Back to Journals » International Journal of General Medicine » Volume 15
Nontuberculous Mycobacteria Lung Disease (NTM-LD): Current Recommendations on Diagnosis, Treatment, and Patient Management
Authors Pathak K, Hart S, Lande L
Received 20 June 2022
Accepted for publication 5 August 2022
Published 1 October 2022 Volume 2022:15 Pages 7619—7629
DOI https://doi.org/10.2147/IJGM.S272690
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kriti Pathak,1,* Stephanie Hart,1,* Leah Lande1,2
1Division of Pulmonary and Critical Care Medicine, Lankenau Medical Center, Wynnewood, PA, USA; 2Lankenau Institute for Medical Research, Wynnewood, PA, USA
*These authors contributed equally to this work
Correspondence: Leah Lande, Tel +1 610-642-3796, Email [email protected]
Abstract: Nontuberculous mycobacteria (NTM) are a group of ubiquitous environmental bacteria that can be found in soil, dust, and water. Mycobacterium avium complex (MAC) is the most common pathogen and the one most associated with chronic pulmonary disease. In recent years, the prevalence of Mycobacterium avium complex-related pulmonary disease (MAC-PD) has increased and is an emerging public health concern. This is due to a combination of environmental and geographic factors, dynamic changes in organism virulence and antimicrobial susceptibility, and evolving host susceptibility. Given the dynamic nature of the disease, management of NTM pulmonary disease (NTM-PD) often includes a multimodal approach including antimicrobial therapy, airway clearance techniques, limiting environmental exposures, and reducing susceptibility to NTM through prevention of reflux and maintenance of body weight. This review will explore the most recent concepts in the diagnosis, treatment, and management of individuals with NTM pulmonary infection.
Keywords: non-tuberculous mycobacteria, Mycobacterium avium complex, MAC, Mycobacterium abscessus, environment, bronchiectasis
Epidemiology
In the United States over the past two decades, numerous reports have shown that the prevalence rates for NTM infections are increasing.1 This is likely multifactorial and due to a larger aging population, increasing environmental exposures, increased incidence of chronic lung disease, and increased physician awareness of NTM.1 There exist over 180 species of NTM, most of which do not cause clinical disease.2 Progressive pulmonary disease is most commonly caused by Mycobacterium avium complex, which includes M. avium, M. intracellulare and M. chimera, as well as Mycobacterium abscessus.3 Individuals who are immunosuppressed or have underlying lung disease such as chronic obstructive pulmonary disease (COPD), bronchiectasis, cystic fibrosis, primary ciliary dyskinesia, and alpha-1-antitrypsin deficiency are at increased risk of acquiring NTM.4,5 The disease is also increasing in incidence in individuals without predisposing factors. Given the ubiquitous nature and the increasing incidence of NTM associated lung disease, it becomes imperative to understand how to adequately diagnose and treat this disease. This paper will review the current recommendations on diagnosis, treatment and overall management of patients with non-tuberculous mycobacterial lung disease.
Signs and Symptoms
The symptoms of NTM-PD are varied and non-specific.6 The most common symptoms include a persistent cough, fatigue, unintended weight loss, dyspnea, hemoptysis, increased mucus production, and night sweats.6,7 Chronic NTM pulmonary infection affects 94% of patients and can cause progressive damage to the lungs and over time can impair lung function.7
There are two main radiographic presentations for NTM-PD which are detected using high resolution CT imaging (HRCT). The less severe form is referred to as nodular bronchiectasis and the more severe form causes cavitary lung disease.3 Nodular bronchiectasis is seen in at least 50% of patients with MAC-PD.8 In this condition, the airways become damaged and subsequently dilate and become scarred (Figure 1). This results in the loss of the ability to effectively clear mucus, which then accumulates within the airways and serves as a nidus for NTM growth as well as for growth of other respiratory pathogens.8 Nodules and bronchiectasis are most frequently encountered in the right middle lobe and lingula.9 Nodular bronchiectasis predominantly affects thin, older women of Caucasian or Asian descent, over half of which have no prior history of smoking or underlying lung disease.7 Additional clinical characteristics associated with increased susceptibility to NTM-PD include scoliosis, pectus excavatum, mitral valve prolapse, and mutations in the CFTR gene.10
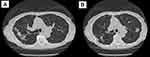 |
Figure 1 (A and B) Nodular bronchiectatic changes in an 80-year-old woman with MAC pulmonary infection. |
Cavitary lung disease occurs as a result of progressive scarring or fibrosis of the lung which results in the formation of cavities (Figure 2). These generally occur within the upper lobes of the lung.11 This condition can cause ongoing fibrosis of the lung and respiratory failure over time. Individuals predominantly affected are prior smokers with underlying COPD or structural lung disease. In addition, individuals with higher disease burden or with M. abscessus lung infection are also more likely to have the cavitary form of NTM infection.11
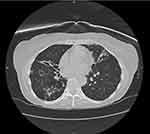 |
Figure 2 Cavitary bronchiectatic changes in a 65-year-old woman with MAC pulmonary infection. |
While pulmonary manifestations of NTM remain the most encountered, these infections can also involve the skin, bones, and lymph nodes.7 Disseminated infections rarely occur but are a risk in individuals who are immunocompromised.7
Diagnosis
A diagnosis of NTM-PD requires characteristic symptoms in addition to clinical evaluation with laboratory testing. The American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) have published joint guidelines, most recently updated in 2020, which outline the diagnostic criteria for NTM-PD. As per these guidelines, the affected individuals must meet a combination of clinical, radiographic, and microbiologic criteria to establish a formal diagnosis of NTM, as outlined in Table 1.12
 |
Table 1 Clinical, Radiographic, and Microbiologic Criteria for the Diagnosis of NTM |
While chest x-rays can be used to examine the lung, high resolution CT imaging is able to give sharper and more detailed images of the lungs and is preferable for evaluation of NTM-PD.
The diagnosis of NTM-PD requires microbiologic confirmation from a laboratory with the capability to perform accurate identification of NTM species and in vitro drug susceptibility testing. NTM infections have been classified into rapidly growing and slowly growing mycobacteria.12 Rapidly growing mycobacteria usually grow in culture within a week and include: M. abscessus, M. chelonae, and M. fortuitum. M. abscessus has three subspecies which include M. abscessus abscessus, M. abscessus massiliense, and M. abscessus bolletii. It is important to distinguish between the subspecies of M. abscessus as certain subspecies carry mutational resistance genes, which then affect treatment options. Slowly growing mycobacteria include the most common species, MAC, as well as M. kansasii. On liquid media, these take 10–14 days to grow; however, on solid media, can take up to 2–6 weeks to grow. M. kansasii is pathogenic and often presents as upper lobe predominant cavitary disease.12
Positive cultures for NTM must be interpreted cautiously as these organisms can be present without causing overt lung infection. Additionally, given their ubiquitous presence in the environment, they can sometimes contaminate laboratory specimens.
Treatment
The management of NTM pulmonary disease (NTM-PD) is complex and warrants a multimodal approach that includes antimicrobial therapy in carefully selected patients, mucus clearance techniques, implementation of environmental and lifestyle modifications to limit exposure to NTM, maintenance of a healthy body weight, and minimizing gastroesophageal reflux.12,13 First, we will discuss antibiotic therapy, as this is the mainstay of treatment for NTM-PD. Later, we will discuss adjunctive therapies and ways in which to reduce environmental exposure to NTM.
When discussing treatment of NTM-PD, it is important to note that not all patients will require therapeutic intervention. When making the decision on whether to treat a patient with NTM-PD, multiple considerations must be made. These include microbiologic, radiographic, and clinical findings. A discussion between the clinician and patient is necessary as treatment regimens may be difficult to tolerate due to adverse effects and require patients to adhere to a lengthy duration of therapy, highlighting the importance of assessing a patient’s symptom burden and disease severity and weighing this against potential risks of treatment.
As mentioned above, NTM-PD may manifest radiographically as nodular bronchiectatic or fibrocavitary disease. The latter form of disease has been associated with more rapid progression and increased disease severity, so treatment is generally recommended for this cohort. This is in contrast to patients with nodular bronchiectatic disease, as these patients tend to have variable clinical presentations so the decision to treat is often made on a case-by-case basis. Factors that may lead a clinician to recommend therapy include severe symptomatology, progressive radiographic changes, or a high burden of organisms as indicated by smear positivity.12 The latter has been demonstrated in studies to be an independent predictor of radiographic progression.14,15 Conversely, an elderly patient with multiple comorbidities and relatively mild disease may not benefit from treatment. If the decision has been made to observe a patient rather than treat with antibiotics, the patient must be regularly assessed for disease progression and, if this occurs, initiation of therapy should be reconsidered.7 Predictors of disease progression include low body mass index (specifically, a lower visceral fat ratio), the presence of pulmonary cavitation (felt to be related to higher bacterial burden in the cavity), serum albumin <3.5 g/dL (reflective of severe inflammation) and forced vital capacity (FVC) <80% predicted.16
Treatment Regimens and Antimicrobial Susceptibility Testing
Antibiotics are the mainstay of treatment for NTM-PD. Treatment regimens vary depending on which species of NTM is isolated (more specifically, slowly growing versus rapidly growing species), severity of disease, as well as in vivo antibiotic susceptibility testing. Recommendations for treatment have been outlined in a recently updated clinical practice guideline published by the American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) in 2020.12 Treatment will be subdivided below based on the most common species that cause NTM-PD. It should be mentioned that there have been no large-scale clinical trials that have tested these treatment regimens, with the exception of a recently published trial investigating inhaled liposomal amikacin suspension, which will be discussed in more detail below. Current recommendations are based on small series of patients and expert opinion. Table 2 summarizes dosing guidelines for drug use in the management of NTM-PD. This was modified from the ATS/IDSA 2020 clinical practice guideline. Please note that some medications require dosage adjustment for hepatic or renal impairment and this is not reflected in Table 2.
 |
Table 2 Dosing Guidelines for Antimicrobials Used More Commonly in the Treatment of NTM-PD |
Susceptibility-based treatment is preferred over empiric antibiotic therapy. In patients with MAC and M. abscessus, susceptibility testing should be performed for macrolides and amikacin.12 Acquired macrolide resistance occurs due a 23S rRNA gene mutation and acquired resistance to amikacin is due to a mutation in the 16S rRNA gene.12 Specific to M. abscessus is the need for at least a 14-day incubation period to evaluate for potential inducible resistance to macrolides via the erythromycin resistance methylase (erm(41)) gene.12 More on this will be discussed below. In patients with M. kansasii, susceptibility testing should be done for rifampicin and clarithromycin and, if resistance to rifampicin is encountered, susceptibility testing for other antibiotics such as amikacin and linezolid is recommended.13 There is insufficient data to confirm the benefit of susceptibility testing for other antibiotics used in the treatment of NTM-PD, but it is often used to guide therapy in macrolide-resistant organisms.
Treatment of MAC
ATS/IDSA guidelines suggest a three-drug regimen consisting of a macrolide, ethambutol, and a rifamycin to treat MAC-PD.12 Retrospective studies have reported sputum conversion rates of 75–86% with macrolide-containing regimens.17,18 Macrolide susceptibility has been consistently shown to be a predictor of treatment success; thus, macrolides are the foundation of MAC-PD therapy, specifically clarithromycin and azithromycin. Further proof of the superiority of macrolides in the treatment of MAC-PD is the reduced rate of conversion of sputum cultures to negative and increased mortality when macrolides are dropped from treatment regimens.19 No head-to-head studies have been performed comparing clarithromycin and azithromycin; however, azithromycin is preferred due to its improved tolerability, less drug–drug interactions, and equal efficacy.18,20 Ethambutol is important as a companion drug for preventing macrolide resistance from emerging.12 The role of rifamycin is less clear but is thought to further reduce the development of macrolide resistance.
Two randomized controlled trials have evaluated the efficacy of two-drug compared to three-drug regimens. A more recent study published in 2014 evaluated the combination of clarithromycin and ethambutol with or without rifampicin (taken daily for 12 months) in the treatment of MAC-PD and found improved rates of sputum culture conversion in those treated with two-drug regimens (55% vs 40.6% in intention-to-treat analysis).21 The study has been criticized due to its unblinded nature as well as small sample size (59 patients) with loss of patients during the study, resulting in the study being underpowered to detect a between-group difference in rates of macrolide resistance. Serum clarithromycin concentrations are lowered by the rifampicin-mediated induction of CYP3A4. In this study, the dose of clarithromycin used was below that recommended by the ATS/IDSA, thus the addition of rifampicin may have led to ineffective serum levels of clarithromycin, potentially influencing the results. According to the ATS/IDSA NTM guidelines, the currently available literature is insufficient to determine the risk of acquired macrolide resistance with a two-drug rather than a three-drug regimen, reiterating the recommendation for three-drug treatment regimens.12 Macrolide monotherapy is strongly advised against, due to the increased risk of developing macrolide resistance.22,23
Intermittent dosing of therapy (ie, three times weekly) is recommended for patients with non-severe nodular bronchiectatic disease.12 Three times weekly therapy in this patient population has been shown to be as effective as daily therapy with regard to symptomatic and radiographic improvement as well as sputum conversion rates, although no randomized controlled trials have been done to specifically evaluate this.12,18 Importantly, intermittent therapy has not been shown to increase the rates of macrolide resistance.12,15 Escalation to daily therapy is advised in those patients who fail to respond to intermittent dosing (ie, those who fail to convert sputum cultures to negative), as this has been shown to achieve success in an additional 30% of patients.24
For patients with severe nodular bronchiectatic or fibrocavitary MAC-PD, daily triple-drug therapy is recommended over three times weekly therapy.12 The addition of a parenteral aminoglycoside is suggested for patients with extensive cavitary disease, those who progress despite daily three-drug therapy, and those with macrolide-resistant MAC-PD.12 The recommended dosing interval is intramuscular streptomycin or intravenous amikacin 10–15mg/kg three times a week for at least 2–3 months, although the dosing of amikacin in studies has varied and often requires dosage adjustment based on patient tolerability.12,25,26 Lower doses at 5–8mg/kg may be considered in those older than age 50, with weight <50kg, or in those receiving >3 months of parenteral therapy.13 Although not studied in any randomized controlled trials, experts recommend monitoring of serum drug concentrations for patients on amikacin or streptomycin who are at risk for ototoxicity and nephrotoxicity.
Inhaled amikacin has also been recommended as add-on therapy for patients with MAC-PD who have failed to convert sputum cultures to negative despite at least 6 months of guideline-directed treatment (refractory MAC-PD).12,27 A more recent prospective randomized controlled trial (CONVERT) published in 2018 investigated the safety and efficacy of amikacin liposome inhalation suspension (ALIS) added to standard three-drug regimens in 336 patients with refractory MAC-PD.27 The investigators found that 29% of patients treated with ALIS achieved culture conversion compared to only 8.9% of patients on guideline-directed therapy alone.27 Patients treated with ALIS experienced more primarily respiratory adverse events, including hoarseness, cough, dyspnea, and bronchospasm; however, serious adverse events were comparable between groups, including respiratory infections and hemoptysis.27 Audiologic adverse effects were similar between groups.27 Of note, most adverse effects were mild to moderate in severity and typically reported in the first month of treatment with ALIS, with less incidence thereafter.27 Based on results of this study, ALIS was FDA approved for difficult-to-treat MAC-PD at a dosage of 590mg daily.27
Treatment of M. abscessus
Mycobacterium abscessus is a unique species of NTM in that certain strains of M. abscessus not only develop mutational macrolide resistance via the 23S rRNA gene but can also induce macrolide resistance in the presence of a macrolide, thus macrolide susceptibility testing becomes very important when managing patients with M. abscessus, as briefly mentioned above.19 Inducible or mutational resistance is conveyed through a functional erm(41) gene, which may be found in M. abscessus subspecies M. abscessus and M. bolletii.12,19 The erm(41) gene is non-functional in subspecies M. massiliense, which renders this subspecies susceptible to macrolides.12,19 Not surprisingly, subspecies without inducible macrolide resistance were more likely to convert sputum cultures to negative and thus have improved treatment outcomes compared to those with macrolide resistance, highlighting the importance of macrolide therapy in treatment of M. abscessus.12,35,36
A macrolide-containing three-drug regimen is preferred in patients without inducible or mutational macrolide resistance.12 In strains with inducible resistance, a macrolide-containing regimen is still suggested for its immunomodulatory effects alone but should not be considered an active drug in the treatment regimen, which should consist of at least 3 additional drugs active against the subspecies in the initial phase of treatment and at least 2–3 active drugs in the continuation phase of therapy, guided by in vitro susceptibility testing.12 Therapy is recommended to be given daily and should be continued for >12 months, although the optimal treatment duration is not clear.12 A typical treatment regimen includes induction therapy with ≥2 parenteral antibiotics (choices include amikacin, imipenem, tigecycline) for 6–8 weeks, followed by maintenance therapy with clofazimine and inhaled amikacin, with or without azithromycin.12
Treatment of M. Kansasii
Rifampicin-susceptible strains of M. kansasii pulmonary disease (PD) should be treated with rifampicin, ethambutol, and either isoniazid or a macrolide.12 Unlike with MAC-PD, patients with M. kansasii PD should not be treated with parenteral aminoglycosides, as the previously mentioned three-drug regimens have been shown to be highly effective in achieving culture conversion and thus the risk of adverse effects with amikacin or streptomycin likely outweigh any potential benefit.12 The ATS/IDSA panel do suggest that a parenteral aminoglycoside may be considered in patients with M. kansasii PD only if a rifampicin cannot be used or in very severe disease.12
Second-Line Therapies
Macrolide resistance presents a challenge when treating patients with MAC-PD. As alluded to above, the development of macrolide-resistant MAC-PD conveys poorer patient outcomes and increased mortality.23 In this patient group, the addition of second-line drugs is usually necessary. Options include clofazimine, bedaquiline, and delamanid.
Two observational studies performed in the last decade have evaluated the safety, efficacy, and clinical and microbiologic outcomes in patients treated with clofazimine, which is an oral bactericidal antimicrobial agent primarily used in the treatment of leprosy and which has been shown to have excellent in vitro activity against MAC and other species of NTM.13,28 Clofazimine has been shown to be safe and tolerable in the treatment of NTM-PD.29,30 Most commonly, patients report skin discoloration while taking the medication, although this typically does not lead to drug discontinuation.29 One particular study evaluating outcomes found that 95% of patients treated with a clofazimine-containing regimen converted from positive to negative sputum cultures in a mean of 4.5 ± 4.2 months.31
Bedaquiline is a diarylquinolone used to treat multidrug-resistant tuberculosis (MDR-TB) and has shown promise as salvage therapy for MAC and M. abscessus in small case series.32,33 In patients treated with bedaquiline, rifabutin rather than rifampin should be used if a rifamycin is needed, because both bedaquiline and rifampin are metabolized by cytochrome P-450.13
Much like bedaquiline, delamanid is a novel drug used in the treatment of MDR-TB and has demonstrated in vitro activity against certain strains of NTM but in vivo activity has not been tested.34 There have not been any clinical trials published to date regarding the use of either drug in treatment of NTM-PD.
In patients with rifampicin-resistant M. kansasii disease or are unable to tolerate one of the first-line drugs, second-line treatment includes the addition of a fluoroquinolone.12 Recommendations for intermittent versus daily dosing are similar for M. kansasii PD as with MAC-PD.12
Role of Surgery
Surgical resection by a surgeon experienced in mycobacterial lung disease may be considered in patients with localized disease, such as focal bronchiectasis or cavitary disease, and in patients with refractory severe symptoms (ie, hemoptysis) who fail to respond to medical management.12,37,38 Previous studies evaluating surgical outcomes after adjunctive surgery have found variable rates of sputum culture conversion and post-operative complications, ranging from 57–100% and 0–46%, respectively.12,37 Improved outcomes have been achieved in patients with negative sputum cultures pre-operatively and in patients who undergo a partial lung resection rather than a pneumonectomy.12,37 However, the optimal surgical candidate and timing of surgical invention has not been clearly defined and the long-term outcomes of patients undergoing adjunctive surgery are not yet known.
Treatment Duration, Therapeutic Goals, and Surveillance
Once initiated, the recommended duration of treatment for NTM-PD is at least 12 months after sputum cultures convert from positive for infection to negative (referred to as culture conversion); however, the optimal duration of therapy is not known.12,16 The goal of treatment includes both clinical and radiologic improvement and achievement of negative sputum cultures whilst limiting treatment-associated adverse effects. Sputum cultures should be monitored at regular intervals. Close monitoring of patients while on treatment for NTM-PD is very important in order to identify treatment failures and medication side effects early. As alluded to above, patients may have difficulty adhering to therapy so it is prudent to ask about a patient’s adherence to all prescribed medications at regular intervals. It is important to assess for adverse effects of treatment in patients with NTM. Some of the most common side effects include adverse gastrointestinal, audiologic (hearing loss, tinnitus), and hepatotoxic effects, thus it is important to monitor patients clinically and with regular audiologic examinations as well as routine bloodwork, including liver function testing.12 Medications such as macrolides and clofazimine may prolong the QT interval, so a baseline EKG should be obtained and repeated at regular intervals.12 Ethambutol may cause changes to visual acuity and/or color vision, so patients taking this medication should be referred for routine ophthalmologic examination.12 Patients should also be counseled to monitor for peripheral neuropathy from ethambutol. Rifampin can cause cytopenias and hepatotoxicity so regular bloodwork to monitor liver function tests and complete blood counts should be performed. Patients on clofazimine should be made aware of the potential for skin tanning and dryness.12 Streptomycins may cause nephrotoxicity and/or derangements in electrolytes, so serum creatinine and metabolic profiles should be monitored in these patients.12
Adjunctive Therapies
Effective mucus clearance using a multimodal approach is an important part of the management of NTM-PD. This can be achieved through the use of non-pharmacologic methods such as aerobic exercise, manual chest percussion, postural drainage maneuvers, chest wall oscillation vests, and positive expiratory pressure devices or through inhaled medications, such as beta agonists followed by nebulized hypertonic saline or N-acetyl cysteine. Oral mucolytics such as guaifenesin are also useful in some patients.
Prevention
The recurrence rate of NTM-PD has been reported to be as high as 30–50%.18 A study published in 2015 evaluated risk factors for recurrence after successful treatment of NTM-PD and found increased rates of recurrence in patients with the nodular bronchiectatic form of NTM-PD compared to those with fibrocavitary disease.39 In this study and others, the majority of recurrences have been attributed to re-infection with a new genotype rather than recurrent infection with the same isolate.18,39 Lessening exposure to NTM may reduce the risk of initial infection and recurrence after treatment.
Since most reinfections come from repeated environmental exposures, an effort should be made by susceptible patients to reduce environmental exposures to NTM, which include house dust,40,41 garden soils,42,43 and water sources such as showerheads,44,45 sink faucets,46–48 underground water pipes,49–51 indoor swimming pools,52 hot tubs,53 continuous positive airway pressure machines,54 and humidifiers.55 There have been no studies proving that limiting environmental exposure to NTM will reduce disease burden, but it appears to be prudent to try to reduce exposures through some of the following household and lifestyle modifications.
Household Plumbing
Granular activated charcoal filters should not be used on faucets and showerheads as NTM can accumulate on their surface, and they do not filter out mycobacteria. Instead, specialized 0.2 micrometer filters can be used, which do filter out NTM, but also can become clogged and need to be changed frequently. NTM have been isolated in high numbers from refrigerator taps and ice machines, and avoiding these should be considered in individuals at risk for NTM infection. Showerheads and shower aerosols have also been found to harbor high concentrations of NTM, and are likely the predominant mode of exposure of individuals to NTM in the environment.45,46,56–58 NTM adhere more to the biofilm of plastic showerheads than metal, and these can be cleaned manually on a regular basis, and then disinfected by immersing in undiluted bleach for 30 minutes or inserting into boiling water. Showerheads with larger diameter holes likely produced less aerosol-containing NTM than those that produce a mist with smaller particles. Finally, patients should be instructed to minimize the amount of time spent in the shower and to ventilate the bathroom as much as possible.
Hot water heaters are likely serving as incubators for NTM within the home environment. They should be drained on a regular basis, as the sediment which accumulates at the bottom of the tank contains the highest concentration of NTM. In addition, the hot water heater temperature should be kept at 120°F, as tanks kept at this temperature were shown to have less NTM growth than those kept at or below 110° F.59
Changes in Individual Behaviors and Habits
Tabletop humidifiers as well as humidifiers attached to central heating units have been shown to harbor NTM and should be avoided.58 Continuous positive airway pressure machine humidifiers should be filled with either sterile water or previously boiled water which has then cooled to room temperature.54 Hot tubs and steam rooms should be avoided, and patients should consider avoiding the use of indoor pools,52,53 as this has been associated with NTM infection in individuals with cystic fibrosis,60 with the air above the pool containing more NTM than the pool water itself.61 Individuals who garden should be counseled to moisten potting soil prior to using it in order to decrease the generation of dust aerosols, and to consider wearing an N95 mask while gardening, as NTM have been found in natural and commercial soil and peat and their aerosols, with concentrations as high as one million organisms per gram.40,42,62 Caution should also be used with exposure to household and vacuum cleaner dust which have been shown to contain NTM.40,41
Association with Gastroesophageal Reflux Disease
It has been hypothesized that MAC organisms may be ingested through drinking water and subsequently aspirated into the respiratory tract in the setting of reflux, particularly silent nocturnal reflux.63 Such reflux and aspiration may also result in local damage to the airways which then may predispose individuals to MAC-PD after environmental aerosol exposure. In order to try to mitigate the possible contribution of reflux to MAC-PD, drinking water can be sterilized by 10 minutes of boiling and measures to try to prevent gastroesophageal reflux can be employed, such as not eating immediately prior to lying down, avoiding foods that aggravate reflux and sleeping with the head of bed elevated above 30 degrees.64,65 Finally, since patients with a lower body mass index have an increased susceptibility to NTM infection, trying to avoid weight loss is an essential aspect of the management of NTM infection.66
Conclusion
The diagnosis and management of MAC-PD requires a comprehensive understanding of the genetic and environmental risk factors that predispose individuals to infections and progressive lung disease. Furthermore, a multimodal approach to treatment including antimicrobial therapy, mucous clearance regimens, attenuation of reflux, and minimizing environmental exposures is crucial to achieving long-term control of disease.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Winthrop KL, Marras TK, Adjemian J, et al. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008–2015. Ann American Thorac Soc. 2020;17(2):178–185. doi:10.1513/AnnalsATS.201804-236OC
2. Aksamit T, Carreon M, Daley C, et al. Nontuberculous mycobacteria (NTM). CHEST Foundation; 2021. Available from: https://foundation.chestnet.org/lung-health-a-z/nontuberculous-mycobacteria-ntm/.
3. Ryu YJ, Koh WJ, Daley CL. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians’ perspectives. Tuberc Respir Dis. 2016;79(2):74–84. doi:10.4046/trd.2016.79.2.74
4. Prince DS, Peterson DD, Steiner RM, et al. Infection with mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321:863–868. doi:10.1056/NEJM198909283211304
5. Daley CL, Winthrop KL. Mycobacterium avium complex: addressing gaps in diagnosis and management. J Infect Dis. 2020;222(4):199–211. doi:10.1093/infdis/jiaa354
6. Nontuberculous mycobacteria (NTM) infections. Centers for disease control and prevention; 2019. Available from: https://www.cdc.gov/hai/organisms/ntm/clinicians.html.
7. Lande L Nontuberculous mycobacterial lung disease. The National Organization for Rare Disorders; 2015. Available from: https://rarediseases.org/rare-diseases/nontuberculous-mycobacterial-lung-disease/.
8. Lee G, Lee KS, Moon JW, et al. Nodular bronchiectatic mycobacterium avium complex pulmonary disease: natural course on serial computed tomographic scans. Ann Am Thorac Soc. 2013;10(4):299–306. doi:10.1513/AnnalsATS.201303-062OC
9. Levin DL. Radiology of pulmonary mycobacterium avium-intracellulare complex. Clin Chest Med. 2002;23(3):603–612. doi:10.1016/S0272-5231(02)00009-6
10. Kim R, Greenberg D, Ehrmantraut M, et al. Pulmonary nontuberculous mycobacterial disease prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2013;178:1066–1074. doi:10.1164/rccm.200805-686OC
11. Kwon YS, Koh WJ. Diagnosis and treatment of nontuberculous mycobacterial lung disease. J Korean Med Sci. 2016;31(5):649–659. doi:10.3346/jkms.2016.31.5.649
12. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71(4):e1–e36. doi:10.1093/cid/ciaa241
13. Lande L, George J, Plush T. Mycobacterium avium complex pulmonary disease: new epidemiology and management concepts. Curr Opin Infect Dis. 2018;31(2):199–207. doi:10.1097/QCO.0000000000000437
14. Pan S, Shu C, Feng J, et al. Treatment for Mycobacterium avium complex lung disease. J Formos Med Assoc. 2020;119(1):S67–S75. doi:10.1016/j.jfma.2020.05.006
15. Hwang JA, Kim S, Jo KW, et al. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49(3):1600537. doi:10.1183/13993003.00537-2016
16. Kim SJ, Yoon SH, Choi SM, et al. Characteristics associated with progression in patients with of nontuberculous mycobacterial lung disease: a prospective cohort study. BMC Pulm Med. 2017;17(5):1–8. doi:10.1186/s12890-016-0349-3
17. Jeong BH, Jeon K, Park HY, et al. Intermittent antibiotic therapy for nodular bronchiectatic mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015;191:96–103. doi:10.1164/rccm.201408-1545OC
18. Wallace RJ
19. Nash KA, Brown-Elliott BA, Wallace RJ
20. Rubinstein E. Comparative safety of the different macrolides. Int J Antimicrob Agents. 2001;18(Suppl 1):S71–6. doi:10.1016/S0924-8579(01)00397-1
21. Miwa S, Shirai M, Toyoshima M, et al. Efficacy of clarithromycin and ethambutol for mycobacterium avium complex pulmonary disease: a preliminary study. Ann Am Thorac Soc. 2014;11:23–29. doi:10.1513/AnnalsATS.201308-266OC
22. Nash KA, Inderlied CB. Genetic basis of macrolide resistance in mycobacterium avium isolated from patients with disseminated disease. Antimicrob Agents Chemother. 1995;39(12):2625–2630. doi:10.1128/AAC.39.12.2625
23. Park Y, Lee EH, Jung I, et al. Clinical characteristics and treatment outcomes of patients with macrolide-resistant mycobacterium avium complex pulmonary disease: a systematic review and meta-analysis. Respir Res. 2019;20(1):1–10. doi:10.1186/s12931-019-1258-9
24. Koh WJ, Jeong BH, Jeon K, et al. Response to switch from intermittent therapy to daily therapy for refractory nodular bronchiectatic mycobacterium avium complex lung disease. Antimicrob Agents Chemother. 2015;59:4994–4996. doi:10.1128/AAC.00648-15
25. Yagi K, Ishii M, Namkoong H, et al. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect Dis. 2017;17:558. doi:10.1186/s12879-017-2665-5
26. Olivier KN, Shaw PA, Glaser TS, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc. 2014;11:30–35. doi:10.1513/AnnalsATS.201307-231OC
27. Griffith DE, Eagle G, Thomson R, et al.; CONVERT Study Group. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198(12):1559–1569. doi:10.1164/rccm.201807-1318OC
28. National Center for Biotechnology Information. PubChem compound summary for CID 2794, Clofazimine. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Clofazimine.
29. Martiniano SL, Wagner BD, Levin A, et al. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest. 2017;152:800–809. doi:10.1016/j.chest.2017.04.175
30. Garrelts JC. Clofazimine: a review of its use in leprosy and mycobacterium avium complex infection. DICP. 1991;25(5):525–531. doi:10.1177/106002809102500513
31. Jarand J, Davis JP, Cowie RL, et al. Long-term follow-up of mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest. 2016;149:1285–1293. doi:10.1378/chest.15-0543
32. Philley JV, Wallace RJ, Benwill JL, et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest. 2015;148(2):499–506. doi:10.1378/chest.14-2764
33. Gil E, Sweeney N, Barrett V, et al. Bedaquiline as treatment for disseminated nontuberculous mycobacteria infection in 2 patients co-infected with HIV. Emerg Infect Dis. 2021;27(3):944–948. doi:10.3201/eid2703.202359
34. Kim DH, Jhun BW, Moon SM, et al. In vitro activity of bedaquiline and delamanid against nontuberculous mycobacteria, including macrolide-resistant clinical isolates. Antimicrob Agents Chemother. 2019;63(8):e00665–19. doi:10.1128/AAC.00665-19
35. Koh WJ, Jeon K, Lee NY, et al. Clinical significance of differentiation of mycobacterium massiliense from mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405–410. doi:10.1164/rccm.201003-0395OC
36. Koh WJ, Jeong BH, Kim SY, et al. Mycobacterial characteristics and treatment outcomes in mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64:309–316. doi:10.1093/cid/ciw724
37. Kim JY, Park S, Park IK, et al. Outcomes of adjunctive surgery for nontuberculous mycobacterial pulmonary disease. BMC Pulm Med. 2021;21(312):1–9. doi:10.1186/s12890-021-01679-0
38. Mitchell JD. Surgical treatment of pulmonary nontuberculous mycobacterial infections. Thorac Surg Clin. 2019;29(1):77–83. doi:10.1016/j.thorsurg.2018.09.011
39. Lee BY, Kim S, Hong Y, et al. Risk factors for recurrence after successful treatment of mycobacterium avium complex lung disease. Antimicrob Agents Chemother. 2015;59(6):2972–2977. doi:10.1128/AAC.04577-14
40. Dawson D. Potential pathogens among strains of mycobacteria isolated from house-dusts. Med J Aust. 1971;1:679–681. doi:10.5694/j.1326-5377.1971.tb87787.x
41. Torvinen E, Torkko P, Rintala ANH. Real-time PCR detection of environmental mycobacteria in house dust. J Microbiol Methods. 2010;82:78–84. doi:10.1016/j.mimet.2010.04.007
42. De Groote MA, Pace NR, Fulton K, Falkinham JO. Relationships between mycobacterium isolates from patients with pulmonary mycobacterial infection and potting soils. Appl Environ Microbiol. 2006;72:7602–7606. doi:10.1128/AEM.00930-06
43. Iivanainen E, Martikainen P, Raisanen M, Katila M. Mycobacteria in coniferous forest soils. FEMS Microbiol Ecol. 1997;23:325–332. doi:10.1016/S0168-6496(97)00040-8
44. Falkinham JO, Iseman M, de Haas P, van Soolingen D. Mycobacterium avium in a shower linked to pulmonary disease. J Water Health. 2008;6:209–213. doi:10.2166/wh.2008.232
45. Feazel L, Baumgartner L, Peterson K, et al. Opportunistic pathogens enriched in showerhead biofilms. PNAS. 2009;106:16393–16399. doi:10.1073/pnas.0908446106
46. Thomson R, Tolson C, Carter R, et al. Isolation of NTM from household water and shower aerosols in patients with NTM pulmonary disease. J Clin Microbiol. 2013;51:3006–3011. doi:10.1128/JCM.00899-13
47. Slosarek M, Kubin M, Jaresova M. Water-borne household infections due to mycobacterium enoki. Central Eur J Publ Hlth. 1993;1:78–80.
48. Donohue MJ, Mistry JH, Donohue JM, et al. Increased frequency of nontuberculous mycobacteria detection at potable water taps within the United States. Environ Sci Technol. 2015;49(10):6127–6133. doi:10.1021/acs.est.5b00496
49. Thomson R, Carter R, Tolson C, et al. Factors associated with the isolation of nontuberculous mycobacteria (NTM) from a large municipal water system in Brisbane, Australia. BMC Micro. 2013;13:89. doi:10.1186/1471-2180-13-89
50. Torvinen E, Suomalainen S, Lehtola M, et al. Mycobacteria in water and loose deposits of drinking water distribution systems in Finland. Appl Environ Microbiol. 2004;70:1973–1981. doi:10.1128/AEM.70.4.1973-1981.2004
51. Covert T, Rodgers M, Reyes A, Stelma JG. Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol. 1999;65:2492–2496. doi:10.1128/AEM.65.6.2492-2496.1999
52. Iivanainen E, Arbeit RD, Ristola M, et al. Isolation of mycobacteria from indoor swimming pools in Finland. APMIS. 1999;107:193–200. doi:10.1111/j.1699-0463.1999.tb01544.x
53. Mangione EJ, Huitt G, Lenaway D, et al. Nontuberculous mycobacterial disease following hot tub exposure. Emerg Infect Dis. 2001;7:1039. doi:10.3201/eid0706.010623
54. Assi MA, Beg JC, Marshall WF, et al. Mycobacterium gordonae pulmonary disease associated with a continuous positive airway pressure device. Transpl Infect Dis. 2007;9:249–252. doi:10.1111/j.1399-3062.2007.00202.x
55. Falkinham JO. Ecology of nontuberculous mycobacteria – where do human infections come from? Semin Respir Crit Care Med. 2013;34:95–102. doi:10.1055/s-0033-1333568
56. Falkinham JO. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis. 2011;17:419–424. doi:10.3201/eid1703.101510
57. Nishiuchi Y, Maekura R, Kitada S, et al. The recovery of mycobacterium avium-intracellulare complex (MAC) from the residential bathrooms of patients with pulmonary MAC. Clin Infect Dis. 2007;45:347–351. doi:10.1086/519383
58. Lande L, Peterson D, Sawicki J, et al. Municipal water supply as a major source for mycobacterium avium pulmonary disease: a comparison of household and respiratory isolates. Am J Respir Crit Care Med. 2013;187:A5100.
59. Lande L, Kwait R, Williams M. Hot water heaters are serving as incubators for nontuberculous mycobacteria in the home environment. Am J Respir Crit Care Med. 2015;191:A5270.
60. Prevots DR, Adjemian J, Fernandez AG, et al. Environmental risks for nontuberculous mycobacteria. Individual exposures and climatic factors in the cystic fibrosis population. Ann Am Thorac Soc. 2014;11:1032–1038.
61. Angenent L, Kelley S, Amand A, et al. Molecular identification of potential pathogens in water and air of a hospital therapy pool. PNAS. 2005;102:4860–4865. doi:10.1073/pnas.0501235102
62. Ichiyama S, Shimokata K, Tsukamura M. The isolation of mycobacterium avium complex from soil, water and dusts. Microbiol Immunol. 1998;32:733–739. doi:10.1111/j.1348-0421.1988.tb01434.x
63. Lande L, Rains E, Kwait R, et al. Detection of aspiration in patients with mycobacterium avium complex pulmonary infection. Am J Respir Crit Care Med. 2017;195:A5067.
64. Scott DR, Simon RA. Supraesophageal reflux: correlation of position and occurrence of acid reflux – effect of head-of-bed elevation on supine reflux. J Allergy Clin Immunol Pract. 2015;3:356–361. doi:10.1016/j.jaip.2014.11.019
65. Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomized trial. Lancet. 1999;354:1851–1858. doi:10.1016/S0140-6736(98)12251-1
66. Kartalija M, Outski AR, Bryan CL, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med. 2013;187:197–205. doi:10.1164/rccm.201206-1035OC
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
