Back to Journals » Cancer Management and Research » Volume 15
Preoperative Prognostic Nutritional Index Predict Survival in Patients with Resectable Adenocarcinoma of the Gastroesophageal Junction: A Retrospective Study Based on Propensity Score Matching Analyses
Received 14 April 2023
Accepted for publication 22 June 2023
Published 5 July 2023 Volume 2023:15 Pages 591—599
DOI https://doi.org/10.2147/CMAR.S415618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Siqi Xu,* Huide Zhu,* Zhiwei Zheng
Department of Pharmacy, Cancer Hospital of Shantou University Medical College, Shantou, 515041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiwei Zheng, Email [email protected]
Background: This study evaluated the value of PNI to predicting relapse-free survival (RFS) and overall survival (OS) in patients with resectable gastroesophageal junction adenocarcinoma (AGE).
Methods: Between 2016 and 2020, there were 236 resectable AGE patients underwent a retrospective review via propensity score matched (PSM) analysis. The PNI values were computed for each patient prior to surgery [PNI= 10×albumin (gr/dL) + 0.005×total lymphocyte count (mm3)]. By using disease progression and mortality as the end points, a receiver operating characteristic(ROC) curve was plotted to identify the PNI cut-off value. Kaplan-Meier curves and Cox proportional hazard models were used for survival analysis.
Results: The ROC curve indicated that the ideal cutoff value was 45.60. After propensity score matching, there were 143 patients in our retrospective study, which included 58 patients in the low-PNI group and 85 patients in the high-PNI group. When compared to the low PNI group, the high PNI group substantially increased RFS and OS (p< 0.001, p=0.003, respectively) according to the Kaplan-Meier analysis and Log rank test. Advanced pathological N stage (p=0.011) and poor PNI (p=0.004) were also significant risk factors for a shorter OS, according to a univariate analysis. Multivariate analysis revealed that the N0 plus N1 group had an endpoint mortality risk that was 0.39 times lower than the N2 plus N3 group’s (p=0.008). In comparison to the high PNI group, the hazard of endpoint mortality was 2.442 times greater in the low PNI group (p = 0.003).
Conclusion: PNI is a simplistic and practical predictive predictor of the RFS and OS time in patients with resectable AGE.
Keywords: prognostic nutritional index, resectable adenocarcinoma esophagogastric junction cancer, relapse-free survival, overall survival, prognosis
Introduction
Adenocarcinoma of the gastroesophageal junction (AGE) are those 5 cm above or below the gastroesophageal junction.1 Multiple studies have shown a sharp increase in the incidence of AGE, especially in Western countries.2,3 From 2008 to 2014, the annual incidence of AGE in East Asia was 0.6–1.7 per 100,000 population. However, the annual incidence of AGE in the United States was 2.0–2.2 per 100,000 population between 1991 and 2010.4 Despite improvements in AGE therapeutic intervention, surgical operation remains the primary option of treatment, although overall survival (OS) remains disappointing.5 Therefore, there is ongoing interest in finding a simplified and practical prognostic marker to identify the risks of disease progression and postoperative death in AGE patients.
The prognostic nutritional index(PNI), which was established by the Japanese researcher Onodera,6 is a nutritional evaluation index based on the levels of peripheral blood lymphocyte count and serum albumin. The PNI has recently been revealed to be a prognostic marker in a variety of gastrointestinal malignancies, including gastric cancer and esophageal squamous cell carcinoma.7,8 So far, few studies have been conducted to investigate the function of PNI in predicting the prognosis of AGE undergoing surgical resection. Furthermore, multiple studies have found different optimal critical values of PNI for predicting cancer prognosis.9,10 Thus, the goal of this study was to determine the predictive value of PNI in patients with resectable AGE in terms of relapse-free survival (RFS) and overall survival (OS).
Materials and Methods
Patients
A cohort of 236 resectable AEJ patients conducted a retrospective study at Shantou University Medical College’s cancer hospital between 2016 and 2020. For the purpose of minimizing biases, we used propensity score matching. Patients who were included in the analysis fulfilled the following requirements: (1) AGE confirmed by histopathology; (2) surgery or preceded by adjuvant chemotherapy before surgery; (3) curative gastro-oesophageal resection with R0 resection (en bloc resection with histologically free margins); (4) carry out all necessary laboratory tests (such as routine blood tests, and routine biochemical tests) and (5) American Society of Anesthesiology (ASA) grade of 1–2.
Within one week prior of the surgery, a regular blood test was used to obtain albumin and lymphocyte counts. Medical records were used to obtain the clinical-pathological traits and pathological information for the patients.
Variable Definition
The following formula was used to determine the PNI and BMI: PNI = 10× albumin (gr/ dL) +0.005 ×total lymphocyte count (mm3). Body mass index (BMI) = body weight (kg)/height (m2).
The TNM stage was base on the AJCC eighth edition criteria. T1 indicates tumor invasion of the lamina propria, mucosal muscle layer or submucosa; T2 indicates tumor invasion of the intrinsic muscular layer; T3 indicates tumor invasion of fibrous membrane; T4 indicates tumor invasion of adjacent structures, such as pleura, pericardium, aorta, vertebral body, trachea, etc.
RFS refers to the interval between the conclusion of the initial course of therapy and the occurrence of a recurrent malignancy or the final assessment point during clinical follow-up.
Drawing the receiver operating characteristic curve(ROC)with disease progression and mortality as the endpoints allowed for the decision of the optimal PNI cut-off value. The Youden index=(sensitivity + specificity)-1 was then computed to get the optimal PNI cut-off value.
Follow Up
Following surgery, patients were followed on every 3 months for the first two years, every 12 months for the third to fifth years, and then once a year after that. The hematological examination (containing physical examination, blood routine, blood biochemistry) were part of the follow-up procedures or an optional chest CT scan, and the final cut follow-up time was December 2022. RFS and OS were the main endpoints.
Statistical Analysis
The IBM SPSS program, version 22, was used to perform the statistics. The measurement results were compared using a one-way ANOVA and an independent sample T-test. The chi-square test was used to compare data from an enumeration. The prognostic connection between PNI and RSF or OS was examined using the multivariate Kaplan-Meier method and the Cox regression model. Statistics were considered significant at P < 0.05.
In our retrospective study, we used PSM on the low-PNI and high-PNI groups to adjust for differences in patient background and minimize selection bias. A logistic regression model containing the variables age, weight, BMI, tumor diameter, differentiation, pathological T stage, pathological N stage was used to determine the propensity score. A one-to-two match between the two groups was performed using the nearest-neighbor matching approach. The caliper size was 0.20. Postoperative results were compared between the two groups after matching. The Mann–Whitney U-test was used to compare patient characteristics and postoperative results, and the Chi-square test was used to analyze categorical data.
Results
The Result of ROC for the Best PNI Cut-off Value
The ability of the preoperative PNI to predict RFS and OS was evaluated using the area under the ROC curve (AUC) estimation method. The optimum cut-off value in this study was determined to be 45.6 when the Youden index reached its maximum (YI =0.390), based on disease progression and death (sensitivity: 86.1%; specificity: 52.9%; AUC: 0.775). Patients were divided into two groups based on their PNI values: low- PNI group (< 45.6) and high- PNI group (≥ 45.6). (Figure 1).
 |
Figure 1 Receiver operating characteristic curve was plotted to determine the best cut-off value for the PNI. |
Relationship Between PNI and Clinicopathological Characteristics in Patients
Table 1 provides a summary of the relationships between the PNI and clinicopathological characteristics of the 236 individuals with AGE before and after propensity score matching. Before propensity score matching, there were 171 men and 65 women. The age ranged from 33 to 72 years, with a mean of 43.68± 4.62.
 |
Table 1 Relationships Between PNI and Clinicopathological Before and After Propensity Score Matched |
There were 169 patients (71.61%) in the high PNI group and 67 patients (28.39%) in the low PNI group based on a PNI cut-off value of 45.6. Age (p<0.001), BMI (p =0.001), tumor diameter (p=0.001), pathological T stage (p=0.022), pathological N stage (p < 0.001) and pathological stage (p=0.006) were all strongly correlated with PNI values.
After propensity score matching, there were 143 patients in our retrospective study, which included 58 patients in the low-PNI and 85 patients in the high-PNI groups. There were no significant differences between the low and high PNI groups in terms of age (p=0.158), gender (p=0.699), BMI (p=0.762), tumor diameter (p=0.814), differentiation (p=0.183), pathological T stage (p=0.788), pathological N stage (p =0.935), and pathological stage (p=0.742). The PSM standard deviation distribution histogram was shown in Figure S1.
Survival Analysis in Propensity Score Matched Patients Stratified by PNI
Patients with high PNI had a considerably better RFS than those with low PNI, according to the Kaplan-Meier analysis and Log rank test (p<0.001) (Figure 2). When compared to patients with low PNI, those with high PNI had a significantly better OS (p=0.003)(Figure 3). The low PNI group had 5-year OS rates of 46.9%, while the high PNI group had 5-year OS rates of 71.30%.
 |
Figure 2 Kaplan-Meier curves of RFS based on PNI group in 143 patients with AGE. |
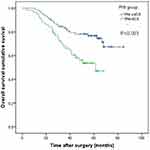 |
Figure 3 Kaplan-Meier curves of OS based on PNI group in 143 patients with AGE. |
Univariate and Multivariate Analyses of the AGE Prognostic Predictors After Propensity Score Matching
After propensity score matching, among 143 patients, univariate analysis revealed that advanced pathological N stage (p=0.011) and lower PNI (p=0.004) were significant predictors for shorter OS. Multivariate analysis showed the risk of endpoint mortality was 0.399 times lower in the N0 plus N1 group than in the N2 plus N3 group (p=0.008). In comparison to the high PNI group, the hazard of endpoint mortality was 2.442 times greater in the low PNI group (p = 0.003) (Table 2).
 |
Table 2 Prognostic Factors for Overall Survival in Patients with AGE After Propensity Score Matching |
Discussion
AGE has a high morbidity over the world, and treatment options include surgery, systemic treatment, and radiation.11 For patients with early operable AGE, surgery remains the preferred option. However, there is a significant risk of postoperative recurrence.12 Although the tumor-node-metastasis (TNM) stage might assess the prognosis of AGE, the TNM classification only considers the extent of the tumor, regardless of its biological characteristics or the host’s anticancer response. It will consequently become more crucial to identify a simple, reliable, and repeatable factor that might accurately predict a patient’s prognosis for AGE.
According to recent research, preoperative malnutrition and an immunosuppressive state might contribute to poor survival in cancer patients.13,14 Among them, the PNI is a valuable indicator to reflect on the nutritional and immunological state of patients with various malignant tumor types.15,16 The PNI includes blood albumin and lymphocyte count values, both of which are frequently tested in clinical practice, particularly before surgical procedures. Hepatocytes generate the nutritional component albumin, which is controlled by proinflammatory cytokines including interleukin-1 (IL-1) and IL-6 as well as tumor necrosis factor (TNF), both of which have a detrimental effect on catabolic metabolism. These inflammatory cytokines, which are produced by the tumor or the host, are crucial for the growth of new blood vessels, as well as for the development of carcinogenesis and the progression of disease.17 Lymphocytes are a crucial component of the immune system. Lymphocytes are essential components of cell-mediated immunity, with cytokine-mediated cytotoxicity inhibiting tumor cell proliferation and invasion.18 Low lymphocyte counts indicate a problem or poor immunity in the body, which affects the outcomes in patients.19
PNI was initially utilized to anticipate surgical hospitalization, delayed tissue repair, and anastomotic leakage.20 However, the preoperative PNI may be a more reliable tool for assessing the physiological state of cancer patients recently, particularly in cases of gastroesophageal cancers.21–23 Nogueiro et al discovered that PNI was shown to considerably affect the OS and disease-free survival of patients with gastric cancer. PNI can be used to predict which patients are more likely to have postoperative morbidity and death.24 Little study has been conducted to date on the association between PNI and OS or RFS in resectable AGE. As a result, our study used propensity score matching analyses to perform a retrospective analysis to assess the prognostic significance of PNI in resectable AGE.
In the current investigation, we discovered a correlation between PNI and pathological N stage and the prognosis of patients with resectable AGE. In patients with resectable AGE, multivariate analysis revealed that pathological N stage and PNI were independent prognostic variables. Comparing patients with high and low PNI, those with high PNI had considerably better RSF and OS. These results indicate that PNI, in patients undergoing gastric-esophagectomy surgery, is a straightforward and practical prognostic marker for the RSF and OS.
There is no universal cut-off value that applies to all patients, and there is ongoing discussion on the PNI’s ideal cut-off value for predicting postoperative survival in patients with malignant tumors.25–27 As a result, we attempted to evaluate the PNI’s prognostic importance and establish an ideal cutoff value that would better predict RFS and OS in AGE patients. An appropriate cutoff value for the preoperative PNI was found at 45.6 by the use of a ROC analysis based on RSF and OS. At this cut-off value, this investigation showed the presence of substantial correlations between a low PNI value and tumor-related parameters, such as a low BMI, a deeper tumor depth, lymph node metastases, and an advanced pTNM stage. Relationships between PNI and BMI before propensity score matching showed that in the low PNI group the BMI was 20.19±2.21 while in the high PNI group the BMI was 21.30±2.24 (P=0.001).
Previous research has shown that BMI can help predict survival outcomes in individuals with certain malignancies.28 In addition, gastroesophageal junction adenocarcinoma is known to be associated with significant weight loss at presentation and has worse outcomes than other types of subside.29,30 Underweight patients may identify a group of individuals who could not actually accept therapeutic intervention or may identify a more aggressive cancer, leading to a poor prognosis.31 Additionally, PSM analysis was carried out to lessen bias resulting from differing distributions of covariables (age, BMI, tumor diameter, tumor size and Pathological T stage, Pathological N stage, and pathological stage) between patients in the low PNI and high PNI groups. Our findings support the idea that a low PNI value in those with more severe or advanced malignancies is an indication of ongoing inflammatory and nutrition.32
According to our knowledge, the current analysis is the first propensity score matching investigation to examine the predictive usefulness of the PNI in AGE with different primary tumor locations and other subgroups. However, the present study had a number of drawbacks. First, the study’s patient population was constrained because it was a single-center retrospective analysis. Furthermore, we emphasized on preoperative PNI values but did not analyze dynamic changes in PNI values along the course of the disease. Thirdly, PSM methods are widely acknowledged for their effectiveness in addressing causal inference issues. However, their utility is limited when it comes to predictive modeling, as the predictions generated may only be applicable to individuals resembling the propensity score matched group. Finally, the results of our study suggest that a preoperative PNI cutoff value of 45.6 is appropriate for predicting survival outcomes based on the use of a ROC analysis with RSF and OS. However, it is important to note that this study serves as an internal validation, and thus requires further validation with external cohorts possessing diverse demographic characteristics. In order to ensure the representativeness of our results within the wider population, it is crucial that future studies incorporate external cohorts to enhance the generalizability and replicability of our findings. This would enable the validation of our results within a broader range of populations and increase the reliability of our conclusions.
Conclusion
The PNI represents a convenient and effective prognostic predictor for both RFS and OS in patients diagnosed with resectable AGE. In light of its simplistic yet practical nature, the utilization of PNI could prove to be a valuable addition to the clinical evaluation and management of individuals with this condition.
Data Sharing Statement
Data are available on reasonable request.
Ethics Approval and Patient Consent for Publication
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee from The Shantou University Medical College Cancer Hospital (No.2022054). Given that this was a retrospective study, the requirement for informed consent was waived by the Ethics Committees of The Shantou University Medical College Cancer Hospital. We declare that we will maintain the confidentiality of the research object’s personal information.
Author Contributions
Siqi Xu and Huide Zhu share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Science and Technology Planning Project of Shantou city, China (grant no. (2022)81-132).
Disclosure
The authors declare that no commercial or financial relationships existed that might be interpreted as a possible conflict of interest during the research.
References
1. Chevallay M, Bollschweiler E, Chandramohan SM, et al. Cancer of the gastroesophageal junction: a diagnosis, classification, and management review. Ann NY Acad Sci. 2018;1434(1):132–138. doi:10.1111/nyas.13954
2. Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 2017;26(2):107–118. doi:10.1097/CEJ.0000000000000249
3. Xie SH, Lagergren J. Time trends in the incidence of oesophageal cancer in Asia: variations across populations and histological types. Cancer Epidemiol. 2016;44:71–76.
4. Manabe N, Matsueda K, Haruma K. Epidemiological review of gastroesophageal junction adenocarcinoma in Asian countries. Digestion. 2022;103(1):29–36.
5. van Velzen M, Derks S, van Grieken N, Haj Mohammad N, van Laarhoven H. MSI as a predictive factor for treatment outcome of gastroesophageal adenocarcinoma. Cancer Treat Rev. 2020;86:102024.
6. Onodera T, Goseki N, Kosaki G. 栄養不良がん患者の消化管手術における予後栄養指標 [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. Japanese.
7. Sánchez Y, Vaca-Paniagua F, Herrera L, et al. Nutritional indexes as predictors of survival and their genomic implications in gastric cancer patients. Nutr Cancer. 2021;73(8):1429–1439.
8. Zheng Z, Zhu H, Cai H. Preoperative prognostic nutritional index predict survival in patients with resectable esophageal squamous cell carcinoma. Front Nutr. 2022;9:824839.
9. Hirahara N, Tajima Y, Fujii Y, et al. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer. 2018;18(1):285.
10. Huang YY, Liang SH, Hu Y, Liu X, Ma GW. Prognostic value of preoperative nutritional assessment and neutrophil-to-lymphocyte ratio in patients with thymic epithelial tumors. Front Nutr. 2022;9:868336.
11. Greally M, Agarwal R, Ilson DH. Optimal management of gastroesophageal junction cancer. Cancer. 2019;125(12):1990–2001.
12. Narayan RR, Poultsides GA. Advances in the surgical management of gastric and gastroesophageal junction cancer. Transl Gastroenterol Hepatol. 2021;6:16.
13. Hirahara N, Matsubara T, Mizota Y, Ishibashi S, Tajima Y. Prognostic value of preoperative inflammatory response biomarkers in patients with esophageal cancer who undergo a curative thoracoscopic esophagectomy. BMC Surg. 2016;16(1):66.
14. Mimatsu K, Fukino N, Ogasawara Y, Saino Y, Oida T. Utility of inflammatory marker- and nutritional status-based prognostic factors for predicting the prognosis of stage IV gastric cancer patients undergoing non-curative surgery. Anticancer Res. 2017;37(8):4215–4222.
15. Jiang P, Li X, Wang S, Liu Y. Prognostic significance of PNI in patients with pancreatic head cancer undergoing laparoscopic pancreaticoduodenectomy. Front Surg. 2022;9:897033.
16. Kazi M, Gori J, Sasi S, et al. Prognostic nutritional index prior to rectal cancer resection predicts overall survival. Nutr Cancer. 2022;74(9):3228–3235.
17. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437.
18. Kawabe T, Sher A. Memory-phenotype CD4+ T cells: a naturally arising T lymphocyte population possessing innate immune function. Int Immunol. 2022;34(4):189–196.
19. Yamamoto A, Toiyama Y, Okugawa Y, et al. Clinical implications of the preoperative lymphocyte C-reactive protein ratio in esophageal cancer patients. Surg Today. 2021;51(5):745–755.
20. Shimakawa T, Asaka S, Sagawa M, et al. 食道癌手術における術前栄養スクリーニングの現状と評価 [Nutritional screening before surgery for esophageal cancer - current status and evaluation results]. Gan to Kagaku Ryoho. 2014;41(10):1301–1303. Japanese.
21. Konishi T, Kosuga T, Inoue H, et al. Significance of preoperative prognostic nutritional index in the perioperative management of gastric cancer. J Gastrointest Surg. 2022;26(3):558–569.
22. Takechi H, Fujikuni N, Tanabe K, et al. Using the preoperative prognostic nutritional index as a predictive factor for non-cancer-related death in post-curative resection gastric cancer patients: a retrospective cohort study. BMC Gastroenterol. 2020;20(1):256.
23. Kang J, Yang G, Wang D, Lin Y, Wang Q, Luo H. the clinical application value of the prognostic nutritional index for the overall survival prognosis of patients with esophageal cancer: a robust real-world observational study in China. Comput Math Methods Med. 2022;2022:3889588.
24. Nogueiro J, Santos-Sousa H, Pereira A, et al. The impact of the prognostic nutritional index (PNI) in gastric cancer. Langenbecks Arch Surg. 2022;407(7):2703–2714.
25. Matsuura S, Morikawa K, Ito Y, et al. The geriatric nutritional risk index and prognostic nutritional index predict the overall survival of advanced non-small cell lung cancer patients. Nutr Cancer. 2022;74(5):1606–1613.
26. Yan D, Shen Z, Zhang S, et al. Prognostic values of geriatric nutritional risk index (GNRI) and prognostic nutritional index (PNI) in elderly patients with Diffuse Large B-Cell Lymphoma. J Cancer. 2021;12(23):7010–7017.
27. Xu S, Cao S, Geng J, Wang C, Meng Q, Yu Y. High prognostic nutritional index (PNI) as a positive prognostic indicator for non-small cell lung cancer patients with bone metastasis. Clin Respir J. 2021;15(2):225–231.
28. Yu Y, Wu H, Qiu J, et al. A nutrition-related factor-based risk stratification for exploring the clinical benefits in the treatment of patients with locally advanced esophageal squamous cell carcinoma receiving definitive chemoradiotherapy: a retrospective cohort study. Front Nutr. 2022;9:896847.
29. Pan YP, Kuo HC, Hsu TY, et al. Body mass index-adjusted body weight loss grading predicts overall survival in esophageal squamous cell carcinoma patients. Nutr Cancer. 2021;73(7):1130–1137.
30. Zhang HL, Yang YS, Duan JN, et al. Prognostic value of preoperative weight loss-adjusted body mass index on survival after esophagectomy for esophageal squamous cell carcinoma. World J Gastroenterol. 2020;26(8):839–849.
31. Wu CY, Lin YH, Lo WC, et al. Nutritional status at diagnosis is prognostic for pharyngeal cancer patients: a retrospective study. Eur Arch Otorhinolaryngol. 2022;279(7):3671–3678.
32. Bullock AF, Greenley SL, McKenzie G, Paton LW, Johnson MJ. Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: systematic review, narrative synthesis and meta-analysis. Eur J Clin Nutr. 2020;74(11):1519–1535.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
