Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Plasma Extracellular Vesicle miRNA Profiles Distinguish Chronic Obstructive Pulmonary Disease Exacerbations and Disease Severity
Authors O'Farrell HE , Bowman RV, Fong KM , Yang IA
Received 29 June 2022
Accepted for publication 25 October 2022
Published 4 November 2022 Volume 2022:17 Pages 2821—2833
DOI https://doi.org/10.2147/COPD.S379774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Hannah E O’Farrell, Rayleen V Bowman, Kwun M Fong, Ian A Yang
Faculty of Medicine, the University of Queensland, Brisbane, QLD, Australia
Correspondence: Hannah E O’Farrell, Faculty of Medicine, the University of Queensland, Brisbane, QLD, Australia, Tel +61 07 3139 4110, Email [email protected]
Purpose: Molecular biomarkers for chronic obstructive pulmonary disease (COPD) severity have been difficult to identify. We aimed to assess extracellular vesicle miRNAs’ potential as a blood biomarker in discriminating disease severity in participants with COPD.
Patients and Methods: Plasma extracellular vesicles (EVs) were obtained from two COPD cohorts (n = 20 during an exacerbation event, n = 20 during stable state), with varying disease severity (GOLD stages). The miRCURY LNA miRNA Serum/Plasma assay, specific to 179 targets, was used to evaluate EV miRNA expression. The miRNAs that were significantly dysregulated were further assessed for discriminatory power using ROC curve analysis, as well as their role in relevant biological pathways.
Results: One miRNA was significantly dysregulated between moderate GOLD participants compared to severe/very severe GOLD participants, with an AUC of 0.798, p = 0.01 for miR-374b-5p. Five miRNAs were significantly dysregulated between exacerbating and stable COPD participants, with miR-223-3p resulting in the highest AUC (0.755, p = 0.006) for a single miRNA, with a combination of three miRNAs (miR-92b-3p, miR-374a-5p and miR-106b-3p) providing the highest discriminatory power (AUC 0.820, p = 0.001). The “cytokine–cytokine receptor interaction” (hsa04060 pathway) was the most significant KEGG pathway enriched for three out of the five miRNAs associated with COPD exacerbations.
Conclusion: This initial small-scale study suggests that the bioactive cargo (miRNAs) in plasma EVs holds specific biological information for the severity of airflow obstruction and COPD exacerbations, warranting further investigation.
Keywords: COPD, exacerbations, extracellular vesicles, miRNAs
Introduction
Pathological hallmarks of chronic obstructive pulmonary disease (COPD) include chronic inflammation and remodeling of the small airways, as well as lung parenchyma destruction, resulting in incompletely reversible airflow limitation.1,2 Now recognized as the third leading cause of death worldwide,3 COPD also causes significant morbidity, highlighting the urgent need to identify and reduce underlying risk factors.4 A number of factors influence COPD development and progression, with the best known being cigarette smoking.5
Clinical symptoms including breathlessness, chronic cough, sputum production and exacerbations are defining characteristics of COPD.6 A number of patients suffer from exacerbations (defined as the worsening of symptoms above a patient’s usual baseline) and may require pharmacological intervention and/or hospitalisation.7 Pharmacological interventions aimed at reducing symptoms, exacerbation frequency and severity include the use of inhaled bronchodilators (to overcome airflow obstruction), corticosteroids (as anti-inflammatory agents) and antibiotics (bacterial infections).5
Exacerbations are serious complications that accelerate lung function decline, reduce quality of life and significantly worsen survival outcomes.8 Given the lack of curative treatments for COPD, reducing exacerbations is a significantly important treatment goal in preventing rapid disease progression. Many potential biomarkers have been tested for the prediction of COPD exacerbations and overall disease progression;9,10 however, as COPD is widely recognised as a complex heterogeneous disease, additional biomarkers are still needed.
Extracellular vesicles (EVs) are nanosized lipid bilayer particles released by most cells and detected in a variety of bodily fluids including blood, urine and sputum.11,12 EVs have been highlighted for their potential as novel disease biomarkers due to a number of favourable characteristics, including their stability in bodily fluids, reflecting the microenvironment and physiological state of their parental cell and the ability to package and transport disease-specific biomolecules (eg, DNA, RNA, miRNAs and proteins).13 In particular, short non-coding RNAs known as microRNAs (miRNAs) play a key role in regulating gene expression14 as well as being highly stable in plasma.15 Studies have highlighted that specific miRNAs can be selectively exported to EVs,16 while others attest to their influence on recipient cell biological processes.17 The stability and accessibility of plasma EV miRNAs has made them ideal targets for biomarker studies.18,19
This small-scale study aimed to identify plasma EV miRNA biomarkers from COPD participants that could distinguish between mild and severe disease (GOLD COPD stage), and between stable disease and exacerbation. Identifying such plasma EV miRNAs, which are then further validated in larger independent patient cohorts, could have significant translational application in the clinical management of COPD.
Materials and Methods
Cohort Demographics
Participants during an exacerbation of COPD (n = 20) were recruited consecutively within 4 days of admission to The Prince Charles Hospital, Brisbane, Australia. Inclusion was based on a clinical diagnosis of severe COPD exacerbation (acute deterioration in COPD symptoms requiring hospitalisation), as determined by a thoracic physician.20 Patients with other comorbid lung disease including predominant asthma based on a physician diagnosis, lung cancer, and interstitial lung disease were excluded.20 Twenty other patients (outpatients with COPD who had stable symptoms for at least 4 weeks) were recruited as a participant in stable state.20
Participant demographics and clinical data including smoking history, lung function, GOLD staging and COPD Assessment Test (CAT) were obtained from medical records and at the time of patient recruitment. Table 1 lists clinical characteristics of participants.
 |
Table 1 Clinical Characteristics of Exacerbating (n = 20) and Stable (n = 20) COPD Participants |
Blood Collection and Processing
Peripheral blood was obtained from 40 COPD participants: 20 participants during an exacerbation event and a separate group of 20 participants in a stable state. Processing of peripheral blood to separate plasma from blood cell fraction was performed as previously described.18
Characterisation of Plasma EVs
Transmission Electron Microscopy
Transmission Electron Microscopy (TEM) on re-suspended plasma EVs was outsourced to the Centre for Microscopy and Microanalysis, The University of Queensland. Full details can be found in the online Supplementary Material methods section in ‘Characterisation of Plasma Extracellular Vesicles’.
Western Blot of EV Markers
Re-suspended plasma EVs in 1X PBS were quantified for protein concentration using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific, USA) before undergoing Western blotting for EV specific markers as previously described.18
Plasma EV Isolation and RNA Extraction
EVs were isolated from thawed plasma samples using the miRCURY Exosome Serum/Plasma Kit (QIAGEN, Hilden, Germany), and total RNA was extracted using the miRNeasy Mini Kit (QIAGEN, Hilden, Germany) as previously described.18
Plasma EV miRNA Profiling
Reverse transcription was performed using the miRCURY LNA RT kit (QIAGEN, Hilden, Germany), followed by miRNA gene expression assessment using the miRCURY LNA miRNA Serum/Plasma Focus PCR Panels (QIAGEN, Hilden, Germany) as previously described.18 Further details can be found in the online Supplementary Material methods section in ‘Quantitative PCR using miRCURY LNA Serum/Plasma Focus PCR Panels’.
Statistical Analyses
Statistical analyses were executed using Statistical Package for Social Science (SPSS V.27.0) (IBM, NY, USA), DIANA-miRPath v.3.0 (8) and GeneGlobe (http://www.giagen.com/geneglobe) as previously described.18 Full details are available on the online Supplementary Material methods section in “Statistical Analyses”.
Results
Clinical Characteristics of COPD Participants During an Exacerbation Event versus Stable State
Clinical characteristics were assessed between the 20 patients experiencing an exacerbation and a separate group of 20 patients who were deemed to be in a stable state (Table 1). Both cohorts consisted of older adults and former smokers with severe GOLD classification, based on spirometry. Clinical characteristics including gender, age, smoking history, and pack years were not significantly different between exacerbating and stable cohorts.
Clinical characteristics that were significantly decreased in the exacerbating cohort compared to the stable cohort included lung function indicators (FEV1% predicted, FEV1, FVC; p = 0.006, p = 0.001 and p = 0.0005, respectively), while more severe GOLD stages (p = 0.021), CAT score (p = 0.001) and inflammatory blood marker HS-CRP (p = 0.026) were significantly increased in the exacerbating cohort compared to the stable cohort.
Characterisation of Plasma EVs from Exacerbating COPD Participants and Those in Stable State
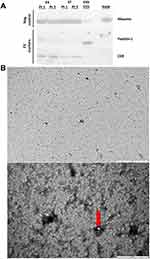
Plasma EVs were isolated from four COPD participants, two during an exacerbation event and two other participants whilst stable, and characterised using Western blotting. Analysis of established “EV-markers” showed presence of protein CD9 and minimal quantities of flotillin-1 in plasma EV from both participants, with neither marker detectable in raw plasma (Figure 1A). An exosome standard from human plasma as a positive control showed EV protein flotillin-1 and a minimal band of CD9. Albumin (negative control) was also present in EV samples from both participants, at similar levels to raw plasma, highlighting this contaminating protein co-isolation with the selected EV isolation method, as the exosome standard (positive control) only identified a minimal band.
Plasma EVs from one exacerbating COPD patient were visualised using TEM (Figure 1B), with a widefield image (5µm) representing a very concentrated sample. A close-up image at 500nm, highlights the very concentrated sample, with multiple small homogeneous lipid membrane particles present (a clearer vesicle is highlighted by the red arrow), below the 100nm size range, suggesting that these small vesicles are within the exosome size range.
Plasma-Derived EV miRNA Profiling Identified Significantly Dysregulated miRNAs Differentially Represented Between Exacerbating COPD Participants and Those in Stable State
QIAGEN’s miRCURY LNA miRNA Serum/Plasma Focus PCR panel was used to test for 179 miRNAs specific to human serum and plasma (including internal controls) on plasma-derived EVs. An unsupervised clustergram of miRNA expression is visualised in each patient sample (Figure 2). Candidate miRNAs that could differentiate between 1) Exacerbating COPD participants (test cohort) and participants at stable state (control cohort) and 2) GOLD stages (control cohort = GOLD 2 (moderate); test cohort = GOLD 3 (severe) and GOLD 4 (very severe)) were identified (Table 2).
 |
Table 2 Dysregulation of miRNA Targets That Were Significantly Different Between Exacerbating and Stable State COPD Cohorts, as Well as GOLD Classified Cohorts |
The miRNA expression from plasma EVs identified and distinguished a number of targets that were significantly dysregulated between the exacerbating and stable state cohorts, as well as between the different GOLD stage cohorts. The plasma EV miRNA expression in the exacerbating cohort compared to the stable cohort identified one significantly under-expressed miRNA (hsa-miR-34a-5p, fold change −2.84, p = 0.025) and 4 significantly over-expressed miRNAs, including hsa-miR-92b-3p, hsa-miR-223-3p, hsa-miR-374a-5p and hsa-miR-106b-3p (fold change range 1.58–2.78, p-value range of 0.007–0.032). The plasma EV miRNA expression in the GOLD stage 2 cohort (moderate) compared to the GOLD stage 3 and 4 (severe/very severe) cohort identified 1 significantly over-expressed miRNA (hsa-miR-374b-5p, fold change 1.91, p = 0.009).
The GeneGlobe online platform and TargetScan prediction software were used as previously described18 to identify genes regulated by the miRNAs differentially represented in plasma EVs between the disease states of interest (Table 3). miR-34a-5p (significantly under-expressed in the exacerbating cohort compared to the stable state cohort) regulates HCN3 and NAV1 genes, while the MEF2D gene was identified as a target for three of the miRNAs overexpressed in the exacerbating cohort. miR-374b-5p (significantly over-expressed in the severe/very severe GOLD stage participants compared to moderate GOLD stage participants) was found to regulate the ACVR2B gene.
 |
Table 3 The Top miRNA-Regulated Genes Targeting miRNAs That Were Identified as Significantly Dysregulated in the Exacerbating and Stable State COPD Cohorts, as Well as GOLD Classified Cohorts |
Clinical Correlates of Dysregulated EV miRNAs
Correlations (Spearman Rank) and significant differences (Mann–Whitney U) were assessed between the identified miRNAs and appropriate clinical characteristics between exacerbating participants compared to stable participants (Table S1), as well as severe/very severe GOLD stage participants compared to moderate GOLD stage participants (Table S2). For the 5 miRNAs significantly over-expressing in the exacerbating participants compared to stable participants, one miRNA significantly correlated with pack years (miR-106b-3p, when increased pack years decreased), three miRNAs with lung function parameters FEV1 (miR-223-3p, miR-374a-3p, miR-34a-5p, when miRNAs increased, FEV1 decreased), while two miRNAs (miR-92b-3p and miR-223-3p) correlated with FVC (when miRNAs increased, FVC decreased). One miRNA (miR-223-3p) is significantly correlated with the inflammatory marker, fibrinogen (miR-223-3p, when increased, fibrinogen levels increased), while two miRNAs (miR-106b-3p and miR-34a-5p) correlated with CAT scores (when miRNAs increased, CAT scores increased). No miRNAs were significantly associated with smoking history, while one miRNA (miR-223-3p) was significantly associated with gender (miR-223-3p, p = 0.04, increased in females) (Table S3). For severe/very severe GOLD stage participants compared to moderate GOLD stage participants, it was found that miR-374b-5p was significantly correlated with FEV1 only (when miRNA increased, FEV1 decreased), while no significant associations were observed for smoking history or gender (Table S3).
Biomarker Potential of Plasma EV miRNAs by ROC Analysis
ROC curve analysis was used to evaluate the utility of plasma EV miRNAs in discriminating both severity of COPD (using GOLD classification) and clinical state of COPD (exacerbation or stable state). A total of 5 miRNAs identified in the primary analyses were dysregulated in exacerbating participants compared to stable state participants. The highest area under the curve (AUC) for a single miRNA was achieved with miR-223-3p (AUC = 0.755; 95% confidence interval (CI) = 0.602–0.908; p = 0.006) (Figure 3A and B). From the correlation analysis (Table S1), the top miRNAs with the highest individual AUC values that were not significantly correlated with each other included miR-106b-3p, miR-374a-5p and miR-92b-3p. These miRNAs were further assessed as a combination in a logistic regression model (Figure 3C). This model was then assessed using ROC curve analysis, with the AUC improving to 0.820 (standard error = 0.066; CI = 0.690–0.950; p = 0.001) (Figure 3D).
A total of 1 miRNA was identified in the primary analyses as significantly dysregulated between moderate GOLD participants compared to severe/very severe GOLD participants. The AUC for miR-374b-5p miRNA was 0.798 (standard error 0.084; 95% CI 0.634–0.962; p = 0.01) (Figure 4).
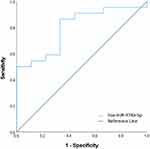 |
Figure 4 Discriminatory power assessed using ROC curve analysis for miR-374b-5p that was significantly over-expressed in severe/very severe GOLD participants compared to moderate GOLD participants. |
Biological Pathways Associated with miRNAs Differentially Represented in Plasma EVs in Exacerbating Compared to Stable State Participants
The five miRNAs differentially represented in EVs of exacerbating participants compared with stable participants were found to be enriched in seven KEGG pathways (Table 4). The “cytokine–cytokine receptor interaction” (hsa04060) was the most significant KEGG pathway targeted by the most miRNAs, with 9 enriched target genes, as well as the most genes validated by the miRNA-regulated gene targets from the GeneGlobe analysis.
 |
Table 4 Predicted Biological Pathways Enriched by the Significantly Dysregulated miRNAs Identified in Exacerbating COPD Participants Compared to Stable State |
Discussion
This small-scale study verified that EVs could be isolated from plasma and that the pattern of dysregulation of EV miRNAs was different depending on the clinical state and severity of COPD. These EV miRNA signatures are specific to each cohort and have translational potential as sensitive biomarkers that can sufficiently discriminate between steady state and exacerbation in an individual whose symptoms (based on CAT and lung function parameters) are less sensitive of informing whether an exacerbation is acute. Further, KEGG pathway analysis based on the identified candidate EV miRNAs specific to COPD state supports their involvement in biologically relevant pathways.
Biological Pathways Associated with miRNAs Differentially Represented in Plasma EVs in Exacerbating Compared to Stable State Participants
The five miRNAs differentially represented in EVs of exacerbating participants compared with stable participants were found to be enriched in seven KEGG pathways (Table 4). The “cytokine–cytokine receptor interaction” (hsa04060) was the most significant KEGG pathway targeted by the most miRNAs, with 9 enriched target genes, as well as the most genes validated by the miRNA-regulated gene targets from the GeneGlobe analysis. This result highlights that the molecular function of these differentially expressed EV miRNAs is significantly enriched in cytokine activity, which is in line with previous studies,21–23 further suggesting their crucial role during an exacerbation event and orchestrating a chronic inflammatory response.21
Significantly Dysregulated miRNAs Discriminate Exacerbating COPD Participants from Stable State Participants, as Well as Between GOLD Classifications with Discriminatory Power
Exacerbations are a major cause of disease progression, morbidity and mortality, and given the lack of curative treatments, earlier intervention for exacerbation prevention is highly relevant.13 EVs are highly stable in circulating bodily fluids and package cellular information indicative of their parental cell, which can be exchanged and influence recipient cell pathophysiology. Studies have reported various circulating miRNAs that are involved in the development and progression of COPD, including emphysema severity24 (lists of studied circulating miRNAs have been extensively detailed in previous reviews25,26). Additionally, recent reports by Fujita et al describe how miRNAs involved in COPD pathogenesis are modulated through EV-mediated transfer.27
miR-223-3p was one of the four plasma EV miRNAs that we found to be represented at high levels in exacerbating cases. Using the same miRCURY LNA miRNA array chemistry, Ezzie et al found miR-223 to be higher in lung tissue in COPD than in smokers without COPD.28 miR-223-3p appears to regulate inflammation, cell differentiation, proliferation and cell death.29 miR-223 over-expression reduces pro-inflammatory responses,30 and has been proposed to modulate the balance of neutrophilic/eosinophilic responses by inhibiting PARP-1 levels.31 However, in neutrophilic asthma participants, elevated miR-223 appears to be insufficient to reduce inflammation.32 Similarly, in COPD exacerbating participants, high levels of miR-223 in circulating EVs may be insufficient to suppress pro-inflammatory responses.30
Tan et al reported a significant increase in plasma exosomes in exacerbating COPD participants compared to stable state and suggested that EVs are involved in the inflammatory process of COPD exacerbations.33 Our finding of the positive correlation between miR-223-3p and the inflammatory blood marker, fibrinogen, would be consistent with such a role for EV miR-233-3p. As we also found a negative correlation between EV miR-233-3p and indices of lung function, miR-233-3p could potentially represent a link between inflammation and COPD severity.
While exacerbations are a significant phenotype that contributes to disease severity and progression, the GOLD classification for COPD diagnosis requires spirometry, with the severity of airflow limitation categorising patients with either mild or very severe disease.7 As COPD is recognised as a heterogeneous disease, and not all patients can access lung function tests in a timely way, the additional use of biomarkers to assess for COPD severity such as COPD severity-related EV miRNAs from plasma, may be a promising additional strategy for disease evaluation. Our results identified miR-374b-5p as significantly over-expressed in our severe/very severe GOLD COPD cohort compared to our moderate GOLD COPD cohort. Involvement of miR-374b-5p and its over-expression in severe GOLD COPD has yet to be described.
miRNA Regulated Target Genes and Relevant Biological Pathways in COPD
Target gene MEF2D was regulated by 3 out of the 4 significantly over-expressed miRNAs in exacerbating participants compared to stable state COPD participants by GeneGlobe TargetScan analysis. MEF2D has been reported to play an important role in cell differentiation, proliferation and apoptosis control, as well as being implicated as pathogenic in several diseases other than COPD.34,35 In COPD participants, it was previously reported to be correlated with lower levels of physical activity, reduced capacity related to fatigue, muscle wasting, as well as breathlessness.36 In COPD, skeletal muscle weakness is often observed and acutely worsens during an exacerbation, particularly if the patient is bed bound.37,38 Skeletal muscle differentiation is controlled by transcription factors including MEF2D. Systemic inflammation is a proposed mechanism contributing to acute muscle weakness,39 and Zhu et al reported that inflammatory conditions lead to increased expression of MEF2D.
KEGG pathway analysis of miRNAs differentially represented in EV cargo of exacerbating participants compared to stable participants shows that these miRNAs are enriched in the cytokine–cytokine receptor interaction, which is consistent with previous studies of dysregulated RNA expression and signaling pathways in lung tissue from participants with COPD.22,23 Chronic inflammation is a hallmark of COPD in which cytokines play critical roles in the recruitment and activation of inflammatory cells,40 including neutrophils and eosinophils, which are more prominent during exacerbations.41–43
In summary, our results support the premise that early detection of dysregulated EV miRNAs in COPD may predict which participants are on a rapid deterioration pathway.
Limitations
There are potential limitations in this preliminary study that need to be considered. Firstly, the use of a precipitation-based method for EV isolation, while yielding high volumes of EVs, also co-isolated contaminating proteins (Albumin), as reported in previous studies,44 with the potential to interfere with downstream EV characterization. Nevertheless, for assessment of miRNA expression, this method has support.45 Additionally, the target gene analysis performed was through using QIAGEN’s Geneglobe gene regulation tool for identifying candidate miRNA-regulated genes. This was therefore a predictive tool only, as we were unable to experimentally validate these miRNA–mRNA interactions, particularly as it was EV miRNA that was assessed and not total mRNA.
Our results require prospective validation in larger cohorts, with EV miRNA analysis being repeated in each case before, during and after exacerbations rather than studying separate stable and exacerbating cohorts as here, so as to reduce extraneous biases. Furthermore, all stages of disease severity should be represented (as most of the cases reported here had GOLD stage severe or very severe disease).
Conclusion
This preliminary study identified a total of five miRNAs that were significantly differently packaged between participants with stable COPD and those experiencing an exacerbation, with miR-223-3p resulting in the highest AUC (0.755) for a single miRNA and the combination of miR-106b-3p, miR-374a-5p and miR-92b-3p further improving discriminatory power (AUC 0.820). One miRNA (miR-374b-5p) was significantly over-expressed in severe/very severe GOLD stage COPD participants compared to moderate GOLD stage COPD participants (AUC 0.798).
These results highlight the biomarker potential that EV bioactive cargo (miRNA) within plasma holds for defining and characterising exacerbations and disease severity in COPD. This warrants further investigation in larger participant cohorts to verify the translational potential. Future studies could explore the effects on EV production and the selective packaging of miRNAs due to treatments such as corticosteroids, antibiotics and mucolytics.
Ethics Statement
The study was conducted in accordance with the principles of the Declaration of Helsinki. Protocols and patient recruitment were approved by the Human Research Ethics Committee for the Metro North Hospital and Health Service (HREC/15/QPCH/259, HREC/17/QPCH/54 and LNR/2019/QPCH/52409) and The University of Queensland (2016000248 and 2019001147). All patients provided written informed consent.
Acknowledgments
The authors acknowledge the participants for their donation for this study and the staff of The Prince Charles Hospital. The authors would also like to acknowledge Dr Matthias Floetenmeyer at the Centre for Microscopy and Microanalysis, The University of Queensland, for assistance with Electron Microscopy.
Disclosure
Professor Kwun M Fong reports grants from NHMRC/MRFF, CCQ, ACRF, and TPCH Foundation; non-financial support from MeVis Veolity and Olympus, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(6):1304–1309. doi:10.1164/ajrccm.163.6.2009116
2. Cosio Piqueras MG, Cosio MG. Disease of the airways in chronic obstructive pulmonary disease. Eur Respir J. 2001;18(34 suppl):41s. doi:10.1183/09031936.01.00234601
3. Organization WH. Chronic obstructive pulmonary disease (COPD). Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
4. WHO. COPD predicted to be third leading cause of death in 2030. Available from: http://www.who.int/respiratory/copd/World_Health_Statistics_2008/en/.
5. GOLD. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Apr2.pdf.
6. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi:10.1164/rccm.200703-456SO
7. GOLD. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease - 2020 Report. Available from: https://goldcopd.org/wp-content/uploads/2020/03/GOLD-2020-POCKET-GUIDE-ver1.0_FINAL-WMV.pdf.
8. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
9. Miller BE, Tal-Singer R, Rennard SI, et al. Plasma Fibrinogen Qualification as a Drug Development Tool in Chronic Obstructive Pulmonary Disease. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Am J Respir Crit Care Med. 2016;193(6):607–613. doi:10.1164/rccm.201509-1722PP
10. Keene JD, Jacobson S, Kechris K, et al. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med. 2017;195(4):473–481. doi:10.1164/rccm.201607-1330OC
11. Benedikter BJ, Wouters EFM, Savelkoul PHM, Rohde GGU, Stassen FRM. Extracellular vesicles released in response to respiratory exposures: implications for chronic disease. J Toxicol Environ Health. 2018;1–19. doi:10.1080/10937404.2018.1466380
12. El Andaloussi S, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Perspective. Nat Rev Drug Discov. 2013;12:347. doi:10.1038/nrd3978
13. O’Farrell HE, Yang IA. Extracellular vesicles in chronic obstructive pulmonary disease (COPD). J Thorac Dis. 2019;11(Suppl 17):S2141–S2154. doi:10.21037/jtd.2019.10.16
14. Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17–24. doi:10.1016/j.gpb.2015.02.001
15. Muth DC, Powell BH, Zhao Z, Witwer KW. miRNAs in platelet-poor blood plasma and purified RNA are highly stable: a confirmatory study. BMC Res Notes. 2018;11(1):273. doi:10.1186/s13104-018-3378-6
16. Nolte-’t Hoen ENM, Buermans HPJ, Waasdorp M, Stoorvogel W, Wauben MHM. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):9272–9285. doi:10.1093/nar/gks658
17. Pattarayan D, Thimmulappa RK, Ravikumar V, Rajasekaran S. Diagnostic potential of extracellular microRNA in respiratory diseases. Clin Rev Allergy Immunol. 2018;54(3):480–492. doi:10.1007/s12016-016-8589-9
18. O’Farrell HE, Bowman RV, Fong KM, Yang IA. Plasma extracellular vesicle miRNAs can identify lung cancer, current smoking status, and stable COPD. Int J Mol Sci. 2021;22(11):5803. doi:10.3390/ijms22115803
19. Yao B, Qu S, Hu R, et al. A panel of miRNAs derived from plasma extracellular vesicles as novel diagnostic biomarkers of lung adenocarcinoma. FEBS Open Bio. 2019;9(12):2149–2158. doi:10.1002/2211-5463.12753
20. O’Farrell HE, Shaw JG, Goh F, et al. Potential clinical utility of multiple target quantitative polymerase chain reaction (qPCR) array to detect microbial pathogens in patients with chronic obstructive pulmonary disease (COPD). J Thorac Dis. 2019;11(Suppl 17):S2254–s2265. doi:10.21037/jtd.2019.10.39
21. Zhao J, Cheng W, He X, et al. Chronic obstructive pulmonary disease molecular subtyping and pathway deviation-based candidate gene identification. Cell J. 2018;20(3):326–332. doi:10.22074/cellj.2018.5412
22. Bi H, Zhou J, Wu D, et al. Microarray analysis of long non-coding RNAs in COPD lung tissue. Inflammation Res. 2015;64(2):119–126. doi:10.1007/s00011-014-0790-9
23. Huang X, Li Y, Guo X, et al. Identification of differentially expressed genes and signaling pathways in chronic obstructive pulmonary disease via bioinformatic analysis. FEBS Open Bio. 2019;9(11):1880–1899. doi:10.1002/2211-5463.12719
24. Savarimuthu Francis SM, Davidson MR, Tan ME, et al. MicroRNA-34c is associated with emphysema severity and modulates SERPINE1 expression. BMC Genomics. 2014;15:88. doi:10.1186/1471-2164-15-88
25. De Smet EG, Mestdagh P, Vandesompele J, Brusselle GG, Bracke KR. Non-coding RNAs in the pathogenesis of COPD. Thorax. 2015;70(8):782. doi:10.1136/thoraxjnl-2014-206560
26. Salimian J, Mirzaei H, Moridikia A, Harchegani AB, Sahebkar A, Salehi H. Chronic obstructive pulmonary disease: microRNAs and exosomes as new diagnostic and therapeutic biomarkers. J Res Med Sci. 2018;23:27. doi:10.4103/jrms.JRMS_1054_17
27. Fujita Y, Araya J, Ito S, et al. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J Extracellular Vesicles. 2015;4:28388. doi:10.3402/jev.v4.28388
28. Ezzie ME, Crawford M, Cho J-H, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67(2):122. doi:10.1136/thoraxjnl-2011-200089
29. Yuan X, Berg N, Lee JW, et al. MicroRNA miR-223 as regulator of innate immunity. J Leukoc Biol. 2018;104(3):515–524. doi:10.1002/jlb.3mr0218-079r
30. Roffel MP, Bracke KR, Heijink IH, Maes T. miR-223: a key regulator in the innate immune response in asthma and COPD. Front Med. 2020;7:196. doi:10.3389/fmed.2020.00196
31. Hageman GJ, Larik I, Pennings HJ, Haenen GR, Wouters EF, Bast A. Systemic poly(ADP-ribose) polymerase-1 activation, chronic inflammation, and oxidative stress in COPD patients. Free Radic Biol Med. 2003;35(2):140–148. doi:10.1016/s0891-5849(03)
32. Ghonim MA, Pyakurel K, Ibba SV, et al. PARP is activated in human asthma and its inhibition by olaparib blocks house dust mite-induced disease in mice. Clin Sci. 2015;129(11):951–962. doi:10.1042/cs20150122
33. Tan DBA, Armitage J, Teo T-H, Ong NE, Shin H, Moodley YP. Elevated levels of circulating exosome in COPD patients are associated with systemic inflammation. Respir Med. 2017;132:261–264. doi:10.1016/j.rmed.2017.04.014
34. McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci. 2002;27(1):40–47. doi:10.1016/s0968-0004(01)
35. Zhu HX, Shi L, Zhang Y, et al. Myocyte enhancer factor 2D provides a cross-talk between chronic inflammation and lung cancer. J Transl Med. 2017;15(1):65. doi:10.1186/s12967-017-1168-x
36. Natanek SA, Gosker HR, Slot IG, et al. Pathways associated with reduced quadriceps oxidative fibres and endurance in COPD. Eur Respir J. 2013;41(6):1275–1283. doi:10.1183/09031936.00098412
37. Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58(9):752–756. doi:10.1136/thorax.58.9.752
38. Troosters T, Probst VS, Crul T, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(10):1072–1077. doi:10.1164/rccm.200908-1203OC
39. Wouters EFM, Creutzberg EC, Schols AMWJ. Systemic effects in COPD. Chest. 2002;121(5 Supplement):127S–130S. doi:10.1378/chest.121.5_suppl.127S
40. Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118(11):3546–3556. doi:10.1172/JCI36130
41. Siva R, Green RH, Brightling CE, et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29(5):906. doi:10.1183/09031936.00146306
42. Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi:10.1056/NEJMoa032158
43. Fujimoto K, Yasuo M, Urushibata K, Hanaoka M, Koizumi T, Kubo K. Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J. 2005;25(4):640–646. doi:10.1183/09031936.05.00047504
44. Ding M, Wang C, Lu X, et al. Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling. Anal Bioanal Chem. 2018;410(16):3805–3814. doi:10.1007/s00216-018-1052-4
45. Andreu Z, Rivas E, Sanguino-Pascual A, et al. Comparative analysis of EV isolation procedures for miRNAs detection in serum samples. J Extracellular Vesicles. 2016;5(1):31655. doi:10.3402/jev.v5.31655
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.