Back to Journals » Journal of Inflammation Research » Volume 16
Predictive Value of Systemic Inflammation Score for Contrast-Associated Acute Kidney Injury and Adverse Outcomes Among Patients Undergoing Elective Percutaneous Coronary Intervention
Authors Zeng JL, Xiang YF, Zhang LW, Chen LC, Chen JH, Liang WJ, You Z, Wang CX, Lin ZJ, Lin KY, Guo Y
Received 4 May 2023
Accepted for publication 29 June 2023
Published 8 July 2023 Volume 2023:16 Pages 2845—2854
DOI https://doi.org/10.2147/JIR.S419831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Ji-Lang Zeng,1– 3,* Yi-Fei Xiang,1– 3,* Li-Wei Zhang,1– 3,* Li-Chuan Chen,1– 3 Jun-Han Chen,1– 3 Wen-Jia Liang,1– 3 Zhebin You,2– 4 Chang-Xi Wang,1– 3 Zhi-Jie Lin,1– 3 Kai-Yang Lin,1– 3 Yansong Guo1– 3
1Department of Cardiology, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou, People’s Republic of China; 2Fujian Provincial Key Laboratory of Cardiovascular Disease, Fujian Provincial Center for Geriatrics, Fujian Provincial Clinical Research Center for Severe Acute Cardiovascular Diseases, Fuzhou, People’s Republic of China; 3Fujian Heart Failure Center Alliance, Fuzhou, People’s Republic of China; 4Fujian Key Laboratory of Geriatrics, Department of Geriatric Medicine, Fujian Provincial Hospital, Fujian Provincial Center for Geriatrics, Fujian Medical University, Fuzhou, Fujian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yansong Guo; Kai-Yang Lin, Fujian Provincial Hospital, Dongjie Street 134, Fuzhou, Fujian, 350001, People’s Republic of China, Tel +86-13559355708, Fax +86-591-87557768, Email [email protected]; [email protected]
Purpose: Prior research has demonstrated a key role of systemic inflammatory state in the pathogenesis and progression of contrast-associated acute kidney injury (CA-AKI). Recently, the systemic inflammation score (SIS) has been introduced to evaluate the inflammatory status, utilizing the lymphocyte-to-monocyte ratio (LMR) and albumin. The primary objective of this study was to determine whether the SIS can predict CA-AKI and long-term prognosis in patients undergoing elective percutaneous coronary intervention (PCI).
Patients and Methods: A total of 5726 patients who underwent elective PCI were included from January 2012 to December 2018. The primary outcome was CA-AKI, defined as an increase in serum creatinine (SCr) ≥ 0.3 mg/dl or ≥ 50% than baseline SCr within 48 h after the PCI procedure. The secondary outcome was long-term mortality. All patients were classified into low- and high-SIS groups.
Results: During hospitalization, 349 (6.1%) patients developed CA-AKI. Multivariate logistic regression analysis showed that patients in the high SIS group had a 1.47-fold higher risk of developing CA-AKI than those in the low SIS group [odds ratio (OR): 1.50, 95% confidence interval (CI): 1.12– 2.01, P =0.006]. Furthermore, the SIS showed the greatest prediction performance for CA-AKI compared with other inflammatory hematological ratios. In the multivariate Cox regression analysis, the high SIS group was found to be closely associated with long-term mortality [hazard ratio (HR): 1.58, 95% CI: 1.26– 1.97, P < 0.001, vs low SIS group]. The Kaplan-Meier curve analysis also demonstrated a difference in long-term mortality between the two groups (Log rank test, P < 0.001).
Conclusion: The SIS was closely associated with CA-AKI and long-term mortality in patients after elective PCI. Thus, more attention should be paid to exploring the potential benefits of anti-inflammatory strategies in preventing CA-AKI and improving the prognosis of patients undergoing PCI.
Keywords: contrast-associated acute kidney injury, mortality, percutaneous coronary intervention, systemic inflammation score
Introduction
Contrast-associated acute kidney injury (CA-AKI) is a prevalent complication in patients who undergo percutaneous coronary intervention (PCI). The occurrence and development of CA-AKI not only leads to prolonged hospitalization and increased healthcare costs, but is also significantly associated with numerous adverse cardiovascular and renal outcomes, such as heart failure, renal replacement therapy, and major adverse cardiovascular events (MACE).1–4 Given the limited availability of effective treatments for CA-AKI, identifying modifiable high-risk factors is crucial for implementing preventative strategies and improving prognosis.
Prior research has demonstrated a key role of systemic inflammatory state in the pathogenesis and progression of CA-AKI.5 Several hematological parameters, such as the lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII), have been demonstrated to be closely associated with CA-AKI and other inflammation-related diseases.6–8 Recently, the systemic inflammation score (SIS), which was constructed based on LMR and albumin, has been developed and shown to be a novel prognostic indicator in several types of cancer.9 However, the relationship between SIS and CA-AKI remains unknown. Here, we aimed to investigate the predictive ability of SIS for CA-AKI in patients undergoing elective PCI treatment, and further evaluate its prognostic value for long-term outcomes.
Materials and Methods
Study Population
We conducted a retrospective, single-center study at Fujian Provincial Hospital. Patients who underwent elective PCI were continuously enrolled from January 2012 to December 2018. Exclusion criteria were as follows: (1) Lacking any one of the data on complete blood routine count and pre- or post-procedural serum creatinine (SCr); (2) End-stage renal disease [estimated glomerular filtration rate (eGFR) <15 mL/min/1.73m2] or dialysis; (3) Cancer with a life expectancy no more than 1 year; (4) Contrast medium (CM) exposure within 7 days pre-procedure or allergic to CM; (5) Treated with nephrotoxic drugs within the 48 h prior to the procedure. Lastly, 5726 patients were included in the analysis.
Protocol
Lymphocyte, monocyte, and albumin levels were measured at admission, and SCr was measured at admission and the 2 consecutive days after the PCI procedure. Other clinical data including demographic characteristics, comorbidities, medical history, and laboratory tests were obtained from electronic medical records. PCI and perioperative management were performed by at least 2 experienced interventional cardiologists during hospitalization based on current guidelines. All patients were injected with 0.9% normal saline at a rate of 1 mL/kg/h for hydration throughout the perioperative period for 12 hours (0.5 mL/kg/h for patients with heart failure).
Definition of Blood Inflammatory Markers
The SIS consisted of two components: albumin and LMR. According to previous studies, albumin and LMR were analyzed as categorical variables. Albumin was dichotomized based on its reference range lower limit of 40 g/L (reference range, 40–55 g/L), while LMR was dichotomized using the receiver operating characteristic (ROC) curve-determined optimal cutoff value of 3.63 [area under the curve (AUC): 0.597, 95% confidence interval (CI): 0.565–0.628]. Based on these cutoff values and previous studies, we defined SIS as follows: Patients who exhibited high albumin levels (>40 g/L) and high LMR (>3.63) were assigned a score of 0; patients with either high albumin levels (>40 g/L) or high LMR (>3.63) were assigned a score of 1; patients with both low albumin levels and low LMR (≤40 g/L and ≤3.63, respectively) were assigned a score of 2. Subsequently, to better understand the severity of inflammation in each patient, we further classified the patients into low SIS group (score of 0) and high SIS group (score of 1 or 2) (Supplementary Table 1). Additionally, neutrophil-to-lymphocyte ratio (NLR), PLR, SII, and systemic inflammation response index (SIRI) were calculated based on the following formulas: NLR = neutrophil count/lymphocyte count; PLR = platelet count/lymphocyte count; SII = (neutrophil count × platelet count)/lymphocyte count, and SIRI = (neutrophil count × monocyte count)/lymphocyte count.
Outcomes and Follow-Up
The primary outcome of the study was CA-AKI, defined as an increase in SCr ≥0.3 mg/dl or ≥50% than baseline SCr within 48 h after the PCI procedure according to the Acute Kidney Injury Network (AKIN).10 The secondary outcome was long-term mortality. All patients were followed up by trained doctors through outpatient visits or telephone surveys after discharge, and 242 (4.2%) patients were lost to follow-up at the beginning of the study.
Statistical Analysis
Continuous variables were presented as mean ± SD (normal distribution) or median and interquartile range (skewed distribution), and categorical variables were reported as numbers and proportions. Differences between the low- and high-SIS groups were identified using the ANOVA test (normal distribution), Mann–Whitney U-test (skewed distribution), or chi-squared test or Fisher’s exact test (categorical variables).
Univariate logistic regressions were performed to identify CA-AKI-related risk factors, and factors with P <0.05 or significant clinical significance were retained in the multivariable logistic regressions. To exclude confounding factors, Model 1 adjusted for age >75 years and gender. Model 2 adjusted for Model 1 plus hypertension, diabetes, congestive heart failure (CHF), atrial fibrillation, chronic kidney disease (CKD) and hypotension. Model 3 was further adjusted for acute myocardial infarction (AMI), anemia and contrast medium volume >150 mL based on model 2. To test for multicollinearity, the variance inflation factor (VIF) method is utilized. A VIF value ≥5 indicated the presence of multicollinearity. Furthermore, the relationship between SIS and CA-AKI was further analyzed by subgroup analysis and interaction tests, and the results were expressed through forest plots. To assess the correlation between SIS and other inflammatory hematological ratios (including NLR, PLR, SII, and SIRI), Spearman correlation coefficients were utilized. ROC analysis and Delong’s test were performed to compare the diagnostic performance of SIS and other inflammatory hematological ratios. Multivariate Cox proportional hazard models were subsequently used to assess the predictive value of SIS for long-term prognosis. Survival analysis between the two groups was assessed using the Kaplan-Meier method and Log rank tests. All data analyses were performed in R version 4.2.1. P <0.05 was considered statistically significant.
Results
Baseline Characteristics of the Study Population
Table 1 displayed the baseline characteristics of the patients in the low- and high-SIS groups. The overall mean age was 65.3 ± 10.4 years, and 1228 (21.4%) were female. Compared to patients in the low SIS group, those in the high SIS group were found to be elder, predominantly male, and had lower levels of hemoglobin, albumin, and left ventricular ejection fraction. Besides, they showed a higher proportion of CHF, elevated n-terminal pro-brain natriuretic peptide (NT-proBNP) levels, and were more frequently treated with diuretics. In terms of inflammatory hematological ratios, patients in the high SIS group exhibited significantly higher levels of NLR, PLR, SII, and SIRI. Additionally, it was observed that SIS had positive and moderate correlations with NLR (r=0.511, P <0.0001), PLR (r=0.334, P <0.0001), SII (r=0.423, P <0.0001), and SIRI (r=0.616, P <0.0001). The baseline characteristics of patients in the non-CA-AKI and CA-AKI groups were presented in Supplementary Table 2.
 |
Table 1 Baseline Characteristics Between Low- and High-SIS Groups |
Predictive Value of the SIS for CA-AKI
During hospitalization, 349 (6.1%) patients developed CA-AKI, and a significantly higher incidence of CA-AKI was observed in the high SIS group (8.2% vs 3.5%, P <0.001, vs low SIS group) (Figure 1).
 |
Figure 1 Incidence of CA-AKI in the low- and high-SIS group. Abbreviations: CA-AKI, contrast-associated acute kidney injury; SIS, systemic inflammation score. |
In order to further evaluate the predictive value of the SIS for CA-AKI, we established three logistic regression models (Table 2). In model 1, the risk of developing CA-AKI was 2.40-fold higher in the high SIS group after adjustment for demographics factors [odds ratio (OR): 2.40, 95% confidence interval (CI): 1.87–3.10, P <0.001, vs low SIS group]. In model 2, after adjustment for covariates in Model 1 plus several comorbidities, the relationship with CA-AKI remained significant in the high SIS group (OR: 2.13, 95% CI: 1.64–2.79, P <0.001). Similar results were observed after fully adjusted covariates in model 3 (OR: 1.50, 95% CI: 1.11–2.01, P =0.006). Through subgroup analysis and interaction tests, we further tested the stability of the results (Supplementary Figure 1). It was shown that there were no interaction effects of various covariates on the association between SIS and CA-AKI (all P for interaction >0.05).
 |
Table 2 Predictive Value of the SIS for CA-AKI |
Comparison with Other Inflammatory Hematological Ratios
Figure 2 displayed the ROC analysis used to predict CA-AKI. Among the 5 inflammatory markers, the SIS had the greatest AUC (AUC: 0.641, 95% CI: 0.612–0.670). By using Delong’s test to compare the AUCs, we observed that the AUC of the SIS was significantly better than that of the PLR (ΔAUC: 0.082, P <0.001), SII (ΔAUC: 0.038, P =0.019), and SIRI (ΔAUC: 0.027, P =0.045), and was roughly comparable to the NLR (ΔAUC: 0.022, P =0.146).
Follow-Up
During a median follow-up of 34 months, 496 (9.0%) patients died. In the multivariate Cox proportional hazard models adjusted for confounders, the high SIS group had a 1.58-fold higher risk of long-term mortality than the low SIS group [hazard ratio (HR): 1.58, 95% CI: 1.26–1.97, P <0.001] (Table 3). Similarly, the Kaplan-Meier curves also revealed that there were significant differences in the long-term mortality between the two groups (Log rank test, P <0.001) (Figure 3).
 |
Table 3 Predictive Value of the SIS for Long-Term Mortality |
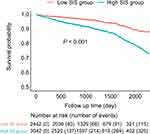 |
Figure 3 Mortality between patients in the low- and high-SIS group. Abbreviation: SIS, systemic inflammation score. |
Sensitivity Analysis
To check the robustness of the results, we conducted a sensitivity analysis using another classical definition of CA-AKI, defined as an increase in SCr of ≥0.5 mg/dl or ≥25% within 48 h after the PCI procedure according to the European Society of Urogenital Radiology (ESUR).11 The outcomes displayed good coincidence with primary analysis (Supplementary Table 3).
Discussion
Our research revealed that patients in the high SIS group had a greater likelihood of developing CA-AKI after elective PCI than those in the low SIS group. This observation persisted even after taking into account potential confounders. Furthermore, the results of the ROC analysis indicated that the SIS had a superior predictive capability for CA-AKI in comparison to other inflammatory hematological ratios. Additionally, we observed that patients in the high SIS group had an appreciably higher long-term mortality rate.
With the widespread use of CM in the field of interventional cardiology, CA-AKI has become a crucial issue in clinical practice, and it is usually regarded as the third leading cause of hospital-acquired AKI.12,13 So far, there is no effective treatment available for CA-AKI, thereby risk prediction and effective prevention strategies are crucial to reducing its incidence. Although the pathological mechanisms of CA-AKI are complex and not completely understood, increasing evidence suggests that the inflammation process may play a crucial role in its development and progression.14 Firstly, inflammatory responses are commonly observed in patients with CA-AKI, as demonstrated by increased levels of inflammatory cytokines, such as neutrophil gelatinase-associated lipocalin (NGAL) and interleukin-6 (IL-6), in plasma and urine.15,16 Secondly, animal and cell experiments have demonstrated that injecting CM can stimulate the secretion of inflammatory cytokines and chemokines in renal endothelial and tubular cells, resulting in the subsequent induction of leukocytes migration to the kidney.17,18 As the main inflammatory cells, leukocytes (such as lymphocytes and monocytes) promote the release of pro-inflammatory cytokines and proteases which can lead to the further aggravation of renal injury in experimental animals.19 Alternatively, the administration of Antithrombin III (ATIII) or renalase (enzymes that have been shown to inhibit leucocytes activation and NF-κB pathway) significantly reduced kidney injury in experimental animals.17,18 Besides, numerous clinical studies suggested that the presence of inflammation, as evaluated through circulating blood cells, was closely associated with unfavorable outcomes. For example, Hayiroğlu et al emphasized the potential role of the inflammatory process in the progression of heart failure and further confirmed the strong predictive value of SII for long-term mortality in heart failure patients.20 Moreover, it has been reported that LMR and NLR were significant risk factors for AKI and adverse cardiovascular prognosis.21–23
As a comprehensive indicator composed of albumin, the predictive value of SIS for CA-AKI can also be partly explained by the biological functions of albumin. Albumin is abundant in human plasma and performs crucial physiological functions like maintaining osmotic pressure, facilitating substance transport, and reflecting the nutritional status of the body. In addition, albumin functioned as a negative acute-phase protein, and its concentrations had a negative correlation with inflammation severity.24 Basic research further indicated that albumin may exert anti-inflammatory effects by inhibiting leukocyte adhesion and the NF-κB pathway.25 Another major function of albumin was its antioxidant properties.26 Oxidative stress was considered to be involved in the pathogenesis of CA-AKI.27 Albumin can clear oxygen radicals by isolating reactive oxygen species and regulating intracellular signaling pathways, thereby reducing oxidative damage.26,28 Furthermore, a recent retrospective study conducted by Murat et al demonstrated that a lower albumin level was an independent predictive factor for CA-AKI following PCI.29 Another meta-analysis including 8 studies with a total of 18,687 patients also indicated a negative correlation between albumin levels and the risk of CA-AKI.30 In addition, several retrospective and prospective studies reported the association between albumin and poor outcomes among patients with cardiovascular diseases.31–33
To the best of our knowledge, this study was the first to explore the correlation between the SIS and CA-AKI. The SIS is a composite score composed of the LMR and albumin, LMR represented the balance between the pro-inflammatory and anti-inflammatory responses, while albumin indicated the nutritional status and affects the degree of inflammation. The use of SIS can provide a more accurate and comprehensive assessment of the inflammatory state of the body. Our research also showed that the SIS was a superior predictor of CA-AKI when compared to other inflammatory hematological ratios. Additionally, in contrast to traditional risk factors for CA-AKI such as glomerular filtration rate and CM volume, SIS is a simple a simple, cost-effective, and readily accessible biomarker for clinicians. Overall, SIS may serve as a valuable tool for risk stratification and early detection of CA-AKI following the PCI procedure.
Despite its potential benefits, there were a few limitations that must be acknowledged in this study. Firstly, it was a single-center retrospective study conducted on the Chinese population. Thus, there is a need for further multicenter prospective studies to validate our findings. Secondly, due to the retrospective design of our study, some potential confounding factors could not be fully adjusted for. Thirdly, changes in measurement time may result in the missed measurement of peak SCr levels, potentially leading to an underestimation of the true incidence of CA-AKI. Fourthly, we were unable to analyze kidney-related deaths as they were missing from our follow-up information.
Conclusion
In summary, our study suggested that the SIS was a robust independent predictor of increased CA-AKI incidence and long-term mortality risk in patients undergoing elective PCI. Further studies are needed to clarify the underlying mechanisms and explore the potential benefits of anti-inflammatory strategies in preventing CA-AKI and improving the prognosis of patients undergoing PCI.
Abbreviations
CA-AKI, contrast-associated acute kidney injury; PCI, percutaneous coronary intervention; SIS, systemic inflammation score; CM, contrast medium; eGFR, estimated glomerular filtration rate; CHF, congestive heart failure; CKD, chronic kidney disease; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index.
Data Sharing Statement
The datasets produced and analyzed in this study are not publicly accessible due to confidentiality concerns, but can be obtained from the corresponding author upon request, subject to review and approval by the Study’s Committee.
Ethics Approval and Informed Consent
This study followed the Helsinki Declaration principles and ethical approval was granted by the Fujian Provincial Hospital ethics committee (Ethical approval number: K2019-07-011). The need for patient consent to review their medical records was waived by the Fujian Provincial Hospital Ethics Committee. The waiver was granted based on the following reasons: (1) The retrospective design of the study; (2) The importance of the study’s purpose; (3) The minimal risk to patients; (4) The absence of adverse effects on patient rights and health, and (5) The assurance that patient data confidentiality would be strictly protected throughout the research process. To ensure patient data confidentiality, all personal identifiers have been anonymized and removed from the dataset.
Patient Data Confidentiality Statement
All personal identifiers, such as names, telephone numbers, and other direct identifiers, have been anonymized and removed from the dataset to ensure the privacy and confidentiality of the patients.
Funding
This research was supported by the National Natural Science Foundation of China General Program (Grant numbers: 81873495 and 82171569), the Heart Failure Center Research Foundation of Fujian Provincial Hospital (supported by the Fujian Provincial Department of Finance), and the National Key Clinical Specialty Construction Project of China (Cardiovascular Medicine 2021). The funding source had no role in this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Weisbord DS, Palevsky PM, Kaufman JS, et al. Contrast-associated acute kidney injury and serious adverse outcomes following angiography. J Am Coll Cardiol. 2020;75(11):1311.
2. James MT, Samuel SM, Manning MA, et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6(1):37–43. doi:10.1161/CIRCINTERVENTIONS.112.974493
3. Azzalini L, Candilio L, McCullough PA, Colombo A. Current risk of contrast-induced acute kidney injury after coronary angiography and intervention: a reappraisal of the literature. Can J Cardiol. 2017;33(10):1225–1228. doi:10.1016/j.cjca.2017.07.482
4. Hayıroğlu Mİ, Bozbeyoglu E, Yıldırımtürk Ö, Tekkeşin Aİ, Pehlivanoğlu S. Effect of acute kidney injury on long-term mortality in patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock who underwent primary percutaneous coronary intervention in a high-volume tertiary center. Turk Kardiyol Dern Ars. 2020;48(1):1–9. doi:10.5543/tkda.2019.84401
5. Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy: from pathophysiology to preventive strategies. Can J Cardiol. 2016;32(2):247–255. doi:10.1016/j.cjca.2015.05.013
6. Karauzum I, Karauzum K, Acar B, et al. Predictive value of lymphocyte-to-monocyte ratio in patients with contrast-induced nephropathy after percutaneous coronary intervention for acute coronary syndrome. J Transl Int Med. 2021;9(2):123–130. doi:10.2478/jtim-2021-0024
7. Velibey Y, Oz A, Tanik O, et al. Platelet-to-lymphocyte ratio predicts contrast-induced acute kidney injury in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2017;68(5):419–427.
8. Jiang H, Li D, Xu T, et al. Systemic immune-inflammation index predicts contrast-induced acute kidney injury in patients undergoing coronary angiography: a cross-sectional study. Front Med. 2022;9:841601. doi:10.3389/fmed.2022.841601
9. Chang Y, An H, Xu L, et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015;113(4):626–633. doi:10.1038/bjc.2015.241
10. Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
11. Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast media safety committee, European Society of Urogenital Radiology (ESUR). Eur Radiol. 1999;9(8):1602–1613. doi:10.1007/s003300050894
12. Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Wiviott SD. Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the national cardiovascular data registry. Circulation. 2012;125(3):497–504. doi:10.1161/CIRCULATIONAHA.111.039909
13. Fähling M, Seeliger E, Patzak A, Persson PB. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol. 2017;13(3):169–180. doi:10.1038/nrneph.2016.196
14. McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51(15):1419–1428. doi:10.1016/j.jacc.2007.12.035
15. Mårtensson J, Martling CR, Bell M. Novel biomarkers of acute kidney injury and failure: clinical applicability. Br J Anaesth. 2012;109(6):843–850. doi:10.1093/bja/aes357
16. Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. 2013;28(2):254–273. doi:10.1093/ndt/gfs380
17. Lu Z, Cheng D, Yin J, et al. Antithrombin III protects against contrast-induced nephropathy. EBioMedicine. 2017;17:101–107. doi:10.1016/j.ebiom.2017.02.009
18. Wang F, Yin J, Lu Z, et al. Limb ischemic preconditioning protects against contrast-induced nephropathy via renalase. EBioMedicine. 2016;9:356–365. doi:10.1016/j.ebiom.2016.05.017
19. Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi:10.1155/2009/137072
20. Hayiroğlu Mİ, Çınar T, Çinier G, et al. Evaluating systemic immune-inflammation index in patients with implantable cardioverter defibrillator for heart failure with reduced ejection fraction. Pacing Clin Electrophysiol. 2022;45(2):188–195. doi:10.1111/pace.14436
21. Jiang F, Lei J, Xiang J, et al. Monocyte-to-lymphocyte ratio: a potential novel predictor for acute kidney injury in the intensive care unit. Ren Fail. 2022;44(1):1004–1011. doi:10.1080/0886022X.2022.2079521
22. de Hond TAP, Ocak G, Groeneweg L, et al. Hematological ratios are associated with acute kidney injury and mortality in patients that present with suspected infection at the emergency department. J Clin Med. 2022;11(4):1017. doi:10.3390/jcm11041017
23. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic Immune Inflammation Index (SII), System Inflammation Response Index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. 2023;12(3):1128. doi:10.3390/jcm12031128
24. Don BR, Kaysen G. Poor nutritional status and inflammation: serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi:10.1111/j.0894-0959.2004.17603.x
25. Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res. 2002;55(4):820–829. doi:10.1016/S0008-6363(02)00492-3
26. Belinskaia DA, Voronina PA, Shmurak VI, Jenkins RO, Goncharov NV. Serum albumin in health and disease: esterase, antioxidant, transporting and signaling properties. Int J Mol Sci. 2021;22(19):10318. doi:10.3390/ijms221910318
27. Börekçi A, Gür M, Türkoğlu C, et al. Oxidative stress and paraoxonase 1 activity predict contrast-induced nephropathy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2015;66(4):339–345. doi:10.1177/0003319714533588
28. Bourdon E, Blache D. The importance of proteins in defense against oxidation. Antioxid Redox Signal. 2001;3(2):293–311. doi:10.1089/152308601300185241
29. Murat SN, Kurtul A, Yarlioglues M. Impact of serum albumin levels on contrast-induced acute kidney injury in patients with acute coronary syndromes treated with percutaneous coronary intervention. Angiology. 2015;66(8):732–737. doi:10.1177/0003319714551979
30. Liu L, Lun Z, Wang B, et al. Predictive value of hypoalbuminemia for contrast-associated acute kidney injury: a systematic review and meta-analysis. Angiology. 2021;72(7):616–624. doi:10.1177/0003319721989185
31. Hayıroğlu Mİ, Çınar T, Çinier G, et al. Prognostic value of serum albumin for long-term mortality in patients with dual-chamber permanent pacemakers. Biomark Med. 2022;16(5):341–348. doi:10.2217/bmm-2021-0991
32. Çinier G, Hayıroğlu Mİ, Kolak Z, et al. The value of C-reactive protein-to-albumin ratio in predicting long-term mortality among HFrEF patients with implantable cardiac defibrillators. Eur J Clin Invest. 2021;51(8):e13550. doi:10.1111/eci.13550
33. Chien SC, Chen CY, Leu HB, et al. Association of low serum albumin concentration and adverse cardiovascular events in stable coronary heart disease. Int J Cardiol. 2017;241:1–5. doi:10.1016/j.ijcard.2017.04.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

