Back to Journals » Patient Related Outcome Measures » Volume 14
Patient-Reported Outcomes in Ovarian Cancer: Facilitating and Enhancing the Reporting of Symptoms, Adverse Events, and Subjective Benefit of Treatment in Clinical Trials and Clinical Practice
Authors Campbell R , King MT, Stockler MR , Lee YC, Roncolato FT, Friedlander ML
Received 31 October 2022
Accepted for publication 25 April 2023
Published 8 May 2023 Volume 2023:14 Pages 111—126
DOI https://doi.org/10.2147/PROM.S297301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lynne Nemeth
Rachel Campbell,1 Madeleine T King,1 Martin R Stockler,2 Yeh Chen Lee,2– 4 Felicia T Roncolato,2,5 Michael L Friedlander3,4
1University of Sydney, Faculty of Science, School of Psychology, Sydney, NSW, Australia; 2University of Sydney, NHMRC Clinical Trials Centre, Sydney, NSW, Australia; 3School of Clinical Medicine, Faculty of Medicine and Health, University of New South Wales, Sydney, NSW, Australia; 4Department of Medical Oncology, Prince of Wales and Royal Hospital for Women, Sydney, NSW, Australia; 5MacArthur Cancer Therapy Centre, Campbelltown Hospital, Sydney, NSW, Australia
Correspondence: Rachel Campbell, University of Sydney, Room 325, Brennan-Maccallum Building, Sydney, NSW, 2006, Australia, Tel +61 2 8627 7631, Email [email protected]
Abstract: Patient-reported outcomes (PROs) provide a valid, standardized way of assessing symptoms, adverse events and the subjective benefit of treatment from the patient’s perspective. Assessment of PROs is critical in ovarian cancer due to the high morbidity of the disease and its treatments. Several well-validated PRO measures are available to assess PROs in ovarian cancer. Their inclusion in clinical trials can provide evidence on the benefits and harms of new treatments based on patients’ experiences to guide improvements in clinical practice and health policy. Aggregate PRO data collected in clinical trials can be used to inform patients about likely treatment impacts and assist them to make informed treatment decisions. In clinical practice, PRO assessments can facilitate monitoring of a patient’s symptoms throughout treatment and follow-up to guide their clinical management; in this context, an individual patient’s responses can facilitate communication with their treating clinician about troublesome symptoms and their impact on their quality of life. This literature review aimed to provide clinicians and researchers with a better understanding of why and how PROs can be incorporated into ovarian cancer clinical trials and routine clinical practice. We discuss the importance of assessing PROs throughout the ovarian cancer disease and treatment trajectory in both clinical trials and clinical practice, and provide examples from existing literature to illustrate the uses of PROs as the goals of treatment change in each setting.
Keywords: ovarian cancer, patient-reported outcomes, clinical trials, clinical practice
Introduction
Patient-reported outcomes (PROs), including health-related quality of life (HRQL), provide unique information and insight into the impact of disease and treatment from the perspective of patients. A PRO is “any report of a patient’s health status that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else”.1 PROs include symptoms associated with disease or treatment, functional outcomes (eg physical, cognitive, social or emotional) and multidimensional constructs such as HRQL. Assessing PROs is especially important in ovarian cancer due to the high morbidity associated with the disease and its treatments. The inclusion of PROs in ovarian cancer clinical trials can complement conventional clinical trial endpoints, which are typically progression-free survival and overall survival, by providing additional information on both adverse effects and subjective benefits of treatment which can inform clinical decision-making and guide health policy.2,3 PROs can also be used in routine clinical practice to monitor symptoms of ovarian cancer and its treatment, to guide the management of individual patients, and potentially to improve their outcomes.4 This literature review provides an overview of why and how PROs can be used in ovarian cancer to facilitate and enhance the reporting of symptoms, adverse events, and subjective benefit of treatment in both clinical trials and clinical practice.
The benefits of assessing PROs in ovarian cancer clinical trials and clinical practice are summarized in Box 1 and described in further detail below.
 |
Box 1 Benefits of Assessing PROs in Ovarian Cancer Clinical Trials versus Clinical Practice |
PROs in Clinical Trials
There is wide-spread encouragement from professional societies and regulatory bodies for including PRO endpoints in clinical trials.16 For example, the Food and Drug Administration (FDA) released guidance in 2009 on the use of PRO measures in clinical trials to support medical labelling claims.1 In addition, both the European Society for Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO) have proposed standardized approaches to defining the clinical benefit of new therapies to patients.17,18 These approaches use scores to evaluate the Magnitude of Clinical Benefit (ESMO MCB) or Net Health Benefit (ASCO) that in addition to survival endpoints also include PRO outcomes such as adverse effects and HRQL, assessed using validated PRO measures to assess the value of treatment.17,18 The ESMO MCB scores based on survival outcomes are upgraded if there is evidence to indicate either improved HRQL or delayed deterioration in HRQL or a substantial reduction in adverse events. Professional gynecological oncology societies also strongly encourage the inclusion of PROs in ovarian cancer clinical trials specifically to assess the subjective symptom benefit and adverse effects of treatment although their uptake has been limited.19,20
The inclusion of PRO endpoints in ovarian cancer clinical trials has several important benefits. First, it allows survival benefits to be interpreted in the context of patients’ lived experience of symptoms and impacts on quality of life – both positive and negative. This additional evidence enables clinicians and patients to make more informed decisions about treatment options, based on what other patients in similar situations have experienced and reported.5 The use of aggregate PRO data to inform clinical practice guidelines is important because there is well-documented discordance between clinician and patient-ratings of adverse treatment effects indicating patients are the best informants of their own symptoms.21,22 PRO data collected in clinical trials can enhance clinicians’ understanding of the experience of treatments from patients’ perspectives, helping them communicate realistic expectations about the likely impact of new treatments for ovarian cancer on daily living.5 Importantly, the value women with ovarian cancer place on different treatment outcomes is likely to vary, and the extent to which women will be willing to compromise their HRQL for possible survival gains will differ, particularly as the disease progresses, underscoring the potential benefit of including patient preference sub studies in clinical trials.23 PRO data may also be used to improve the methodology of clinical trials in general.5 For example, in some contexts PRO data has been shown to be an independent prognostic indicator of both progression-free survival and overall survival,6 suggesting PRO scores may be useful for trial stratification purposes or defining the inclusion criteria of trial populations.5 For example, patients with baseline PRO scores indicative of poor prognosis may not be suitable candidates for clinical trials but require supportive care and discussions on treatment decisions which include cessation of systemic therapy in the final weeks or months of life.
Traditionally PROs have most commonly been included as secondary endpoints in cancer clinical trials, typically assessed with HRQL questionnaires, and used to compare the impact of the experimental treatment vs standard of care on mean scores in HRQL. Most trials of maintenance therapy have evaluated the change from baseline in the Functional Assessment of Cancer Therapy – Ovarian Cancer (FACT-O) Trial Outcome Index (TOI) score versus placebo and have not shown any significant difference between the placebo/PARP inhibitor arm.24 There is increasing recognition that there are limitations with these analyses and efforts are being made to include additional PRO outcomes to evaluate the impact/benefit of treatments on patients.24
Despite increased awareness of the value of including PRO endpoints in ovarian cancer clinical trials, their inclusion and reporting to date has been sub-optimal with room for improvement.25–27 Issues identified with previous ovarian cancer clinical trials include a lack of clear pre-specified PRO hypotheses; PRO endpoints not included; insensitive PRO endpoint selection; collection of poor-quality PRO data not suitable for analysis; differences in PROs between treatment arms ignored; and poor quality reporting.25 These lost opportunities highlight the need for closer attention to the PRO components of future ovarian cancer clinical trials at all stages of the research process to ensure the collection and reporting of high-quality PRO data to inform patient-centered care. Kurtz et al emphasize this and collate guidance on how best to incorporate PROs into ovarian cancer clinical trials moving forward.
PROs in Clinical Practice
PROs can also be used at the point of care to guide the management of individual patients. Basch et al conducted a practice changing trial demonstrating weekly electronic PRO symptom monitoring with automated alerts to nurses/clinicians resulted in reduced emergency and hospital visits, improved HRQL, and conferred a 5-month increase in overall survival in outpatients receiving chemotherapy for advanced cancer.28,29 Another randomized trial by Denis et al corroborated these findings in patients with advanced lung cancer by demonstrating home-based electronic PRO symptom monitoring resulted in a 7-month median survival benefit.30 A potential reason for the findings in these trials is that prompt and effective management of patient symptoms through earlier initiation of supportive care can prevent adverse downstream consequences and enable patients to continue to receive cancer-directed therapies.29,31 Systematic reviews have also indicated several important benefits of assessing PROs in oncology clinical practice such as improved symptom control, patient-clinician communication and patient satisfaction.11,32,33 Together these findings provide compelling evidence for the potential value of routine PRO symptom monitoring in the clinical management of women with ovarian cancer as well as in clinical trials.
Typically, using PROs to guide the management of individual patients in routine care involves monitoring a patient’s PRO scores over time throughout treatment and follow-up to determine whether the patient’s PRO scores have changed sufficiently to warrant a change in their management. For example, individual PRO scores may be used to inform decisions around whether a patient requires supportive intervention, dose reduction or escalation, or should cease treatment. However, to facilitate this decision-making, clinicians need to be able to effectively interpret the meaning of changes in a patient’s PRO scores over time. This is challenging as it requires consideration of what degree of change represents real change as opposed to measurement error which can arise due to random or chance factors, or flaws in the assessment instrument,34 and may also be affected by recall bias.35 Thus, when tracking PRO scores over time, it is important to set thresholds that trigger alerts and action in clinic above the bounds of measurement error to avoid “false-positive changes” that do not reflect true change.36 Additional barriers to effective use of PROs in clinical practice include lack of integration of PROs into clinical workflow, inadequate information technology infrastructure to enable easy collection and use of PROs, and clinicians’ inability to action PRO data.37
PRO Measures for Ovarian Cancer
Several rigorously developed PRO measures with proven validity and reliability, developed in accordance with best-practice standards,1,38 are available to assess PROs in ovarian cancer.39 The two most widely used measures to date are both cancer-specific HRQL measures: the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30)40 and the Functional Assessment of Cancer Therapy-General (FACT-G)41 Both measures also have ovarian cancer-specific modules that enable assessment of additional symptoms associated with ovarian cancer and its treatments (eg QLQ-OV2822 and FACT-O42 /FOSI).43,44
A more recent addition is the Measure of Ovarian Symptoms and Treatment concerns (MOST), developed specifically to assess the symptom benefit and adverse effects of treatment in women with recurrent ovarian cancer receiving palliative chemotherapy.45 Another version of MOST (MOST-S26) was recently developed and validated for the purpose of symptom surveillance following first-line treatment for ovarian cancer to complement clinical follow-up.46 Of note, all MOST versions focus exclusively on symptoms and global well-being and are not HRQL measures. Thus, MOST should be administered in combination with relevant FACT or EORTC measures if HRQL is an outcome of interest.47 A patient-reported version of the common terminology criteria for adverse events (PRO-CTCAE) has also been developed to facilitate patient reporting of adverse events.48 The PRO-CTCAE has the potential to provide a systematic flexible approach to capturing symptomatic adverse events in ovarian cancer clinical trials or clinical practice.48
Selecting appropriate PRO measures for use in ovarian cancer research or clinical practice requires careful consideration. PRO instrument selection will depend on whether the purpose of assessment is to measure clinical trial endpoints or assess outcomes in clinical practice, and whether the measurement context is first-line treatment, maintenance therapy, palliative chemotherapy or end of life where appropriate outcomes and endpoints differ considerably. PRO instrument selection for clinical trials should be based on pre-specified PRO hypotheses and the specific research questions that need to be addressed.49,50 The International Society for Quality of Life Research (ISOQOL) developed a set of minimum standards for the selection of PRO measures for use in patient-centered outcome and comparative effectiveness research.38 These include documentation of the characteristics of the conceptual and measurement model, evidence for reliability, validity and interpretability of scores, quality translations, and acceptable patient and investigator burden.38 Key considerations when choosing a PRO measure for use in clinical practice include ease of administration, completion, scoring and interpretation of scores and the availability of meaningful cut-off points to inform interpretation of PRO results and trigger appropriate referral or clinical intervention.51
PROs Across the Disease and Treatment Trajectory
In the next sections of this review article, we discuss the importance of assessing PROs throughout the ovarian cancer disease and treatment trajectory, and provide examples from existing literature to illustrate the uses of PROs as the goals of treatment change in each setting. By doing so, we aim to emphasize the importance of capturing the patients’ voice in ovarian cancer research and clinical practice during both the acute treatment and survivorship phases, and to encourage researchers and clinicians to incorporate meaningful PRO assessments in their future research and clinical practice. As shown in Figure 1, the choice of which PROs to assess – either in clinical trials or clinical practice – should differ depending on whether the context is first-line chemotherapy, maintenance therapy, treatment of recurrent disease, or end of life care, as explained below.
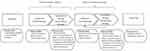 |
Figure 1 Relevant PROs to assess throughout the disease and treatment trajectory in ovarian cancer clinical trials and clinical practice. |
Diagnosis and First-Line Treatment
Although ovarian cancer has previously been characterized as a “silent disease” because the majority of women only present with signs and symptoms at an advanced stage, there is increasing evidence that many women do report recognizable symptoms before diagnosis.52 The most commonly reported symptoms include abdominal pain, abdominal bloating, feeling full quickly, difficulty eating and in some cases urinary symptoms.52
Receiving a diagnosis of ovarian cancer is often a traumatic shock causing considerable distress, particularly because symptoms are often misattributed to other less serious conditions.53 Monitoring PROs from time of diagnosis may help to identify those requiring support for the psychological impact of the diagnosis and disease-related symptoms early in the disease trajectory.
Women diagnosed with ovarian cancer are typically treated with cytoreductive surgery and chemotherapy. The goal of first-line treatment is to eradicate or reduce the volume of disease without severely compromising HRQL.54 Many patients experience burdensome side effects both during and at the end of primary treatment. The most common physical symptoms reported during primary chemotherapy include fatigue, hair loss, trouble sleeping, difficulties concentrating, altered sense of taste, pins and needles, constipation and poor appetite.55 Sexual dysfunction and intimacy issues are also prevalent during chemotherapy with patients reporting worse menopause-related symptoms and body image during first-line chemotherapy than subsequent lines of chemotherapy.56 Patient-reports of the adverse effects of primary treatment and their impact on HRQL are paramount for understanding the harms versus benefits of treatment from the patient’s perspective.
PROs in “Survivorship” and Maintenance Treatment
Many women experience burdensome side-effects after initial treatment that persist long after treatment ends, negatively affecting HRQL.55,57 In a prospective cohort study of Australian women diagnosed with ovarian cancer (the OPAL study), participants completed several PRO measures every 3 months from 6-months post diagnosis for up to 4 years. Results indicated high symptom burden at the end of primary chemotherapy with most symptoms improving by 3 months post-treatment.55,57 However, psychological symptoms only improved slightly over time and 20–30% of patients still reported moderate-to-severe fatigue, trouble sleeping, neurotoxicity and anxiety 3.5 years after treatment completion.55 Younger age, more severe symptoms and prior anxiety or depression increased delayed risk of recovery.57 These findings underscore the importance of long-term monitoring of patient-reported adverse effects of treatment and survivorship concerns following first-line treatment to document and understand the extent of these issues and identify and address persistent troublesome symptoms.
Since the OPAL study was conducted, maintenance therapies with poly (ADP-ribose) polymerase (PARP) inhibitors have received regulatory approval in a number of countries and are rapidly becoming standard of care following first-line chemotherapy. Maintenance therapy may be prescribed for at least two years or longer following chemotherapy or until disease progression when administered in the second-line setting. Three randomized Phase III trials demonstrated PARP inhibitors to be effective at prolonging progression-free survival following complete or partial response to first-line platinum-based chemotherapy.58–60 These trials found no difference in overall HRQL between treatment arms (PARP vs placebo), however PARP inhibitors were predominantly associated with low-grade (clinician-rated) fatigue and nausea58–60 amongst other symptoms. In the SOLO1 trial, low grade (clinician-rated) nausea occurred early in the trial (median time to first onset was about two days) with resolution of first event of nausea in >90% of patients, while low grade (clinician-rated) fatigue occurred a little later (median time to first onset at about three weeks) with resolution in 76% of patients.61 Assessing PROs during maintenance therapy may assist in monitoring symptoms and identifying patients for whom dose adjustment would better balance harm and benefit.
With the advent of maintenance therapy, patients may now be living longer without symptoms/signs of recurrence, but this may come at a cost as prolonged maintenance treatments can be associated with adverse effects that persist and may negatively impact HRQL in the long-term. In clinical trials of maintenance therapies, it is therefore important to assess patient-rated adverse effects and the trade-offs patients are willing to accept to prolong progression-free survival. Treatment inconvenience and non-adherence also warrant consideration in this setting due to the length and complexity of the treatment regimens, and difficulties managing side effects, particularly when patients are asymptomatic, and the immediate benefits of treatment are inapparent.62 It is also important that PRO data are analyzed and presented in a way that reveals subgroups of patients who experience greater intensity of adverse effects, as illustrated by Beesley et al;55 these subgroups may be obscured in trials that only compare mean scores in HRQL, for example in PARP inhibitor versus placebo arms, which are almost always very similar. In addition, global questions about HRQL may not be sensitive enough to detect specific adverse effects, eg nausea and fatigue, and can be influenced by a range of other factors external to treatment. For this reason, outcomes that are more likely to occur directly as a consequence of treatment should be considered as the key PRO.
In clinical trials examining the benefit of maintenance therapies, the inclusion of endpoints, such as quality adjusted progression-free survival (QAPFS) and quality-adjusted time without symptoms of disease progression or toxicity of treatment (Q-TWIST),63 can help quantify and interpret the balance of efficacy and toxicity of treatment from the patients’ perspective. Q-TWIST was first used to evaluate adjuvant therapy for breast cancer64 and has since been used in several other settings including in the evaluation of maintenance therapy for recurrent ovarian cancer as well as the first-line setting.65 For example, the SOLO2 trial demonstrated olaparib maintenance therapy after chemotherapy in patients with platinum-sensitive relapsed ovarian cancer resulted in patient-centered benefits including longer QAPFS and time without significant symptoms of toxicity (TWIST).65 These results supported the primary endpoint of SOLO2 which was a significant prolongation in progression-free survival.65 A number of other trials of maintenance therapy with PARP inhibitors have also reported Q-TWIST or TWIST and QAPFS66–69 which help interpret the benefit to patients of prolongation of the disease-free interval discounted by the impact of adverse effects associated with PARP inhibitors.
Given treatment advances, particularly with respect to maintenance therapies that may continue for up to 3 years, it has become increasingly important to monitor the troublesome side effects of all contemporary treatments including bevacizumab and PARP inhibitors on an ongoing basis throughout follow-up after primary treatment. The aim of clinical follow-up is to identify and manage adverse effects of treatment and psychological distress and to detect symptoms and signs of disease recurrence.70 Although PRO measures are ideally suited for the purpose of standardized symptom assessment and tracking symptoms between follow-up visits, they have not been routinely implemented in ovarian cancer follow-up care. Importantly, administering and reviewing PROs at follow-up consultations may help clinicians to identify patients in need of supportive care and psychosocial intervention to improve HRQL, and potentially facilitate earlier detection of symptomatic recurrence. An example is a Phase 2 trial (ACTRN12620000332921) currently underway in Australia to evaluate the feasibility of remote nurse-led follow-up using a PRO measure (the MOST-S26) to structure consultations versus routine clinic-based follow-up.71 Specifically, this trial will assess whether structured follow-up using MOST-S26 improves emotional well-being and patient satisfaction; does not delay time to diagnosis of recurrence; identifies more patients with psychological distress and improves psychological outcomes; and is cost-effective.71 If proven superior to routine clinic-based follow-up, this novel PRO-based approach has the potential to transform ovarian cancer follow-up care.
PROs in Palliative Chemotherapy
Although many women initially respond to first-line treatment for ovarian cancer, 80% or more will eventually develop recurrent disease and be offered palliative chemotherapy.72 Although the prognosis of patients with platinum sensitive recurrent ovarian cancer (ROC) is superior to those with platinum-resistant disease, with few exceptions treatment is not curative.73 The goal of palliative chemotherapy is to reduce the symptom burden caused by ovarian cancer, improve HRQL, delay disease progression, and possibly prolong survival, with acceptable treatment toxicity. However, the extent to which disease-related symptoms, such as abdominal bloating or abdominal pain, improve in response to palliative chemotherapy is rarely assessed or documented in clinical trials or clinical practice.74 Moreover, the extent and severity of these symptoms is often under-appreciated by treating clinicians.74–76 In recognition of this problem, the Gynecologic Cancer Intergroup (GCIG) released a consensus statement strongly recommending that any clinical trials of palliative chemotherapy should include patient-reported symptom benefit as a key trial endpoint.19
This call to action led to the establishment of the GCIG Symptom Benefit Working group and the GCIG Symptom Benefit Study (ACTRN12607000603415). The first stage of the Symptom Benefit Study aimed to comprehensively document the patient-reported symptom experience of women with platinum resistant/refractory ROC receiving palliative chemotherapy by asking participants to complete five HRQL measures at baseline and before each cycle.74 Results indicated that almost all patients were symptomatic at baseline with approximately 70% reporting ≥9 symptoms.74 Of those who were symptomatic at baseline only 36% to 48% reported a clinically important improvement in symptoms after each cycle of chemotherapy, as evidenced by a reduction in scores on the QLQ-OV28 abdominal/gastrointestinal symptom scales.74 Overall, these results indicated that although chemotherapy improved symptoms in approximately half of the women, a substantial number did not benefit and progressed rapidly.
Stage 2 of the Symptom Benefit Study aimed to determine the proportion of women with platinum resistant/refractory ROC as well as potentially platinum sensitive ROC who had ≥3 lines of prior treatment who benefited from palliative chemotherapy, defined by a clinically significant improvement in HRQL scores and improvement of symptoms. All participants completed the MOST and the EORTC QLQ-C30 every 3–4 weeks before each cycle of chemotherapy, until disease progression. Results demonstrated over 50% of participants reported moderate to severe abdominal and psychological symptoms at baseline.73 Of those who were symptomatic at baseline, 40% reported an improvement within 2 months of starting chemotherapy, while only about 15% reported an improvement in aspects of HRQL.73 Thus, only a relatively small proportion of patients reported improvement in their symptoms and HRQL as a result of palliative chemotherapy, underscoring the importance of tempering patient expectations prior to treatment and initiating timely referral for supportive care to address bothersome symptoms. Overall, the findings from the GCIG Symptom Benefit Study highlighted the critical importance of documenting the extent of patient-rated symptoms at baseline in clinical trials of palliative chemotherapy, and evaluating subjective symptom benefit and HRQL improvement in response to treatment.
The AURELIA trial provides another example of the value of incorporating a pre-specified PRO endpoint to assess symptom benefit in clinical trials examining treatments for ROC.77 The AURELIA trial found that adding bevacizumab to standard chemotherapy for women with recurrent platinum resistant ROC achieved an approximate doubling of the proportion of patients who experienced a 15% improvement in patient-reported abdominal symptoms, assessed using the QLQ-OV28 abdominal symptom subscale.77 Better outcomes with bevacizumab were also observed for global QOL and physical, role and social functioning.77 These PRO results indicated the benefits of bevacizumab extended beyond the prolongation of progression-free survival and included greater improvements in abdominal/GI symptoms and other aspects of HRQL. These findings further highlight the importance of incorporating PRO endpoints in clinical trials to help interpret the primary endpoint of prolonged progression-free survival in the recurrent setting.
Similar to the first-line setting, in trials of maintenance therapies for ROC it is important to assess patient-centered benefits and patient-reported adverse effects to support the primary endpoint of progression-free survival and provide insight into the benefits and harms of treatment from patients’ perspectives.24 In the recurrent setting, patients continue on maintenance therapy until progression which in a subset may be greater than 5 years. This has obvious implications for the ideal duration and frequency of the administration of PRO measures in this setting. It is recommended that PRO measures should be administered up until and beyond disease progression and continue through to the next line of treatment to determine the impact of recurrence and further treatment on patients.24 There is likely to be an additive effect of multiple lines of prior chemotherapy in combination with prolonged maintenance therapy over time, possibly resulting in cumulative toxicity. This should be documented and reported from patients’ perspectives using PRO measures that capture the relevant expected toxicities, such as peripheral neuropathy. In addition to the adverse effects of treatment, patient preferences and trade-offs are also important to assess in this context.24
Another important finding of the GCIG Symptom Benefit Study was that physicians under-recognized patients’ symptoms, as evidenced by differences in the frequency and grading of symptoms by patients versus their treating clinicians.74,78 For example, there was substantial discordance between patient-rated and physician-rated symptoms such as fatigue (93% vs 38%), pain (74% vs 39%) and emotional distress (89% vs 1%).74 Although the discordance between clinician and patient-rated symptoms has been previously well-documented,12,79,80 this finding further underscores the importance of asking patients to rate and grade their own symptoms. It also highlights the need for routine symptom assessment in clinical practice to enable women with ROC to self-report their physical and psychological symptoms in a valid, standardized way, and for clinicians to use this PRO data to improve care and treatment decision-making for these women.
Implementation of routine PRO assessment in clinical practice is associated with many benefits including improved patient-clinician communication, patient satisfaction,33,81 HRQL,28,82 compliance with chemotherapy, fewer emergency visits,28 and longer overall survival.29,30 Of particular note, the seminal randomized controlled trial by Basch et al demonstrated routine web-based PRO symptom monitoring in outpatients with common advanced cancers receiving chemotherapy resulted in improved HRQL and overall survival.28,29 Although this study highlights the potential value of routine PRO assessment in a variety of advanced cancers, further clinical trials are required to examine whether these benefits generalize to women with ROC.
In a recent randomized controlled trial by Donovan et al, women with ROC who participated in nurse-guided or self-directed web-based symptom management interventions reported improved symptom controllability (ie positive expectancies in one’s ability to control symptoms). However, symptom burden and HRQL did not improve in either intervention-arm.83 The authors suggested the lack of improvement in symptom burden might be due to the highly variable nature of symptoms in ROC.83 Moreover, the authors acknowledged patients with high symptom burden had difficulty engaging in self-management interventions. Given the high symptom burden of ROC, future research should extend these efforts and examine the use of novel PRO-based interventions to facilitate enhanced symptom management and potentially improve the HRQL of women with ROC.
PROs in End of Life (EOL)
Women with ROC are a heterogeneous group with variable outcomes. Ultimately, most will develop platinum resistant/refractory disease, with the benefits of chemotherapy diminishing with each successive line of treatment.84 Deciding when to discontinue active treatment is challenging for both clinicians and patients.85 A major difficulty for clinicians is identifying patients who are expected to have a short survival time and are unlikely to benefit from further chemotherapy and should therefore be spared unnecessary treatment toxicity in their last months of life. The American Society of Clinical Oncology recommends against using cancer-directed therapy in patients with low performance status, with no benefit from prior evidence-based interventions and no strong evidence supporting the clinical value of further anti-cancer treatment.86 Despite this, there is evidence that up to 30% of gynecological cancer patients are treated with chemotherapy in their last month of life.87–89 The ability of clinicians to identify patients who have poor prognosis and little to gain from further chemotherapy would help facilitate communication around the role of further chemotherapy and support evidence-based decision-making.
There is strong evidence that HRQL is an independent prognostic factor in a variety of advanced cancers,6–10 indicating PROs may be useful for identifying subgroups of patients at risk of very short survival who should not receive further treatment and for whom supportive care alone may be preferable. The GCIG Symptom Benefit Study found that women with platinum resistant/refractory ROC with low self-reported global health status, role function, or physical function before starting chemotherapy were more likely to stop chemotherapy early, with short overall survival time.13 Similarly, women with potentially platinum sensitive ROC starting third or subsequent line (≥3) chemotherapy with low baseline HRQL (ie low physical, social and role function and global health status) had shorter overall survival, while social function was prognostic for progression-free survival.14 These findings highlight the prognostic value of measuring aspects of HRQL when considering further chemotherapy in ROC to identify women with poor prognosis who are unlikely to benefit from further treatment.
Overall, these findings suggest that patient-reported HRQL at baseline, in addition to clinician-rated performance status and other key clinical indicators, should be considered together when making decisions about whether to continue treatment. This information could be used in discussion with the patient to demonstrate the likelihood of response to treatment and help patients and their families make informed decisions about further treatment. Self-ratings of global health status and physical, social and role function in particular are simple to implement in clinical practice and could improve patient-clinician communication regarding prognosis and help decision-making in women considering further chemotherapy.
Another useful approach to prognostication recently developed by the North-Eastern German Society of Gynecological Oncology (NOGGO) and Working Group Gynecological Oncology (AGO) is the NOGGO-AGO QoL prognostic score for ROC.15 It calculates a risk score on the basis of the platinum-free interval, performance status, age, global QoL and nausea to predict patients at low, medium and high risk of short-term mortality.15 Thus, the NOGGO-AGO QoL prognostic score also provides a useful tool to help identify patients’ at high risk of poor prognosis to support and inform decisions about further chemotherapy. Utilizing PROs/HRQL as prognostic tools in clinical practice is likely to have a multitude of benefits including helping patients and their families to make decisions about treatment; reducing the number of patients who opt for more systemic therapy with low likelihood of clinical benefit thereby ensuring treatment is stopped at the right time; and earlier initiation of supportive care to improve symptom control and quality of life in the final months of life.
Within the context of clinical trials, the use of PROs/HRQL for prognostication could also help to improve clinical trial methodology. Baseline PRO sores on key aspects of HRQL could be used to identify patients who should be receiving supportive care for symptom control rather than participating in a clinical trial. Individuals reporting scores below a pre-specified threshold on key HRQL domains at baseline could be excluded from trial participation and referred for supportive care instead. This approach could potentially also be used to allocate patients to trial arms in trials examining best supportive care compared to chemotherapy. The exclusion of women with low HRQL scores and very poor prognosis is also likely to be important for trial results as the inclusion of patients with a high likelihood of rapid progression may dilute study findings and miss signals of efficacy in clinical trials where a substantial proportion come off the trial within 8 weeks. Finally, given the evidence for the prognostic significance of aspects of HRQL in ROC, trial participants could also be stratified into trial arms based on their baseline HRQL scores to balance treatment groups. Clinician-rated performance status is commonly used as a trial inclusion/exclusion criterion; as a large body of evidence shows patient HRQL scores have greater predictive power over and above clinician-rated performance status, PROs should be considered for this purpose also. PROs are known to be highly correlated with clinician-rated performance status, and as they come directly from the patient, arguably they are a more valid indicator of patient health status.
Aside from their use as a prognostic indicator, prospective assessment of PROs is also critical to understanding patients’ experience of end of life (EOL) care and collecting PRO data in this setting may help to improve the quality of EOL services. The goal of EOL care is to control pain and other symptoms so that the patient is as comfortable as possible.90 Thus, early integration of supportive care for symptom management is essential and may be facilitated by PRO assessments. There are considerable challenges to collecting PRO data at EOL because of the variable location of care at this stage of a patient’s disease (eg hospital inpatient/outpatient, hospice, home) and patients’ potentially being unable or unwilling to complete self-report measures due to their progressive illness. The PEACE study (Palliation in Gyne-oncology: Patient Expectations and Assessment of CarE; ACTRN1262100 1031853)91 is currently underway in Australia and Denmark to examine the feasibility and acceptability of collecting PRO data on satisfaction with EOL care from patients with advanced gynecological malignancies, including women with platinum-resistant/refractory recurrent ovarian cancer; and will also provide preliminary data on both patient and carer satisfaction with EOL care. This PRO data will provide a better understanding of patients’ and carers’ experiences of EOL care and potentially highlight gaps in care where improvements may be needed, which ultimately may help optimize the EOL experience of women dying with advanced gynecological malignancies.
Guidance for Incorporating PROs in Clinical Trials
Guidance is available to assist researchers and clinicians with the PRO aspects of ovarian cancer clinical trials at all stages of the research process from trial design through to reporting. Following this guidance will help to ensure the provision of high-quality PRO evidence to inform patient-centered care and health policy. In this section, we provide a brief overview of some key resources and their uses. These are also recommended by the GCIG Symptom Benefit Committee.24
In 2013, the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement was released,92,93 providing recommendations for a minimum set of items that should be included in a clinical trial protocol. The SPIRIT-PRO is an extension of the original SPIRIT statement providing PRO-specific protocol guidance developed through a rigorous international consensus-based process.50 SPIRIT-PRO recommends 16 items that should be included as a minimum standard in the PRO sections of clinical trial protocols where PROs are a primary or secondary endpoint, and should be used in conjunction with the SPIRIT 2013 statement. SPIRIT-PRO items address PRO-specific aspects of the clinical trial protocol including: the PRO study rationale, objectives and eligibility criteria; PRO domains used to evaluate the intervention; assessment time points; selection of PRO measures and their measurement properties; the PRO data collection plan; translation of PRO measures to other languages; proxy completion; strategies to minimize missing PRO data; and, whether PRO data will be monitored during the active trial phase to inform the clinical care of participants.50
In 2021, a SPIRIT-PRO explanation and elaboration paper was released to promote understanding and facilitate uptake of the SPIRIT-PRO recommendations by providing more detailed guidance on how to use this resource when developing clinical trial protocols.49 It provides a protocol template and useful examples from existing trial protocols to facilitate implementation of the SPIRIT-PRO guidance. A previous evaluation of the content of ovarian cancer clinical trial protocols indicated their PRO content is often incomplete or missing, potentially leading to sub-optimal PRO methodology, highlighting the need for protocol developers to actively follow the SPIRIT-PRO guidance.26 Use of the SPIRIT-PRO guidance early in trial design will help facilitate careful planning and ensure critical aspects about PRO endpoints are included in trial protocols. This, in turn, will likely help facilitate successful conduct of the PRO aspects of ovarian cancer clinical trials and help promote high-quality data, analysis and reporting of PRO endpoints.
Previous research indicates clinical trial staff and coordinators report logistical and practical challenges to collecting PROs in clinical trials such as managing participants with English as a second language, participants’ companions attempting to complete questionnaires on their behalf and difficulties balancing their duty of care against trial requirements. This underscores the importance of providing clear, standardized guidance and training on PRO administration during trial conduct.94,95 Both the European Organization for the Research and Treatment of Cancer (EORTC) guidelines,96 and the QOL Office Checklist of instructions for the administration of PROMs97 provide useful guidance to address these issues and ensure the collection of high quality PRO data in ovarian cancer clinical trials.
For clinical trial settings, PRO analysis strategies are also important. The “Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life endpoints data (SISAQOL)” initiative published recommendations for statistical approaches for analyzing PRO data collected in oncology randomized controlled trials in 2020,98 which apply to ovarian cancer trials. This initiative aimed to address challenges to analyzing PRO data such as using statistically correct analysis methods that are appropriate to address the PRO research question and using appropriate statistical techniques to handle missing PRO data.99 The SISAQOL initiative developed a taxonomy of research objectives that identified a range of PRO endpoints (defined from PRO data in different ways), matched these to appropriate statistical methods, recommended standardized statistical terminology related to missing data, and appropriate ways to manage missing data.98 Use of these recommendations, developed through comprehensive literature review and a structured collaborative process with diverse international stakeholders, is likely to have many benefits. Improved PRO analysis in clinical trials will enable robust high-quality evidence to inform patient choice, aid clinical decision-making, and inform policy. In addition, more standardized approaches to PRO analysis will facilitate better cross-trial comparison of PRO results, yielding more meaningful, reliable and context-specific interpretations across ovarian cancer clinical trials.
The CONSORT-PRO extension (Consolidated Standards of Reporting Trials) was developed to ensure PRO methods and results are clearly described in clinical trial publications100 and should be used as a supplement to the standard CONSORT guidelines.101,102 CONSORT-PRO provides five additional checklist items (extensions) that should be reported in all trial publications where a PRO is a primary or secondary outcome, and elaboration on the original CONSORT checklist items specific to the reporting of PROs.100 Poor reporting of PRO results in trial publications undermines clinicians’ ability to use PRO data in their practice to benefit patients. Use of the CONSORT-PRO checklist to guide high-quality reporting of PRO data should facilitate robust interpretation of ovarian cancer trial results to inform patient care.
Guidance for Incorporating PROs in Clinical Practice
Successful and sustainable implementation of PROs in clinical practice is challenging due to a diversity of factors at the patient, healthcare provider and system level.37 Patient-level barriers include time for patients to complete PROMs, patient inability to complete PROMs, and perceived irrelevance or lack of value of PROMs. Healthcare provider-level barriers include lack of knowledge on how to interpret and integrate PROs into their clinical practice and difficulty using electronic PRO collection systems. Service-level barriers include inability to integrate routine PRO use into clinical workflows, lack of ability to action PRO data and inadequate information technology infrastructure to enable easy PRO collection. Rose and Bezjak103 further highlight logistical challenges to collecting PROs in clinic, such as the advantages and disadvantages of different modes of PRO administration, patient literacy and language fluency and the reading levels of PROMs, and provide a number of practical real-world examples for how these can be addressed.
To help clinicians interested in using PROs in clinic, the International Society for Quality of Life research (ISOQOL) developed a User’s Guide for Implementing PRO assessment in clinical practice.104,105 The User’s Guide, available at www.isoqol.org, provides detailed descriptions of the considerations, options, resource requirements, and relative advantages and disadvantages associated with various alternatives.105 It addresses a number of methodological and practical considerations including (1) identifying the goals for collecting PROs in clinical practice, (2) selecting the patients, setting, and timing of assessments, (3) determining which questionnaire(s) to use, (4) choosing a mode for administering and scoring the questionnaire, (5) designing processes for reporting results, (6) identifying aids to facilitate score interpretation, (7) developing strategies for responding to issues identified by the questionnaires, and (8) evaluating the impact of the PRO intervention on the practice.104,105 This step-by-step guide may be useful for clinicians considering using PROs to aid the management of women with ovarian cancer in their clinical practice.
More recently, priority recommendations were developed for the service-level implementation of PROs into cancer clinical care.106 These recommendations were developed through a two-round modified Delphi survey with key stakeholders including cancer survivors, clinical and research experts, and Information Technology specialists.106 The highest ranked priorities included assessment of current staff capabilities and service requirements to undertake collection of PROs, mapping of workflows and processes to enable collection, and using collected PROs to guide improved health outcomes to ensure sustainability of PRO collection.106 Future PRO initiatives in ovarian cancer contexts should focus on pragmatic application of these recommendations to ensure successful uptake of PROs in clinical practice.
Finally, in 2020 Medical Care published a supplement referred to as a “Methods Toolkit” to address the challenges of personalizing cancer care using PROs.107 The supplement includes 14 papers: 6 describe different methods for interpreting PROs and 8 describe how different PRO systems have addressed interpreting PRO scores and/or acting on PRO results.107 This “Methods Toolkit” is a useful resource for clinicians and researchers aiming to implement routine PRO assessment into clinical practice, providing a comprehensive overview of methodological fundamentals as well as real-world examples to promote personalized patient care in oncology clinical practice settings.
Conclusion
The assessment of PROs is of critical importance and great value throughout the ovarian cancer disease and treatment trajectory, as summarized in Box 1. In clinical trials, PRO data can enrich the interpretation of effects on overall survival and progression-free survival and provide patient-centered evidence to guide clinical practice guidelines and health policy. Assessment of PROS in clinical practice can help clinicians monitor symptoms over time, and facilitate decisions about both treatment and timely referral for supportive care. Importantly, the choice of which PROs to assess – either in clinical trials or clinical practice – should differ depending on whether the context is first-line chemotherapy, maintenance therapy, treatment of recurrent disease, or end of life care, as summarized in Figure 1. The choice of which patient-rated symptoms and adverse effects to assess should be informed by the extent of disease and pertinent treatments, and patient-reported symptom benefit should be routinely assessed in patients receiving palliative chemotherapy. To ensure the collection and provision of high-quality PRO data to promote patient-centered care, it is essential that researchers and clinicians follow available evidence-based guidance to assist with key decisions at all stages of clinical trials research, and implementation of PROs in clinical practice.
Access to PRO Measures for Ovarian Cancer
- FACT-G: https://www.facit.org/measures/FACT-G
- FACT-O: https://www.facit.org/measures/FACT-O
- FOSI: https://www.facit.org/measures/FOSI
- MOST-S26: https://gcigtrials.org/content/most
- MOST-T35: https://gcigtrials.org/content/most
- MOST-T24: https://gcigtrials.org/content/most
- PRO-CTCAE: https://healthcaredelivery.cancer.gov/pro-ctcae/
- QLQ-C30: https://qol.eortc.org/questionnaire/eortc-qlq-c30/
- QLQ-OV28: https://qol.eortc.org/questionnaire/qlq-ov28/
Acknowledgments
We would like to acknowledge the key role of both the Australia New Zealand Gynecological Group (ANZGOG), NHMRC Clinical Trials Centre and the Gynecological Cancer InterGroup (GCIG) in supporting the GCIG Symptom Benefit Study and the development of the Measure of Ovarian Symptoms and Treatment concerns (MOST).
Funding
This review was supported by an NHMRC program grant (APP1092856).
Disclosure
Professor Martin R Stockler reports grants from AMGEN, grants from ASTELLAS, grants from AstraZeneca, grants from Bayer, grants from Beigene, grants from BMS, grants from Medivation, grants from MSD, grants from Novartis, grants from Pfizer, grants from Roche, grants from Sanofi, grants from Tilray, outside the submitted work. Professor Michael L Friedlander reports grants, personal fees, is a member of Advisory boards received fees for lectures and travel from ASTRA ZENECA, personal fees from MSD, personal fees from TAKEDA, personal fees from LILLY, personal fees from EISEI, is an Advisory Board-not compensated for INCYCLIX, personal fees from GSK, grants from Novartis, grants from BEIGENE, outside the submitted work; The authors report no other conflicts of interest in this work.
References
1. Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labelling claims. Available from: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf.
2. Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30(34):4249–4255. doi:10.1200/JCO.2012.42.5967
3. Rivera SC, Kyte DG, Aiyegbusi OL, et al. The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis. Health Qual Life Outcomes. 2019;17(1):156. doi:10.1186/s12955-019-1220-z
4. Ishaque S, Karnon J, Chen G, et al. A systematic review of randomised controlled trials evaluating the use of patient-reported outcome measures (PROMs). Qual Life Res. 2019;28(3):567–592. doi:10.1007/s11136-018-2016-z
5. Au H-J, Ringash J, Brundage M, et al. Added value of health-related quality of life measurement in cancer clinical trials: the experience of the NCIC CTG. Expert Rev Pharmacoecon Outcomes Res. 2010;10(2):119–128. doi:10.1586/erp.10.15
6. Gotay CC, Kawamoto CT, Bottomley A, et al. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. doi:10.1200/JCO.2007.13.3439
7. Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. doi:10.1186/1477-7525-7-102
8. Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10(9):865–871. doi:10.1016/S1470-2045(09)70200-1
9. Quinten C, Martinelli F, Coens C, et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer. 2014;120(2):302–311. doi:10.1002/cncr.28382
10. Grande GE, Farquhar MC, Barclay SIG, et al. Quality of life measures (EORTC QLQ-C30 and SF-36) as predictors of survival in palliative colorectal and lung cancer patients. Palliat Support Care. 2009;7(3):289–297. doi:10.1017/S1478951509990216
11. Campbell R, Ju A, King MT, et al. Perceived benefits and limitations of using patient-reported outcome measures in clinical practice with individual patients: a systematic review of qualitative studies. Qual Life Res. 2022;31(6):1597–1620. doi:10.1007/s11136-021-03003-z
12. Petersen MA, Larsen H, Pedersen L, et al. Assessing health-related quality of life in palliative care: comparing patient and physician assessments. Eur J Cancer. 2006;42(8):1159–1166. doi:10.1016/j.ejca.2006.01.032
13. Roncolato FT, Joly F, O’Connell R, et al. Reducing uncertainty: predictors of stopping chemotherapy early and shortened survival time in platinum resistant/refractory ovarian cancer-the GCIG symptom benefit study. Oncologist. 2017;22(9):1117–1124. doi:10.1634/theoncologist.2017-0047
14. Roncolato FT, O’Connell RL, Joly F, et al. Predictors of progression free survival, overall survival and early cessation of chemotherapy in women with potentially platinum sensitive (PPS) recurrent ovarian cancer (ROC) starting third or subsequent line(>/=3) chemotherapy - The GCIG symptom benefit study (SBS). Gynecol Oncol. 2020;156(1):45–53. doi:10.1016/j.ygyno.2019.10.001
15. Armbrust R, Richter R, Woopen H, et al. Impact of health-related quality of life (HRQoL) on short-term mortality in patients with recurrent ovarian, fallopian or peritoneal carcinoma (the NOGGO-AGO QoL Prognosis-Score-Study): results of a meta-analysis in 2209 patients. ESMO Open. 2021;6(2):100081. doi:10.1016/j.esmoop.2021.100081
16. Kluetz PG, O’Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol. 2018;19(5):e267–e274. doi:10.1016/S1470-2045(18)30097-4
17. Cherny NI, Sullivan R, Dafni U, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 2017;28(11):2901–2905. doi:10.1093/annonc/mdw258
18. Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34(24):2925–2934. doi:10.1200/JCO.2016.68.2518
19. du Bois A, Quinn M, Thigpen T, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(Suppl 8):viii7–viii12. doi:10.1093/annonc/mdi961
20. Joly F, Hilpert F, Okamoto A, et al. Fifth ovarian cancer consensus conference of the gynecologic cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138. doi:10.1016/j.ejca.2017.03.019
21. Quinten C, Maringwa J, Gotay CC, et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst. 2011;103(24):1851–1858. doi:10.1093/jnci/djr485
22. Greimel E, Bottomley A, Cull A, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. Eur J Cancer. 2003;39(10):1402–1408. doi:10.1016/S0959-8049(03)00307-1
23. Havrilesky LJ, Alvarez Secord A, Ehrisman JA, et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer. 2014;120(23):3651–3659. doi:10.1002/cncr.28940
24. Kurtz JE, Gebski V, Sukhin V, et al. Incorporating patient centered benefits as endpoints in randomized trials of maintenance therapies in advanced ovarian cancer: a position paper from the GCIG symptom benefit committee. Gynecol Oncol. 2021;161(2):502–507. doi:10.1016/j.ygyno.2021.02.018
25. Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials-lost opportunities and lessons learned. Ann Oncol. 2016;27(Suppl 1):i66–i71. doi:10.1093/annonc/mdw080
26. Mercieca-Bebber R, Friedlander M, Kok P-S, et al. The patient-reported outcome content of international ovarian cancer randomised controlled trial protocols. Qual Life Res. 2016;25(10):2457–2465. doi:10.1007/s11136-016-1339-x
27. Mercieca-Bebber R, Friedlander M, Calvert M, et al. A systematic evaluation of compliance and reporting of patient-reported outcome endpoints in ovarian cancer randomised controlled trials: implications for generalisability and clinical practice. J Patient Rep Outcomes. 2017;1(1):5. doi:10.1186/s41687-017-0008-3
28. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. doi:10.1200/JCO.2015.63.0830
29. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. doi:10.1001/jama.2017.7156
30. Denis F, Lethrosne C, Pourel N, et al. Randomized trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients. J Natl Cancer Inst. 2017;109(9). doi:10.1093/jnci/djx029.
31. Nipp R, Temel J. The patient knows best: incorporating patient-reported outcomes into routine clinical care. J Natl Cancer Inst. 2017;109(9). doi:10.1093/jnci/djx044
32. Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32(14):1480–1501. doi:10.1200/JCO.2013.53.5948
33. Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:211. doi:10.1186/1472-6963-13-211
34. VandenBos GR. APA Dictionary of Psychology.
35. Schmier JK, Halpern MT. Patient recall and recall bias of health state and health status. Expert Rev Pharmacoecon Outcomes Res. 2004;4(2):159–163. doi:10.1586/14737167.4.2.159
36. King MT, Dueck AC, Revicki DA. Can methods developed for interpreting group-level patient-reported outcome data be applied to individual patient management? Med Care. 2019;57(Suppl5 1):S38–s45. doi:10.1097/MLR.0000000000001111
37. Nguyen H, Butow P, Dhillon H, et al. A review of the barriers to using Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) in routine cancer care. J Med Radiat Sci. 2021;68(2):186–195. doi:10.1002/jmrs.421
38. Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22(8):1889–1905. doi:10.1007/s11136-012-0344-y
39. Bhat G, Karakasis K, Oza AM. Measuring quality of life in ovarian cancer clinical trials-can we improve objectivity and cross trial comparisons? Cancers. 2020;12(11):3296. doi:10.3390/cancers12113296
40. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer. 1993;85(5):365–376. doi:10.1093/jnci/85.5.365
41. Cella D, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy (FACT) scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi:10.1200/JCO.1993.11.3.570
42. Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001;19(6):1809–1817. doi:10.1200/JCO.2001.19.6.1809
43. Cella D, Paul D, Yount S, et al. What are the most important symptom targets when treating advanced cancer? A survey of providers in the National Comprehensive Cancer Network (NCCN). Cancer Invest. 2003;21(4):526–535. doi:10.1081/CNV-120022366
44. Beaumont J, Yount S, Lalla D, et al. Validation of the Functional Assessment of Cancer Therapy-Ovarian (FACT-O) Symptom Index (FOSI) in a Phase II clinical trial of pertuzumab in patients with advanced ovarian cancer. J Clin Oncol. 2007;25(18_suppl):16021. doi:10.1200/jco.2007.25.18_suppl.16021
45. King MT, Stockler MR, O’Connell RL, et al. Measuring what matters MOST: validation of the Measure of Ovarian Symptoms and Treatment, a patient-reported outcome measure of symptom burden and impact of chemotherapy in recurrent ovarian cancer. Qual Life Res. 2018;27(1):59–74. doi:10.1007/s11136-017-1729-8
46. Campbell R, King MT, Ross TL, et al. Development and validation of the measure of ovarian symptoms and treatment concerns for surveillance (MOST-S26): an instrument to complement the clinical follow-up of women with ovarian cancer after completion of first-line treatment. Gynecol Oncol. 2021;163(2):398–407. doi:10.1016/j.ygyno.2021.08.022
47. Campbell R, Costa DSJ, Stockler MR, et al. Measure of Ovarian Symptoms and Treatment concerns (MOST) indexes and their associations with health-related quality of life in recurrent ovarian cancer. Gynecol Oncol. 2022;166(2):254–262. doi:10.1016/j.ygyno.2022.05.024
48. Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9):dju244–dju244. doi:10.1093/jnci/dju244
49. Calvert M, King M, Mercieca-Bebber R, et al. SPIRIT-PRO Extension explanation and elaboration: guidelines for inclusion of patient-reported outcomes in protocols of clinical trials. BMJ Open. 2021;11(6):e045105. doi:10.1136/bmjopen-2020-045105
50. Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA. 2018;319(5):483–494. doi:10.1001/jama.2017.21903
51. Chan EKH, Edwards TC, Haywood K, et al. Implementing patient-reported outcome measures in clinical practice: a companion guide to the ISOQOL user’s guide. Qual Life Res. 2019;28(3):621–627. doi:10.1007/s11136-018-2048-4
52. Goff B. Symptoms associated with ovarian cancer. Clin Obstet Gynecol. 2012;55(1):36–42. doi:10.1097/GRF.0b013e3182480523
53. Power J, Brown L, Ritvo P. A qualitative study examining psychosocial distress, coping, and social support across the stages and phases of epithelial ovarian cancer. Health Care Women Int. 2008;29(4):366–383. doi:10.1080/07399330701876521
54. Chase DM, Wenzel L. Health-related quality of life in ovarian cancer patients and its impact on clinical management. Expert Rev Pharmacoecon Outcomes Res. 2011;11(4):421–431. doi:10.1586/erp.11.41
55. Beesley VL, Ross TL, King MT, et al. Evaluating patient-reported symptoms and late adverse effects following completion of first-line chemotherapy for ovarian cancer using the MOST (Measure of Ovarian Symptoms and Treatment concerns). Gynecol Oncol. 2022;164(2):437–445. doi:10.1016/j.ygyno.2021.12.006
56. Domenici L, Palaia I, Giorgini M, et al. Sexual health and quality of life assessment among ovarian cancer patients during chemotherapy. Oncology. 2016;91(4):205–210. doi:10.1159/000447403
57. Beesley VL, Webber K, Nagle CM, et al. When will I feel normal again? Trajectories and predictors of persistent symptoms and poor wellbeing after primary chemotherapy for ovarian cancer. Gynecol Oncol. 2020;159(1):179–186. doi:10.1016/j.ygyno.2020.07.029
58. Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–2428. doi:10.1056/NEJMoa1911361
59. González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–2402. doi:10.1056/NEJMoa1910962
60. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi:10.1056/NEJMoa1810858
61. Colombo N, Moore K, Scambia G, et al. Tolerability of maintenance olaparib in newly diagnosed patients with advanced ovarian cancer and a BRCA mutation in the randomized phase III SOLO1 trial. Gynecol Oncol. 2021;163(1):41–49. doi:10.1016/j.ygyno.2021.07.016
62. Moss HA, Chen L, Hershman DL, et al. Adherence to PARP inhibitor therapy among women with ovarian cancer. Gynecol Oncol. 2021;163(2):262–268. doi:10.1016/j.ygyno.2021.08.025
63. Gelber RD, Goldhirsch A. A new endpoint for the assessment of adjuvant therapy in postmenopausal women with operable breast cancer. J Clin Oncol. 1986;4(12):1772–1779. doi:10.1200/JCO.1986.4.12.1772
64. Gelber RD, Goldhirsch A, Cavalli F. Quality-of-life-adjusted evaluation of adjuvant therapies for operable breast cancer. The International Breast Cancer Study Group. Ann Intern Med. 1991;114(8):621–628. doi:10.7326/0003-4819-114-8-621
65. Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, Phase 3 randomised trial. Lancet Oncol. 2018;19(8):1126–1134. doi:10.1016/S1470-2045(18)30343-7
66. Oza AM, Lorusso D, Aghajanian C, et al. Patient-centered outcomes in ARIEL3, a Phase III, randomized, placebo-controlled trial of rucaparib maintenance treatment in patients with recurrent ovarian carcinoma. J Clin Oncol. 2020;38(30):3494–3505. doi:10.1200/JCO.19.03107
67. Chase DM, Marín MR, Backes F, et al. Impact of disease progression on health-related quality of life of advanced ovarian cancer patients - Pooled analysis from the PRIMA trial. Gynecol Oncol. 2022;166(3):494–502. doi:10.1016/j.ygyno.2022.06.028
68. Joly F, Chabaud S, Cropet C, et al. Time without symptoms or toxicity (TWiST) in patients with newly diagnosed advanced ovarian cancer receiving maintenance olaparib or placebo plus bevacizumab: analysis of PAOLA-1/ENGOT-ov25 phase III trial. J Clin Oncol. 2022;40(16_suppl):5562. doi:10.1200/JCO.2022.40.16_suppl.5562
69. Friedlander M, Moore KN, Colombo N, et al. Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): a randomised, phase 3 trial. Lancet Oncol. 2021;22(5):632–642. doi:10.1016/S1470-2045(21)00098-X
70. Cancer Australia. Follow up of women with epithelial ovarian cancer: a clinical practice guideline developed by Cancer Australia. 2012.
71. Cohen PA, Webb PM, King M, et al. Getting the MOST out of follow-up: a randomized controlled trial comparing 3 monthly nurse led follow-up via telehealth, including monitoring CA125 and patient reported outcomes using the MOST (Measure of Ovarian Symptoms and Treatment concerns) with routine clinic based or telehealth follow-up, after completion of first line chemotherapy in patients with epithelial ovarian cancer. Int J Gynecol Cancer. 2021;30:245.
72. Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–92. doi:10.1016/S0020-7292(06)60033-7
73. Lee YC, King MT, O’Connell RL, et al. Symptom burden and quality of life with chemotherapy for recurrent ovarian cancer: the Gynecologic Cancer InterGroup-Symptom Benefit Study. Int J Gynecol Cancer. 2022;32:761–768. doi:10.1136/ijgc-2021-003142
74. Friedlander ML, Stockler M, O’Connell R, et al. Symptom burden and outcomes of patients with platinum resistant/refractory recurrent ovarian cancer: a reality check: results of stage 1 of the gynecologic cancer intergroup symptom benefit study. Int J Gynecol Cancer. 2014;24(5):857–864. doi:10.1097/IGC.0000000000000147
75. Hay CM, Courtney-Brooks M, Lefkowits C, et al. Symptom management in women with recurrent ovarian cancer: do patients and clinicians agree on what symptoms are most important? Gynecol Oncol. 2016;143(2):367–370. doi:10.1016/j.ygyno.2016.08.235
76. Chandwani KD, Zhao F, Morrow GR, et al. Lack of patient-clinician concordance in cancer patients: its relation with patient variables. J Pain Symptom Manage. 2017;53(6):988–998. doi:10.1016/j.jpainsymman.2016.12.347
77. Stockler MR, Hilpert F, Friedlander M, et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J Clin Oncol. 2014;32(13):1309–1316. doi:10.1200/JCO.2013.51.4240
78. Friedlander M, Stockler MR, O’Connell R, et al. Is it time to change the primary endpoint in clinical trials in recurrent ovarian cancer (ROC)?: symptom burden and outcomes in patients with platinum resistant/refractory (PRR) and potentially platinum sensitive ROC receiving ≥ 3 lines of chemotherapy (PPS ≥ 3)—the Gynecologic Cancer Intergroup (GCIG) Symptom Benefit Study (SBS). J Clin Oncol. 2015;33(15_suppl):5536.
79. Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22(17):3485–3490. doi:10.1200/JCO.2004.03.025
80. Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–1632. doi:10.1093/jnci/djp386
81. Gilbert A, Sebag-Montefiore D, Davidson S, et al. Use of patient-reported outcomes to measure symptoms and health related quality of life in the clinic. Gynecol Oncol. 2015;136(3):429–439. doi:10.1016/j.ygyno.2014.11.071
82. Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22(4):714–724. doi:10.1200/JCO.2004.06.078
83. Donovan HS, Sereika SM, Wenzel LB, et al. Effects of the WRITE symptoms interventions on symptoms and quality of life among patients with recurrent ovarian cancers: an NRG oncology/GOG study (GOG-0259). J Clin Oncol. 2022;40(13):1464–1473. doi:10.1200/JCO.21.00656
84. Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23(10):2605–2612. doi:10.1093/annonc/mds203
85. Smith H, Smith TJ. The role of chemotherapy at the end of life: “when is enough, enough?”. JAMA. 2008;299(22):2667–2678. doi:10.1001/jama.299.22.2667
86. Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30(14):1715–1724. doi:10.1200/JCO.2012.42.8375
87. von Gruenigen V, Daly B, Gibbons H, et al. Indicators of survival duration in ovarian cancer and implications for aggressiveness of care. Cancer. 2008;112(10):2221–2227. doi:10.1002/cncr.23391
88. Nevadunsky NS, Spoozak L, Gordon S, et al. End-of-life care of women with gynecologic malignancies: a pilot study. Int J Gynecol Cancer. 2013;23(3):546–552. doi:10.1097/IGC.0b013e3182842efa
89. Rochigneux P, Raoul JL, Beaussant Y, et al. Use of chemotherapy near the end of life: what factors matter? Ann Oncol. 2017;28(4):809–817. doi:10.1093/annonc/mdw654
90. National Cancer Institute. End of life care. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/end-of-life-care.
91. Palliation in gyne-oncology: patient expectations and assessment of care. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=382163.
92. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi:10.7326/0003-4819-158-3-201302050-00583
93. Chan A-W, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi:10.1136/bmj.e7586
94. Mercieca-Bebber R, Calvert M, Kyte D, et al. The administration of patient-reported outcome questionnaires in cancer trials: interviews with trial coordinators regarding their roles, experiences, challenges and training. Contemp Clin Trials Commun. 2018;9:23–32. doi:10.1016/j.conctc.2017.11.009
95. Kyte D, Ives J, Draper H, et al. Inconsistencies in quality of life data collection in clinical trials: a potential source of bias? Interviews with research nurses and trialists. PLoS One. 2013;8(10):e76625. doi:10.1371/journal.pone.0076625
96. Young T, De Haes H, Curran D, et al. Guidelines for assessing quality of life in EORTC clinical trials. 1999.
97. QOL Office. Checklist of instructions for the administration of patient reported outcome measures; 2015. Available from: http://www.pocog.org.au/docview.aspx?id=355.
98. Coens C, Pe M, Dueck AC, et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomised controlled trials: recommendations of the SISAQOL consortium. Lancet Oncol. 2020;21(2):e83–e96. doi:10.1016/S1470-2045(19)30790-9
99. Bottomley A, Pe M, Sloan J, et al. Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards. Lancet Oncol. 2016;17(11):e510–e514. doi:10.1016/S1470-2045(16)30510-1
100. Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814–822. doi:10.1001/jama.2013.879
101. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi:10.1186/1741-7015-8-18
102. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi:10.1136/bmj.c869
103. Rose M, Bezjak A. Logistics of collecting patient-reported outcomes (PROs) in clinical practice: an overview and practical examples. Qual Life Res. 2009;18(1):125–136. doi:10.1007/s11136-008-9436-0
104. Aaronson N, Choucair A, Elliott T, et al.User’s guide to implementing patient-reported outcomes assessment in clinical practice. Int Soc Qual Life Res. 2015;2:1–47.
105. Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res. 2012;21(8):1305–1314. doi:10.1007/s11136-011-0054-x
106. Mazariego C, Jefford M, Chan RJ, et al. Priority recommendations for the implementation of patient-reported outcomes in clinical cancer care: a Delphi study. J Cancer Surviv. 2022;16(1):33–43. doi:10.1007/s11764-021-01135-2
107. Snyder C, Brundage M, Rivera YM, et al. A PRO-cision medicine methods toolkit to address the challenges of personalizing cancer care using patient-reported outcomes: introduction to the supplement. Med Care. 2019;57(Suppl5 1):S1–S7. doi:10.1097/MLR.0000000000001089
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
