Back to Journals » Journal of Inflammation Research » Volume 15
Peri-Ulcerative Mucosal Inflammation Appearance is an Independent Risk Factor for 30-Day Rebleeding in Patients with Gastric Ulcer Bleeding: A Multicenter Retrospective Study
Authors Bai Y , Lei C, Zhang N, Liu Y, Hu Z , Li Y, Qi R
Received 16 June 2022
Accepted for publication 12 August 2022
Published 30 August 2022 Volume 2022:15 Pages 4951—4961
DOI https://doi.org/10.2147/JIR.S378263
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Yixuan Bai,1 Chenggang Lei,2 Na Zhang,1 Yuhui Liu,1 Zhengyu Hu,3 Yan Li,4 Ran Qi5
1Department of Digestive Internal Medicine, Affiliated Dalian Friendship Hospital of Dalian Medical University, Dalian, People’s Republic of China; 2Department of Hepatobiliary Surgery, Qingdao Municipal Hospital, Qingdao, People’s Republic of China; 3Department of General Surgery, Shanghai Tenth People’s Hospital, Affiliated to Tongji University School of Medicine, Shanghai, People’s Republic of China; 4Department of Gastroenterology, Shanghai Tenth People’s Hospital, Affiliated to Tongji University School of Medicine, Shanghai, People’s Republic of China; 5Department of General Surgery, Tongji Hospital of Tongji University, School of Medicine, Tongji University, Shanghai, People’s Republic of China
Correspondence: Ran Qi, Department of General Surgery, Tongji Hospital of Tongji University, School of Medicine, Tongji University, 389 Xincun Road, Putuo District, Shanghai, 200092, People’s Republic of China, Email [email protected]
Aim: The aim of this study was to identify clinical endoscopic indicators related to peri-ulcerative mucosal inflammation and to analyze whether the degree of peri-ulcerative mucosal inflammation appearance is an independent risk factor for gastric ulcer rebleeding.
Methods: We conducted a retrospective study that included patients with gastric ulcer bleeding who were hospitalized at three medical centers in China from January 1, 2016 to December 31, 2019. Ulcer rebleeding that occurred within 30 days of successful initial hemostasis was analyzed to determine whether this event was related to the degree of peri-ulcerative mucosal inflammation appearance or other mucosal inflammation-related factors.
Results: We enrolled 1111 patients and determined that GBS-Rebleeding-ROC (P< 0.001), age (P=0.01), use of NSAIDs (P=0.001), bile reflux (P< 0.001), and Helicobacter pylori (P< 0.001) are all risk factors for peri-ulcerative mucosal inflammation appearance. Through multivariate analysis, we determined that severe peri-ulcerative mucosal inflammation appearance (P=0.002) was an independent risk factor for ulcer rebleeding within 30 days. Finally, we developed a risk assessment model using factors associated with mucosal inflammation that may be useful for early prediction of rebleeding.
Conclusion: The risk factors for peri-ulcerative mucosal inflammation appearance were identified. Severe peri-ulcerative mucosal inflammation appearance is an independent risk factor for ulcer rebleeding.
Keywords: gastric ulcer, peptic ulcer bleeding, risk factors, rebleeding, peri-ulcerative mucosal inflammation
Introduction
Upper gastrointestinal bleeding is a common cause of hospitalization in internal medicine, resulting in significant medical expenses each year. According to previous investigations, peptic ulcers, including gastric ulcers and duodenal ulcers, remain the leading cause of upper gastrointestinal bleeding.1 Due to the widespread use of proton pump inhibitors (PPIs), the prognosis for patients with ulcers and associated upper gastrointestinal bleeding has improved significantly. With the improvement of endoscopic hemostasis technology, the success rate of endoscopic hemostasis has increased as well; however, 7.7–20% of patients still experience rebleeding after successful hemostasis with endoscopic therapy or drugs. This may lead to a significant increase in mortality.2,3 In addition, the aging population and complex comorbidities of patients in some countries may aggravate the poor prognosis of upper gastrointestinal bleeding;4 however, the specific cause is not clear. Therefore, determining independent risk factors for ulcer rebleeding is helpful to more accurately predict the prognosis for patients in early stages of this condition. This can be very useful and will aid clinicians in developing the appropriate treatment programs.
A large number of studies have identified some independent risk factors that affect the prognosis of patients with peptic ulcer bleeding. These include large ulcers, the diameter of exposed vessels, anatomical location, treatment methods, and some concomitant diseases.5–10 Previous research has also established a variety of scoring systems in an attempt to assess the prognosis of patients more accurately.11,12 However, the previous research results still cannot allow clinicians to perfectly predict the prognosis of patients with ulcer bleeding.
An ulcer occurs from damage of the digestive tract mucosa and weakened mucosal barrier function which was caused by various factors.13,14 Factors associated with an injury that lead to ulcer formation and interfere with ulcer healing are likely to be causes of ulcer rebleeding. The degree of peri-ulcerative inflammation can reflect the damage and recovery state of the ulcer surface and may be identified as a predictor of ulcer rebleeding. Until now, there is no relevant research or reports on this subject. We conducted this multicenter retrospective study to determine the significance of mucosal inflammation in the evaluation of ulcer rebleeding.
Materials and Methods
Study Population
We conducted a retrospective study involving continuous patients from the Department of Gastroenterology of Qingdao Municipal Hospital, Friendship Hospital affiliated to Dalian Medical University, and Tongji Hospital of Tongji University from January 1, 2016, to December 31, 2019. Gastroscopy was performed within 48 hours of admission and confirmed that the cause of upper gastrointestinal bleeding is gastric ulcer. All patients were given a high-dose esomeprazole regimen (80 mg IV +8 mg/hour continuous infusion for 72 hours), then changed to a standard dose (40 mg) PPI IV twice a day for 3–5 days. This was followed by a standard oral dose until the ulcer was healed.15
Data were collected from inpatient records, endoscopic reports, and follow-up records. The data included the patient mucosal inflammation status indicators (Degree of peri-ulcerative mucosal inflammation appearance and type of gastritis), mucosal injury factors (NSAIDs, Helicobacter pylori, alcohol consumption, bile reflux), and other data such as endoscopic treatment status, age, Glasgow-Blatchford Score (GBS), length of hospital stay, and whether patients experienced rebleeding or death within one month after discharge. The collected data were used to analyze potential risk factors for ulcer rebleeding. After GBS was collected, the ROC curve was used to determine the cutoff value and the two-classification was performed.
Inclusion Criteria
Inclusion criteria for this study were as follows:
- Age greater than or equal to 18
- Signs or symptoms of upper gastrointestinal bleeding
- Confirmed by endoscopy that gastric ulcer bleeding is the cause of upper gastrointestinal bleeding
Exclusion Criteria
Patients that met any of the following criteria were excluded from the study:
- Patients with advanced malignant tumors at the time of admission
- Patients with mental disorders who cannot cooperate with treatment
- Patients with a history of gastric, duodenal, or bile duct surgery
- Patients with a history of gastric cancer or esophageal varices.
- Patients with a history of severe coagulopathy disease.
- Incomplete data or lost to follow-up (34 cases)
Definitions
Upper gastrointestinal bleeding refers to signs or symptoms of upper gastrointestinal bleeding (hematemesis, melanorrhea, or hemoglobin drop of ≥ 2 g/dL from baseline), and or hypovolemic shock. This standard is also used for rebleeding.
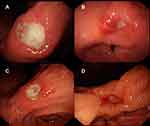
Peri-ulcerative mucosal inflammation appearance refers to the degree of mucosal inflammation within 2 cm of the ulcer edge.
Mild: The mucosa is smooth and soft—with slight hyperemia and edema and without erosion and thickened gastric folds (Figure 1A).
Moderate: The mucosa is obviously hyperemic and edematous—with fibrinoid exudate, without erosion, and with or without thickened gastric folds (Figure 1B).
Severe: The mucosa is severely hyperemic, edematous, and brittle—with fibrinoid exudate and thickened gastric folds and with or without erosion (Figure 1C). The mucosa is obviously hyperemic, edematous, and brittle—with fibrinoid exudate, erosion, and thickened gastric folds (Figure 1D).
Severity grading was confirmed by three associate chief physicians (Yixuan Bai, Yuhui Liu and Na Zhang). All three physicians are skilled in endoscopic procedures and used the same diagnostic criteria.
Helicobacter pylori infection is confirmed due to (1) rapid urease test or (2) histology (biopsies from two sites in the greater curvature of the pyloric antrum and upper-middle part of the gastric corpus) positivity.16
Atrophic gastritis refers to typical endoscopic features, including pale appearance of gastric mucosa, increased visibility of vasculature due to thinning of the gastric mucosa, and loss of gastric folds, and was confirmed by histopathology.17
Bile reflux refers to the mucus lake bile staining or bile spot block when the endoscope is introduced into the gastric cavity.18
Statistical Analysis
All statistical analyses were performed using R 4.0.5 (University of Auckland, NZ). A synthetic minority over-sampling technique (SMOTE) was used to solve the imbalance problem. The Shapiro–Wilk normality test was performed to determine the sample normality. For data that met the normal distribution, t-test or one-way analysis of variance was selected for comparison of differences between groups. For data that did not meet the normal distribution, the Mann–Whitney U-test or the Kruskal–Wallis H-test was selected for comparison of differences between groups. The counted data were compared using Pearson’s chi-square, continuity correction chi-squared test, and Fisher’s exact test.
The influencing factors of P<0.05 in the univariate analysis were also included in the multivariate stepwise logistic regression analysis. The “rms” package was used to construct a rebleeding risk nomogram prediction model. Statistical robustness of the prediction model was evaluated with 1000-fold bootstrapping. The area under the ROC curve (AUC) was used to evaluate the discrimination of the prediction model. The calibration curve and Brier score were used to evaluate the model calibration degree. The sensitivity and specificity of the Yorden index were used to evaluate the application value of the predictive model.
Univariate and multiple stepwise logistic regression were used to analyze the influencing factors of peri-ulcerative mucosal inflammation appearance. Univariate and multivariate analysis were used to evaluate the risk factors that may be related to rebleeding. Stepwise linear regression analysis was used to analyze the influencing factors of hospital stay. P<0.05 was considered statistically significant.
Results
Characteristics of the Study Population
Data on a total of 1111 hospitalized patients were collected from January 1, 2016 to December 31, 2019. Endoscopy was used in all patients to confirm that upper gastrointestinal bleeding was caused by gastric ulcer. Among all cases, there were 425 cases at Tongji Hospital, 236 cases at Dalian Friendship Hospital, and 450 cases at Qingdao Municipal Hospital. The basic clinical characteristics of these cases are shown in Table 1. There were 768 males and 343 females, with an average age of 64.3±17.1 years. Among these, there were 190 patients taking NSAIDs, 106 cases (55.79%) of heart diseases, 41 cases (21.58%) with a history of stroke, 32 cases (16.84%) of inflammatory diseases, 6 cases (3.16%) of rheumatism, and 5 cases of pain or other diseases (2.63%). Of the total, 273 patients successfully stopped bleeding through endoscopic treatment and 255 (93.41%) of these patients did not bleed again within 30 days. Among the 195 patients who reported regular alcohol consumption, 180 (92.31%) were male and 29 (14.87%) had an age of greater than 60. The incidence of rebleeding within 30 days of patients with gastric ulcer bleeding was 4.68% (52). The overall mortality rate was 0.63% (7/1111), of which 71.43% (5/7) patients had rebleeding within 30 days. In hospitalized patients with GBS ≤ 1, there were zero incidences of either rebleeding or death within 30 days.
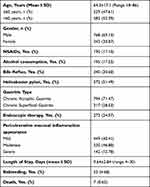 |
Table 1 Baseline Clinical Characteristics of Study Patients (N=1111) |
Parameters Associated with Mucosal Inflammation
We recorded the degree of peri-ulcerative mucosal inflammation appearance and possible influencing factors. Through univariate analysis (Table S1), we found that age (P=0.01), use of NSAIDs (P=0.001), bile reflux (P<0.001), H. pylori (P<0.001), type of gastritis (P<0.001) and GBS-Rebleeding-ROC (P<0.001) were significantly related to the degree of peri-ulcerative mucosal inflammation appearance. The multivariate binary logistic regression analysis by stepwise method was used to detect the influencing factors of moderate inflammation and severe inflammation. Mild inflammation was used as the reference (Table 2).
 |
Table 2 Moderate and Severe Peri-Ulcerative Mucosal Inflammation Appearance Was Analyzed with Mild as Reference |
Independent Risk Factors for 30-Day Ulcer Rebleeding Associated with Mucosal Inflammation
In order to detect the relationship between mucosal inflammation and ulcer rebleeding, the parameters related to gastric mucosal inflammation and 30-day rebleeding were assessed by univariate analysis. The results are shown in Table 3. Except for the type of gastritis (P=0.515) and gender (P=0.086), all other indicators were found to be statistically significantly related to ulcer rebleeding. The factors with statistically significant differences in the univariate analysis were subjected to binary logistic regression analysis. These results are shown in Table 4. Independent risk factors for rebleeding included severe peri-ulcerative mucosal inflammation appearance (P=0.002), being female (P=0.033), age greater than 60 (P=0.001), use of NSAIDs (P=0.004), alcohol consumption (P<0.001), H. pylori (P<0.001) and GBS-Rebleeding-ROC (P<0.001). A nomogram prediction model was established (Figure 2) and the critical value of the nomogram was 241.381. The area under the AUROC curve was 0.767, the sensitivity was 64.7%, the specificity was 77.7%. The accuracy is good, and the calibration plot fits well, as shown in Figure 3. Multiple linear regressions of hospital stay showed that age>60, use of NSAIDs, H. pylori, and severe peri-ulcerative mucosal inflammation appearance were significant influencing factors with a longer length of stay (Table S2).
 |
Table 3 Single Factor Analysis of Ulcer Rebleeding After SMOTE |
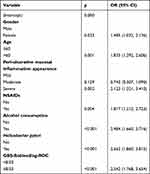 |
Table 4 Multivariate Binary Logistic Regression Analysis of Ulcer Rebleeding |
Discussion
In this study, we used univariate and multivariate analysis to determine the degree of peri-ulcerative inflammation within 2 cm around the ulcer and if this is significantly related to the poor prognosis of gastric ulcer bleeding. Our study also confirmed the risk factors associated with peri-ulcerative inflammation. Although the different types of gastritis and bile reflux can affect the degree of peri-ulcerative mucosal inflammation appearance in these patients, our results do not support these two indicators as predictors of the prognosis of ulcer bleeding.
The past decade has been marked by widespread use of PPIs and the improvement of endoscopic hemostasis technology. Because of this, the mortality rate of patients with upper gastrointestinal bleeding has dropped significantly to 0.5% in China3 and 1.9% in the United States.1 This is compared to the previously reported 4.5–5.4%.19,20 The mortality rate in our study was found to be 0.63%, which is consistent with recently reported data. Compared to the significantly reduced mortality rate, the rate of ulcer rebleeding does not seem to improve significantly. We found that the incidence of ulcer rebleeding was 4.68%, which was similar to the 7.72% previously reported by Bai et al.3 At this point, additional data were needed to determine the adverse prognostic factors of ulcer bleeding and to stratify patients more accurately, which may reduce the incidence of rebleeding and death.
In this study, the ratio of male to female patients was 2.24 and the incidence of ulcer bleeding in males was higher than that in females. Males reported a significantly higher incidence of peptic ulcer bleeding (PUB).21 After gender analysis, our results showed that the risk of ulcer rebleeding for males was statistically different from that of females (P=0.033). Similarly, the results of van Leerdam et al22 also showed that females have a higher risk of rebleeding and death from PUB. It is possible that females have some gender-specific unknown risk factors for ulcer rebleeding.
A peptic ulcer develops from mucosal barrier destruction that is caused by the imbalance of mucosal repair and damage. Although the bleeding state of the ulcer has been graded,23 it is not possible to determine which side of the ulcer mucosa is damaged or healing. The use of PPIs eliminates gastric acid, the most important factor of mucosal damage. However, inflammation in this area did not resolve immediately, which may cause ulcers to heal slowly or lead to rebleeding. Erosion lesions are a manifestation of mucosal inflammation and it is reported to be present in 45% of the ulcerative peptic disease.24 The further development of erosive lesions can lead to ulcers and directly cause upper gastrointestinal bleeding.25 In addition to erosive lesions, other manifestations of mucosal inflammation include hyperemia and edema. Due to telangiectasia and congestion, nutrient and metabolic wastes are not transported efficiently, resulting in slower tissue healing. Massive inflammatory cell infiltration leads to cell damage or tissue edema through direct cytotoxicity or release of cytokines. Furthermore, telangiectasia and edema can make tissue brittle, thus increasing the risk of bleeding. Through endoscopic manifestations, we integrated all the characteristics of mucosal inflammation and graded the degree of mucosal inflammation appearance around the ulcer into three categories: mild, moderate, and severe. We considered the mucous membrane within a 2-cm ring around the ulcer to have the same injury factors as the ulcer surface and have the same tendency to remain damaged or to heal. Multivariate analysis showed that severe peri-ulcerative mucosal inflammation appearance was a significant risk factor for gastric ulcer rebleeding (P=0.002).
We analyzed the factors affecting mucosal inflammation and established an inflammation scoring system. The inflammation score appeared to be a valuable method to predict ulcer rebleeding. Most of the parameters of this score can be quickly obtained through endoscopy and medical history record, which is helpful for clinicians to assess the prognosis of patients in the early phases of disease. The clinical predictive effectiveness of this score still needs to be further verified with additional studies.
The GBS is the most widely used pre-hospital score to assess the prognosis of patients with PUB.26–28 GBS includes the patients’ hemodynamic characteristics, along with complications of the liver and heart. As with age, GBS was included in the study because it is considered to affect the patient’s ability to recover from mucosal inflammation. The predictive effect of GBS on ulcer rebleeding is controversial. A recent, international, multi-center study has shown that GBS helps predict rebleeding.29 However, Wang et al30 and et al31 compared multiple score systems and found that GBS did not have good performance in predicting rebleeding and 30-day mortality. After multivariate analysis, our results show that a high GBS is a significant factor related to ulcer rebleeding, which may be helpful for the prediction of ulcer rebleeding. The latest European guidelines recommend that patients with GBS ≤ 1 be managed through outpatient endoscopy.32 Similarly, our results showed that in patients with a GBS ≤ 1, the incidence of both rebleeding and death were 0.
The length of hospital stay is an important indicator used to evaluate the recovery of patients. Previous studies suggest that GBS and AIMS65 help predict the length of hospital stay.33 Our results found no statistical difference between the length of hospital stay and the GBS (P=0.073). Similarly, the international, multi-center study conducted by Stanley et al29 compared multiple scoring systems and concluded that none of them helped predict the length of hospital stay. Our results suggest that age > 60, use of NSAIDs, H. pylori infection, and severe peri-ulcerative mucosal inflammation appearance are all associated with longer hospital stay. This is important to consider in clinical practice.
There are also certain limitations of this research. First, we did not perform a statistical analysis of mucosal inflammation and death of ulcer patients, since the number of deaths in patients with ulcer bleeding is insufficient. Second, we only analyzed the risk factors of gastric ulcer bleeding, and the analysis results cannot be applied to cases of duodenal ulcer or other causes of upper gastrointestinal bleeding. Third, this study only focuses on the perspective of mucosal inflammation and did not include all factors known to influence ulcer rebleeding.
Conclusion
In summary, our study is the first to prove that the degree of peri-ulcerative mucosal inflammation appearance is an independent risk factor for rebleeding within 30 days of gastric ulcer bleeding. The degree of peri-ulcerative mucosal inflammation appearance can be quickly evaluated and recorded during endoscopy. According to these results, endoscopists may assess the patients’ conditions more quickly and intuitively during endoscopic treatment, and choose aggressive treatment or conservative treatment appropriately. In addition, among the factors that affect ulcer mucosal inflammation, gender, age, use of NSAIDs, H. pylori infection, and GBS are independent risk factors for rebleeding. Considering these risk factors can help clinicians to predict a patient’s prognosis more accurately.
Research Ethics and Consent
This retrospective study was reviewed and approved by the Qingdao Municipal Hospital Medical Ethics Committee (No. 2021-106), Ethics Committee of Shanghai Tongji Hospital (K-2021-014), and Medical Ethics Committee of Dalian Friendship Hospital (YY-LL-2021-046). The informed consent was obtained from the study participants. The guidelines outlined in the Declaration of Helsinki were followed throughout the research process.
Funding
There is no funding to report.
Disclosure
The authors declare no competing interests.
References
1. Wuerth BA, Rockey DC. Changing epidemiology of upper gastrointestinal hemorrhage in the last decade: a nationwide analysis. Dig Dis Sci. 2018;63(5):1286–1293. doi:10.1007/s10620-017-4882-6
2. Jairath V, Kahan BC, Logan RFA, et al. National audit of the use of surgery and radiological embolization after failed endoscopic haemostasis for non-variceal upper gastrointestinal bleeding. Br J Surg. 2012;99(12):1672–1680. doi:10.1002/bjs.8932
3. Bai Y, Du YQ, Wang D, et al. Peptic ulcer bleeding in China: a multicenter endoscopic survey of 1006 patients. J Dig Dis. 2014;15(1):5–11. doi:10.1111/1751-2980.12104
4. Fujishiro M, Iguchi M, Kakushima N, et al. Guidelines for endoscopic management of non-variceal upper gastrointestinal bleeding. Dig Endosc. 2016;28(4):363–378. doi:10.1111/den.12639
5. Camus M, Jensen DM, Kovacs TO, et al. Independent risk factors of 30-day outcomes in 1264 patients with peptic ulcer bleeding in the USA: large ulcers do worse. Aliment Pharmacol Ther. 2016;43(10):1080–1089. doi:10.1111/apt.13591
6. Ishikawa S, Inaba T, Wato M, et al. Exposed blood vessels of more than 2 mm in diameter are a risk factor for rebleeding after endoscopic clipping hemostasis for hemorrhagic gastroduodenal ulcer. Dig Endosc. 2013;25(1):13–19. doi:10.1111/j.1443-1661.2012.01333.x
7. de Groot NL, van Oijen MGH, Kessels K, et al. Reassessment of the predictive value of the Forrest classification for peptic ulcer rebleeding and mortality: can classification be simplified? Endoscopy. 2014;46(1):46–52. doi:10.1055/s-0033-1344884
8. Suceveanu AI, Suceveanu A-P, Parepa I, et al. Reducing upper digestive bleeding risk in patients treated with direct oral anticoagulants and concomitant infection with Helicobacter pylori. Exp Ther Med. 2020;20(6):205. doi:10.3892/etm.2020.9335
9. Lazăr DC, Ursoniu S, Goldiş A. Predictors of rebleeding and in-hospital mortality in patients with nonvariceal upper digestive bleeding. World J Clin Cases. 2019;7(18):2687–2703. doi:10.12998/wjcc.v7.i18.2687
10. Matsuhashi T, Fukuda S, Abe Y, et al. The nature and treatment outcomes of bleeding post-bulbar duodenal ulcers. Dig Endosc. 2021;34:984–993.
11. Monteiro S, Gonçalves TC, Magalhães J, Cotter J. Upper gastrointestinal bleeding risk scores: who, when and why? World J Gastrointest Pathophysiol. 2016;7(1):86–96. doi:10.4291/wjgp.v7.i1.86
12. Saltzman JR, Tabak YP, Hyett BH, et al. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. 2011;74(6):1215–1224. doi:10.1016/j.gie.2011.06.024
13. Holle GE. Pathophysiology and modern treatment of ulcer disease. Int J Mol Med. 2010;25(4):483–491. doi:10.3892/ijmm_00000368
14. Calam J, Baron JH. ABC of the upper gastrointestinal tract: pathophysiology of duodenal and gastric ulcer and gastric cancer. BMJ. 2001;323(7319):980–982. doi:10.1136/bmj.323.7319.980
15. Bai Y, Li ZS. Guidelines for the diagnosis and treatment of acute non-variceal upper gastrointestinal bleeding (2015, Nanchang, China). J Dig Dis. 2016;17(2):79–87. doi:10.1111/1751-2980.12319
16. Kato M, Ota H, Okuda M, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter. 2019;24(4):e12597. doi:10.1111/hel.12597
17. Shah SC, Piazuelo MB, Kuipers EJ, et al. AGA clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology. 2021;161(4):1325–1332. doi:10.1053/j.gastro.2021.06.078
18. Chen L, Zhu G, She L, et al. Analysis of risk factors and establishment of a prediction model for endoscopic primary bile reflux: a single-center retrospective study. Front Med. 2021;8:758771. doi:10.3389/fmed.2021.758771
19. Barkun A, Sabbah S, Enns R, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol. 2004;99(7):1238–1246. doi:10.1111/j.1572-0241.2004.30272.x
20. Marmo R, Koch M, Cipolletta L, et al. Predictive factors of mortality from nonvariceal upper gastrointestinal hemorrhage: a multicenter study. Am J Gastroenterol. 2008;103(7):1639–1647. doi:10.1111/j.1572-0241.2008.01865.x
21. Karaman A, Baskol M, Gursoy S, et al. Epinephrine plus argon plasma or heater probe coagulation in ulcer bleeding. World J Gastroenterol. 2011;17(36):4109–4112. doi:10.3748/wjg.v17.i36.4109
22. van Leerdam ME, Vreeburg EM, Rauws EAJ, et al. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98(7):1494–1499. doi:10.1111/j.1572-0241.2003.07517.x
23. Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2(7877):394–397. doi:10.1016/S0140-6736(74)91770-X
24. Pippa G, Apuzzo M, Bazuro ME, et al. Epidemiological study of duodenal erosive disease; its prevalence and nosological position in relation to ulcerative peptic disease. Scand J Gastroenterol Suppl. 1989;167:32–35. doi:10.3109/00365528909091307
25. Gimiga N, Olaru C, Diaconescu S, et al. Upper gastrointestinal bleeding in children from a hospital center of Northeast Romania. Minerva Pediatr. 2016;68(3):189–195.
26. Mullady DK, Wang AY, Waschke KA. AGA clinical practice update on endoscopic therapies for non-variceal upper gastrointestinal bleeding: expert review. Gastroenterology. 2020;159(3):1120–1128. doi:10.1053/j.gastro.2020.05.095
27. Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the International Consensus Group. Ann Intern Med. 2019;171(11):805–822. doi:10.7326/M19-1795
28. Lau JYW, Yu Y, Tang RSY, et al. Timing of endoscopy for acute upper gastrointestinal bleeding. N Engl J Med. 2020;382(14):1299–1308. doi:10.1056/NEJMoa1912484
29. Stanley AJ, Laine L, Dalton HR, et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. doi:10.1136/bmj.i6432
30. Wang CH, Chen Y-W, Young Y-R, et al. A prospective comparison of 3 scoring systems in upper gastrointestinal bleeding. Am J Emerg Med. 2013;31(5):775–778. doi:10.1016/j.ajem.2013.01.007
31. Liu S, Zhang X, Walline JH, et al. Comparing the performance of the ABC, AIMS65, GBS, and pRS scores in predicting 90-day mortality or rebleeding among emergency department patients with acute upper gastrointestinal bleeding: a prospective multicenter study. J Transl Int Med. 2021;9(2):114–122. doi:10.2478/jtim-2021-0026
32. Gralnek IM, Stanley AJ, Morris AJ, et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) guideline - update 2021. Endoscopy. 2021;53(3):300–332. doi:10.1055/a-1369-5274
33. Abougergi MS, Charpentier JP, Bethea E, et al. A prospective, multicenter study of the AIMS65 score compared with the Glasgow-Blatchford score in predicting upper gastrointestinal hemorrhage outcomes. J Clin Gastroenterol. 2016;50(6):464–469. doi:10.1097/MCG.0000000000000395
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.