;294(1–2):185–192. doi:10.1016/S0009-8981(99)00263-6
71. Wallwork CJ, Parks DA, Schmid-Schönbein GW. Xanthine oxidase activity in the dexamethasone-induced hypertensive rat. Microvasc Res. 2003;66(1):30–37. doi:10.1016/S0026-2862(03)00019-0
72. Pfeffer KD, Huecksteadt TP, Hoidal JR. Xanthine dehydrogenase and xanthine oxidase activity and gene expression in renal epithelial cells. Cytokine and steroid regulation. J Immunol. 1994;153(4):1789–1797. doi:10.4049/jimmunol.153.4.1789
73. Li G, Han L, Ma R, et al. Glucocorticoids increase renal excretion of urate in mice by downregulating urate transporter 1. Drug Metab Dispos. 2019;47(11):1343–1351. doi:10.1124/dmd.119.087700
74. Toncev G, Milicic B, Toncev S, Samardzic G. High-dose methylprednisolone therapy in multiple sclerosis increases serum uric acid levels. Clin Chem Lab Med. 2002;40(5):505–508. doi:10.1515/CCLM.2002.088
75. Hainer BL, Matheson E, Wilkes RT. Diagnosis, treatment, and prevention of gout. Am Fam Physician. 2014;90(12):831–836.
76. Chen N. Comparison of Clinical Characteristics of Different Types of Cushing Syndrome and Analysis of Related Factors of Cardiovascular and Cerebrovascular Diseases. Zhe Jiang University, China; 2019.
77. Li Z, Zhang C, Geng C, Song Y. Metabolic profile differences in ACTH-dependent and ACTH-independent Cushing syndrome. Chronic Dis Transl Med. 2022;8(1):36–40. doi:10.1016/j.cdtm.2021.08.004
78. Faggiano A, Pivonello R, Melis D, et al. Nephrolithiasis in Cushing’s disease: prevalence, etiopathogenesis, and modification after disease cure. J Clin Endocrinol Metab. 2003;88(5):2076–2080. doi:10.1210/jc.2002-021494
79. Garrapa GG, Pantanetti P, Arnaldi G, Mantero F, Faloia E. Body composition and metabolic features in women with adrenal incidentaloma or Cushing’s syndrome. J Clin Endocrinol Metab. 2001;86(11):5301–5306. doi:10.1210/jcem.86.11.8059
80. Hirschberg R, Kopple JD. Evidence that insulin-like growth factor I increases renal plasma flow and glomerular filtration rate in fasted rats. J Clin Invest. 1989;83(1):326–330. doi:10.1172/JCI113878
81. Klinger B, Laron Z. Renal function in Laron syndrome patients treated by insulin-like growth factor-I. Pediatr Nephrol. 1994;8(6):684–688. doi:10.1007/BF00869089
82. Sesti G, Hribal ML, Procopio T, et al. Low circulating insulin-like growth factor-1 levels are associated with high serum uric acid in nondiabetic adult subjects. Nutr Metab Cardiovasc Dis. 2014;24(12):1365–1372. doi:10.1016/j.numecd.2014.06.012
83. Sheng HL, Luan J, Li WY, Jiang YP. Effect of rhGH on GHD in children and analysis of related influencing factors. Biochem Cell Biol Age. 2022;44(6):898–901.
84. Chen YN, Tan X, Deng DM, Kang Q, Zhong LL. Analysis of factors influencing and efficacy of recombinant human growth hormone in the treatment of. Clin Med J. 2022;20(9):67–71.
85. Bu P, Le Y, Zhang Y, Cheng X. Hormonal and chemical regulation of the glut9 transporter in mice. J Pharmacol Exp Ther. 2017;360(1):206–214. doi:10.1124/jpet.116.237040
86. Chung S, Kim GH. Urate transporters in the kidney: what clinicians need to know. Electrolyte Blood Press. 2021;19(1):1–9. doi:10.5049/EBP.2021.19.1.1
87. Mandal AK, Leask MP, Sumpter NA, Choi HK, Merriman TR, Mount DB. Genetic and physiological effects of insulin-like growth factor-1 (IGF-1) on human urate homeostasis. J Am Soc Nephrol. 2023;34(3):451–466. doi:10.1681/ASN.0000000000000054
88. Wang F, Zhong LY. Correlation analysis of serum uric acid level and disease activity in patients with growth hormone-secreting pituitary adenoma. Chinese J of Postgraduates of Med. 2019;42(9):808–811.
89. Janssen J. Mechanisms of putative IGF-I receptor resistance in active acromegaly. Growth Horm IGF Res. 2020;52:101319. doi:10.1016/j.ghir.2020.101319
90. Giustina A, Barkhoudarian G, Beckers A, et al. Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord. 2020;21(4):667–678. doi:10.1007/s11154-020-09588-z
91. Decaux G, Namias B, Gulbis B, Soupart A. Evidence in hyponatremia related to inappropriate secretion of ADH that V1 receptor stimulation contributes to the increase in renal uric acid clearance. J Am Soc Nephrol. 1996;7(5):805–810. doi:10.1681/ASN.V75805
92. Taniguchi K, Tamura Y, Kumagai T, Shibata S, Uchida S. Stimulation of V1a receptor increases renal uric acid clearance via urate transporters: insight into pathogenesis of hypouricemia in SIADH. Clin Exp Nephrol. 2016;20(6):845–852. doi:10.1007/s10157-016-1248-x
93. Decaux G, Musch W. Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol. 2008;3(4):1175–1184. doi:10.2215/CJN.04431007
94. Beck LH. Hypouricemia in the syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1979;301(10):528–530. doi:10.1056/NEJM197909063011005
95. Decaux G, Prospert F, Soupart A, Musch W. Evidence that chronicity of hyponatremia contributes to the high urate clearance observed in the syndrome of inappropriate antidiuretic hormone secretion. Am J Kidney Dis. 2000;36(4):745–751. doi:10.1053/ajkd.2000.17623
96. Kahn AM. Indirect coupling between sodium and urate transport in the proximal tubule. Kidney Int. 1989;36(3):378–384. doi:10.1038/ki.1989.206
97. Decaux G, Dumont I, Waterlot Y, Hanson B. Mechanisms of hypouricemia in the syndrome of inappropriate secretion of antidiuretic hormone. Nephron. 1985;39(3):164–168. doi:10.1159/000183365
98. Irazabal MV, Torres VE, Hogan MC, et al. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80(3):295–301. doi:10.1038/ki.2011.119
99. Wang SH, Zhu HJ, Duan L, et al. Serum uric acid level and its influencing factors in patients with diabetes insipidus. Acta Acad Med Sin. 2023;45(1):44–49.
100. Decaux G, Prospert F, Namias B, Soupart A. Hyperuricemia as a clue for central diabetes insipidus (lack of V1 effect) in the differential diagnosis of polydipsia. Am J Med. 1997;103(5):376–382. doi:10.1016/S0002-9343(97)00165-4
101. Steele TH, Underwood JL, Dudgeon KL. Urate excretion and urine flow in a lithium-induced diabetes insipidus rat model. Pflugers Arch. 1974;346(3):205–213. doi:10.1007/BF00595707
102. Zhang Y, Wang D, Feng Y, Zhang W, Zeng X. Juvenile-onset gout and adipsic diabetes insipidus: a case report and literature review. J Int Med Res. 2018;46(11):4829–4836. doi:10.1177/0300060518800114
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.