Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Metabolic Tumor Volume Measured by 18F-FDG PET/CT is Associated with the Survival of Unresectable Hepatocellular Carcinoma Treated with PD-1/PD-L1 Inhibitors Plus Molecular Targeted Agents
Authors Wang X, Yang X, Wang J, Dong C, Ding J, Wu M, Wang Y, Ding H, Zhang H, Sang X , Zhao H, Huo L
Received 28 December 2022
Accepted for publication 21 March 2023
Published 8 April 2023 Volume 2023:10 Pages 587—598
DOI https://doi.org/10.2147/JHC.S401647
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Xuezhu Wang,1,* Xu Yang,2,* Jingnan Wang,1 Chengyan Dong,3 Jie Ding,1 Meiqi Wu,1 Yanyu Wang,2 Haiyan Ding,4 Hui Zhang,4 Xinting Sang,2 Haitao Zhao,2 Li Huo1
1Department of Nuclear Medicine, Beijing Key Laboratory of Molecular Targeted Diagnosis and Therapy in Nuclear Medicine, State Key Laboratory of Complex Severe and Rare Diseases, Center for Rare Diseases Research, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 2Department of Liver Surgery, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 3GE Healthcare China, Beijing, People’s Republic of China; 4Department of Biomedical Engineering, Tsinghua University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Huo; Haitao Zhao, #1 Shuaifuyuan, Dongcheng District, Beijing, People’s Republic of China, Tel +86 13910801986; +86 13901246374, Email [email protected]; [email protected]
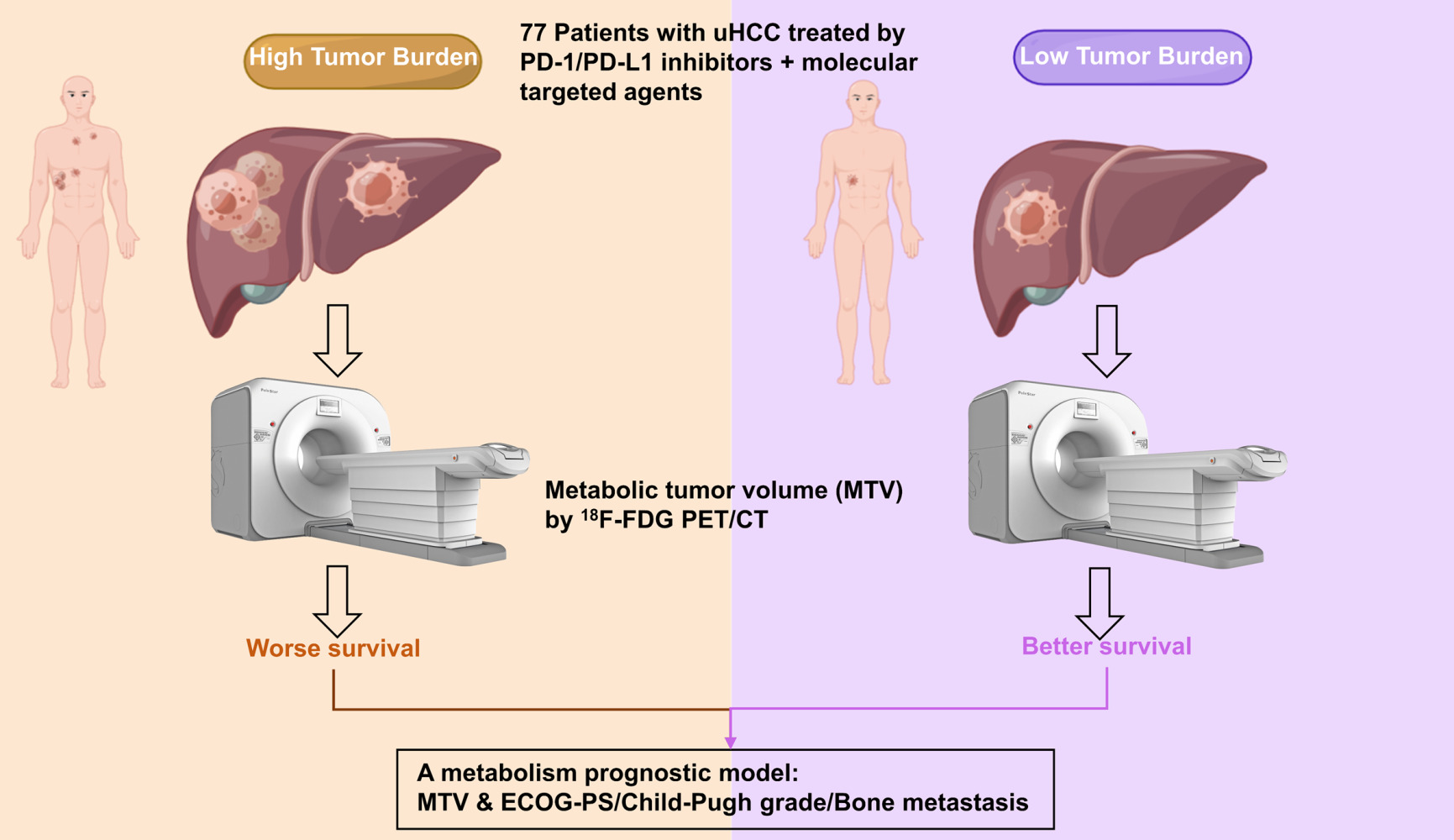
Purpose: The combination of PD-1/PD-L1 inhibitors and molecular targeted agents showed promising efficacy for unresectable hepatocellular carcinoma (uHCC). This study aimed to investigate the prognostic value of metabolic parameters from 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET/CT) in patients with uHCC underwent the combined therapies.
Patients and Methods: Patients with uHCC treated with a combination of immunotherapy and targeted therapy who underwent baseline 18F-FDG PET/CT between July 2018 and December 2021 were recruited retrospectively. The metabolic tumor volume (MTV), total lesion glycolysis (TLG), maximum standardized uptake values (SUVmax), and clinical and biological parameters were recorded. A multivariate prediction model was developed for overall survival (OS) using these parameters together with clinical prognostic factors.
Results: Seventy-seven patients were finally included. The median OS was 16.8 months. We found that a high MTV (≥ 39.65 cm3 as the median value) was significantly associated with OS (P< 0.05). In multivariate analyses for OS, a high MTV, high Eastern Cooperative Oncology Group performance status (ECOG-PS, ≥ 1), Child-Pugh (B-C) grade, and the presence of bone metastasis were significantly associated with poor OS (HR 1.371, HR 3.73, HR 15.384, and HR 2.994, all P< 0.05, respectively). A multivariate prognostic model including MTV and prognostic factors, such as ECOG-PS, Child-Pugh grade, and bone metastasis, further improved the identification of different OS subgroups.
Conclusion: High MTV is an adverse prognostic factor in patients with uHCC treated with a combination of immunotherapy and molecular targeted agents. Integrating PET/CT parameters with clinical prognostic factors could help to personalize immunotherapy.
Keywords: unresectable hepatocellular carcinoma, PD-1 inhibitor, PD-L1 inhibitor, PET/CT, metabolic tumor volume, prognostic model
Graphical Abstract:
Introduction
Immune checkpoint inhibitor therapy or targeted monotherapy has recently shown considerable efficacy in unresectable hepatocellular carcinoma (uHCC).1,2 Moreover, clinical studies have shown that the combination of anti-PD-1/PD-L1 immunotherapy with targeted therapy encourages efficacy in HCC.3–7 However, only 10.7–37.4% of patients achieve an objective response.8 Thus, there is a clear need for better patient selection before immunotherapy.7,9
Despite significant efforts, only a few clinical, histological, and genetic parameters have been identified to reliably predict the efficacy of immunotherapy for uHCC.9–12 In clinical practice, the utility of biomarkers based on tumor tissue is limited by the available tumor tissue due to spatial heterogeneity.18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET/CT), a non-invasive tool, has been shown to be a promising method for screening surveillance after treatment, evaluation of therapy response, and predicting the outcome.13–15 In patients with uHCC, the highest metabolic activity within the tumor in a two-dimensional region of interest (ROI), as well as the metabolic tumor volume (MTV) and total lesion glycolysis (TLG) in a three-dimensional ROI, are considered to provide valuable prognostic information.16,17 However, there is limited data regarding the prognostic relevance of MTV and TLG in uHCC patients treated with immunotherapy.
Therefore, this study aimed to determine the potential prognostic value of baseline PET/CT parameters for predicting the efficacy of a combination of PD-1/PD-L1 inhibitors and targeted therapy for uHCC. We also examined the integration of PET/CT parameters with clinical characteristics to identify which patients could achieve longer survival.
Materials and Methods
Study Design and Patients
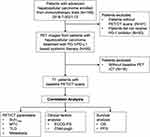
This retrospective study was conducted to investigate the value of standard 18F-FDG PET/CT in predicting outcomes in patients with uHCC undergoing a combination of PD-1/PD-L1 inhibitors and targeted therapy at Peking Union Medical College Hospital (PUMCH). Patients who were scheduled to begin immunotherapy from July 2018 to December 2021 as their first- or later-line systemic treatment for uHCC were retrospectively included in this study. This study was approved by the Ethics Committee of PUMCH, and informed consent was obtained from all participants. The inclusion criteria were as follows: (1) Patients with pathologically confirmed HCC or imaging confirmed as HCC according to the American Association for the Study of Liver Diseases (AASLD) or standard for the diagnosis and treatment of primary liver cancer in 2017 in China,18 (2) patients underwent the combination therapy of a PD-1 inhibitor with a targeted therapy (such as lenvatinib, sorafenib, and apatinib) or PD-L1 inhibitor atezolizumab plus bevacizumab; (3) Eastern Cooperative Oncology Group performance status (ECOG-PS) score of 0–2, and (4) 18F-FDG PET/CT scan pre-immunotherapy or within 3 months post-immunotherapy. The main exclusion criteria includes patients who has no PET/CT imaging during the therapies. This study is registered as NCT03892577.
18F-FDG PET/CT Examination
All 18F-FDG PET/CT scans were performed using a PoleStar m660 PET/CT scanner (SinoUnion Healthcare, Beijing, China) at PUMCH. CT transmission scans (120 kV, 260 effective mA) were conducted for attenuation correction and image fusion.19 Patients were instructed to fast for ≥6 hours before the intravenous injection of 3.70–5.55 MBq/kg of 18F-FDG. Low-dose attenuation CT acquisition was performed (120 kV, 260 mA, 1.8665 mm slice thickness) at 1 h post-administration, followed by a static 3D PET acquisition from vertex to mid-thigh with an image duration of 120 s per bed position, an axial field of view of 22 cm, and a matrix of 192×192 pixels. Three key PET/CT parameters (maximum standardized uptake values, SUVmax; MTV; TLG) were measured using an MIM workstation (MIM Software Inc., USA). Volumetric parameters were defined as described previously.20 The SUVmax was calculated from a 10 mm diameter ROI placed on the highest uptake site of the tumors and for the most intense lesions. To assess tumor burden, MTV was defined as the sum of volumes enclosed by a 41% isocontour around each tumor lesion voxel with the maximum 18F-FDG uptake, as recommended by the European Association of Nuclear Medicine.21 TLG was defined as the average metabolic activity within the tumor multiplied by the tumor volume.
Clinical Characteristics
The following clinical parameters were recorded: age at treatment initiation, gender, immunotherapy start date, prior line systemic target treatments, prior local treatment, current treatment, alpha-fetoprotein (AFP), ECOG-PS, Child-Pugh grade, Barcelona Clinic Liver Cancer (BCLC) stage, numbers of involved organ, extrahepatic spread, presence of bone metastasis, macrovascular invasion, and cirrhosis. Patients were followed up for more than 6 months with regular clinical evaluations. Overall survival (OS) was defined as the time between the start of anti-PD-1/PD-L1 treatment and death due to any cause. Progression-free survival (PFS) was defined as the time from the start of anti-PD-1/PD-L1 treatment to the first documented disease progression or death from any cause based on the Response Evaluation Criteria in Solid Tumors v1.1. Patients were censored at the last follow-up or 1 March 2022, whichever came first.
Statistical Analysis
The normal distribution was assessed using the Kolmogorov–Smirnov test, and continuous variables with a normal distribution are presented as mean ± standard deviation, whereas those with a non-normal distribution are presented as median (interquartile range). For continuous variables, statistical significance was determined using the Student’s t-test or Wilcoxon’s test. A p-value <0.05 for two-sided tests was considered significant. For statistical analysis, all continuous variables were divided into two groups, and specific cutoff values were determined based on median values. Survival functions of patient subgroups defined by SUVmax, MTV, and TLG were estimated using the Kaplan-Meier method and compared using the Log rank test. Univariate analysis was used to identify factors associated with PFS and OS. Prognostic factors identified as significant in the univariate analysis (P<0.1) were then entered into a Cox multivariate regression analysis model. Forward stepwise multivariate regression analysis was performed to identify factors correlated with OS and PFS. In each step, variables with a p-value <0.05 were inputted. The χ2 test was used to analyze the correlations between MTV and clinical characteristics. Statistical analyses were performed using SPSS version 19.0 and R version 4.1.0.
Results
Patient Characteristics
A flow diagram for the inclusion of potentially eligible patients is shown in Figure 1. Overall, 77 patients with uHCC (median age 55 years, range 21–76) were finally included in this retrospective study. Table 1 shows the patients’ clinical and biological characteristics. They were mainly men (84.4%), had cirrhosis (68.8%), and received PD-1 inhibitors (90.9%). Of the 77 patients, 50 patients were treated with local-regional therapy. Baseline 18F-FDG PET/CT scans were also obtained. The median follow-up was 16.8 (5.8–25.4) months. During the follow-up period, 81.8% and 55.8% of patients experienced progression and death, respectively.
 |
Table 1 Characteristics of Patients with Unresectable Hepatocellular Carcinoma |
Association of 18F-FDG PET/CT Parameters with Survival and Clinical Parameters
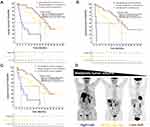
In our study, the cutoff values for the median of SUVmax, MTV, and TLG were 7.12, 39.65 cm3, and 159.2 g, respectively. MTV was significantly associated with OS. The median OS in the high MTV group was 12.8 months (3.7–19.2), while it was 22.8 months (9.6–30.7) in the low MTV group (P=0.044, HR 1.862, 95% CI 1.009–3.437; Figure 2). The prognostic value of TLG had a trend, but it was not significant. The median OS in the high TLG group was 12.8 months (3.73–19.37) compared with 21.5 months (9.58–30.70) in the low TLG group (P=0.056, HR 1.342, 95% CI 0.989–1.823). SUVmax was not significantly associated with OS (16.1 months vs 17.6 months, P=0.37, HR 1.148, 95% CI 0.849–1.553). Moreover, for PFS, the SUVmax, MTV, and TLG were not significantly different (P>0.05, Supplemental Table S1 and Figure S1).
In addition, the clinical features of high and low MTV were investigated. Notably, patients with a high MTV were characterized by being old age (Supplemental Table S2, P<0.05).
Univariate and Multivariate Analyses for PFS and OS
The median OS of 77 patients with uHCC was 16.4 (5.8–25.4) months. In the univariate analysis, high ECOG-PS (≥1), Child-Pugh grade (B-C), the presence of bone metastasis, and a high MTV (≥39.65 cm3) were significantly associated with poor OS (P=0.047; Table 2). Despite a clear trend in the univariate analysis, high TLG (≥159.2 g) was not significantly correlated with poor OS (P=0.059). In the multivariate analysis, a high ECOG-PS, the poor Child-Pugh grade, the presence of bone metastasis, and high MTV remained as independent significant prognostic factors for OS (HR 1.371, 95% CI 1.001–1.878; HR 3.73, 95% CI 1.925–7.231; HR 15.384, 95% CI 14.292–55.556; and HR 2.994, 95% CI 1.425–6.289, respectively; Table 3).
 |
Table 2 Univariate Analysis of Prognostic Factors Associated with Overall Survival in Patients with Unresectable Hepatocellular Carcinoma |
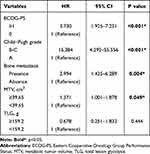 |
Table 3 Multivariate Analysis of Prognostic Factors Associated with Overall Survival in Patients with Unresectable Hepatocellular Carcinoma |
In this cohort, the median PFS was 5.1 (2.6–10.7) months. In the univariate analysis, high ECOG-PS (≥1), the Child-Pugh grade (B-C), and the presence of bone metastasis were significantly associated with poor PFS (P<0.1; Table S2). In the multivariate analysis, a high ECOG-PS and the Child-Pugh grade remained as independent significant prognostic factors for PFS (HR 1.558, 95% CI 1.066–2.276 and HR 2.315, 95% CI 1.377–3.891, respectively; Table S3). However, all PET/CT parameters were not correlated with PFS.
Integrating PET Parameters and Clinical Factors: A Metabolism-Based Prognostic Model
For the multivariate analysis, we developed a prognostic model combining MTV with the ECOG-PS, Child-Pugh grade, and presence of bone metastasis, which were the independent factors for OS. Combining these important clinical factors with MTV provided further patient stratification. Specifically, the three following risk groups were identified: (1) low risk (patients with a low MTV and favorable clinical risk factors); (2) moderate risk (low MTV and unfavorable clinical risk factors or high MTV and favorable clinical risk factors); and (3) high risk (high MTV and unfavorable clinical risk factors). The Kaplan–Meier survival curves of these three groups are shown in Figure 3. The OS in the high-risk group was significantly different from that in all other groups; this combined approach to risk stratification differentiated patients according to survival better than MTV or clinical parameters alone. The median OS in all patients with a high MTV was 12.8 months. For MTV together with ECOG-PS, the median OS of the low, moderate, and high-risk groups were more than 25.5, 15.8, and 4.6 months, respectively. The OS in the high-risk group was significantly shorter than that in the low-risk group (P<0.001) and the moderate-risk group (P<0.001). The OS of the moderate-risk group was not significantly different from that of the low-risk group (P=0.258). Similarly, for MTV with the Child-Pugh grade, the median OS of the low-, moderate-, and high-risk groups were more than 23.4, 12.8, and 2.0 months, respectively. The OS in the high-risk group was significantly shorter than that in the low-risk group (P<0.001) and the moderate-risk group (P<0.001). The OS of the moderate-risk group was significantly shorter than that of the low-risk group (P=0.03). Moreover, for MTV with the presence of bone metastasis, the median OS of the low-, moderate-, and high-risk groups were more than 24.0, 15.6, and 3.4 months, respectively. The OS in the high-risk group was significantly shorter than that in the low-risk group (P<0.001) and the moderate-risk group (P=0.011). The OS of the moderate-risk group was not significantly different from that of the low-risk group (P=0.174).
Discussion
To the best of our knowledge, this is the largest retrospective study to indicate that a high baseline MTV on 18F-FDG PET/CT is predictive of adverse OS in patients with uHCC aiming for treatment with a combination of immunotherapy and targeted therapy. This discriminative and prognostic application of MTV has been used in the past to predict the outcomes of other HCC treatments, including before surgery, transarterial radioembolization, and targeted therapy.22–24 Our study expands these findings to patients with HCC receiving a combination of PD-1/PD-L1 inhibitors and targeted therapy. We propose that it should be taken into consideration when screening appropriate candidates for PD-1/PD-L1 inhibitor-based therapy in future studies.
The metabolic tumor burden is an important prognostic factor for patients treated with PD-1/PD-L1 inhibitor-based immunotherapy. Recently, Ito et al found that melanoma patients with a high baseline MTV of greater than 26.85 cm3 had a significantly shorter OS than those with a low MTV.25 Another cohort with advanced non-small cell lung cancer treated with immunotherapy showed that a high baseline MTV greater than a median of 75 cm3 was correlated with poor OS.26 It was confirmed in our cohort of uHCC patients that those with high MTV (above a cutoff value of a median of 39.65 cm3) had poorer OS. The discrepancy in the above results among the studies is likely associated with the different tumor types, various baseline patient characteristics, and methodological differences. Thus, these findings indicate that MTV should be considered as a predictive biomarker in patients treated with immunotherapy. Wang et al also found that baseline metabolic parameters from 18F-FDG PET could predict the pathological response of 20 HCC patients treated with PD-1 inhibitors and Lenvatinib.15 Emerging evidence has demonstrated that novel methods like granzyme B PET imaging have a higher specificity with immune-related cells such as cytotoxic T cells, which has recently been found to be associated with clinical outcome treated with immunotherapy to immunotherapy via its role in adaptive immunity.27,28 However, the development of the tracer is unfeasible in most hospitals and more multi-center trials are needed to prove its benefits in clinic. 18F-FDG PET has much more clinical applications globally and provides high-sensitivity signals, but the SUV changes may be affected by many factors such as tumor volume, tumor progression, and new detectable lesions, leading to more bias in monitoring immunotherapies.
Our findings suggest a greater impact for prognosticating OS when combining MTV with ECOG-PS and the Child-Pugh grade, which is commonly used to assess liver function in patients with liver disease.29 Many studies have shown that a high Child-Pugh grade is an important factor for poor long-term prognosis in uHCC patients receiving immunotherapy.30 In a cohort of 203 patients with uHCC treated with immunotherapy, the objective response rate was lower in patients with Child-Pugh B grade than in those with A grade (2.8% vs 15.9%; P=0.010), and the median OS was also shorter in Child-Pugh B patients (2.8 vs 10.7 months; HR=2.10; P<0.001).31 The physical condition of the ECOG-PS is also an extremely important risk factor for HCC. Kuo et al reported that a low ECOG-PS was the most important favorable prognostic indicator of OS in multivariate analysis.32 In addition, bone metastasis in HCC may cause significant complications such as spinal cord compression, intractable pain, and pathological fractures, which ultimately lead to a severe decrease in quality of life and a poor outcome.33 We propose that all the above clinical factors could offer timely and direct information to clinical physicians on whether to continue with PD-1 inhibitors and potentially maximize treatment benefits from the combination of a PD-1/PD-L1 inhibitor and targeted therapy in HCC.
Based on these findings, a combination of multiple biomarkers could be an innovative way to improve both prediction and prognostic accuracy.26 We also developed a model combining the ECOG-PS, Child-Pugh grade, and the presence of bone metastasis with MTV at baseline, stratifying the population into three prognostic groups. Patients with poor clinical prognostic factors and a high MTV were characterized by very low OS rates. In our cohort, patients with high clinical prognostic factors (ECOG-PS, Child-Pugh, and bone metastasis) and a high MTV had a short median OS of 2.0–4.6 months; showing they are not suitable for combination immunotherapy considering the cost-effectiveness. Of course, these hypotheses for future applications of combinations of biomarkers in clinical practice and research must be properly tested in prospective randomized clinical trials.
Our study has several potential limitations. First, even if all patients received PD-1/PD-L1-based therapy, there was some heterogeneity in treatment modalities. Moreover, previous studies on the role of 18F-FDG PET/CT in the evaluation of patients with HCC have indicated that 18F-FDG PET/CT metabolic values may depend on the degree of differentiation.34 18F-FDG uptake in well-differentiated HCC is similar to that in normal liver tissue because of the high rate of gluconeogenesis in well-differentiated HCC.35 Thus, some patients with well-differentiated HCC lesions may have a high tumor burden but low metabolic burden from the PET scan, which may influence the results. Finally, this MTV-based prognostic model was constructed based on a small number of uHCC patients; therefore, further larger independent multi-center studies including different ethnicities are necessary to validate the clinical value of our model.
Conclusion
Baseline high MTV on 18F-FDG PET/CT was associated with poor OS following a combination of PD-1/PD-L1 inhibitors and targeted therapy in patients with uHCC. An integrating model combined with clinical prognostic factors, such as the ECOG-PS, Child-Pugh grade, and presence of bone metastasis, may improve the selection of candidate uHCC patients for immunotherapy.
Abbreviations
18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography–computed tomography; AASLD, American Association for the Study of Liver Diseases; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; MTV, metabolic tumor volume; OS, overall survival; PUMCH, Peking Union Medical College Hospital; ROI, region of interest; SUVmax, maximum standardized uptake values; TLG, total lesion glycolysis; uHCC, unresectable hepatocellular carcinoma.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
The study was conducted according to the guidelines of the Declaration of Helsinki of 1975 and approved by the Ethical Committee of PUMCH (IRB protocol number # ZS-1238, date of approval 20 December 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Consent for Publication
All authors gave their consent for publication.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by the National Key Research and Development Program of China (No. 2016YFC0901500, 2020YFC2002702), the National Natural Science Foundation of China (No. 82071967), and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-061, 2022-I2M-C&T-A-003).
Disclosure
The authors declare that they have no competing interests.
References
1. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
2. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
3. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
4. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
5. Chen B, Lei J, Zhao H, et al. Efficacy and safety of TKI plus PD-1 inhibitors in elderly uHCC patients: a retrospective study. J Hepatocell Carcinoma. 2022;9:1171–1185. doi:10.2147/JHC.S387254
6. Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi:10.2147/JHC.S332420
7. D’Alessio A, Rimassa L, Cortellini A, Pinato DJ. PD-1 blockade for hepatocellular carcinoma: current research and future prospects. J Hepatocell Carcinoma. 2021;8:887–897. doi:10.2147/JHC.S284440
8. Ziogas IA, Evangeliou AP, Giannis D, et al. The role of immunotherapy in hepatocellular carcinoma: a systematic review and pooled analysis of 2402 patients. Oncologist. 2021;26(6):e1036–e1049. doi:10.1002/onco.13638
9. Chen S, Huang Z, Jia W, et al. Association of the pretreatment lung immune prognostic index with survival outcomes in advanced hepatocellular carcinoma patients treated with PD-1 inhibitors. J Hepatocell Carcinoma. 2020;7:289–299. doi:10.2147/JHC.S277453
10. Yang X, Hu Y, Yang K, et al. Cell-free DNA copy number variations predict efficacy of immune checkpoint inhibitor-based therapy in hepatobiliary cancers. J Immunother Cancer. 2021;9(5):e001942. doi:10.1136/jitc-2020-001942
11. Li N, Ren SH, Qin YF, et al. Four-pyroptosis gene-based nomogram as a novel strategy for predicting the effect of immunotherapy in hepatocellular carcinoma. Biomed Res Int. 2022;2022:2680110. doi:10.1155/2022/2680110
12. Nan Z, Guoqing W, Xiaoxu Y, et al. The predictive efficacy of Tumor Mutation Burden (TMB) on nonsmall cell lung cancer treated by immune checkpoint inhibitors: a systematic review and meta-analysis. Biomed Res Int. 2021;2021:1780860. doi:10.1155/2021/1780860
13. Yamashige D, Kawamura Y, Kobayashi M, et al. Potential and clinical significance of 18F-fluorodeoxyglucose positron emission tomography/computed tomography for evaluating liver cancer response to lenvatinib treatment. Oncology. 2021;99(3):169–176. doi:10.1159/000510754
14. Lee SM, Kim HS, Lee S, Lee JW. Emerging role of (18) F-fluorodeoxyglucosepositron emission tomography for guiding management of hepatocellular carcinoma. World J Gastroenterol. 2019;25(11):1289–1306. doi:10.3748/wjg.v25.i11.1289
15. Wang G, Zhang W, Chen J, et al. Pretreatment metabolic parameters measured by (18) F-FDGPET to predict the pathological treatment response of HCC patients treated with PD-1 inhibitors and lenvatinib as a conversion therapy in BCLC stage C. Front Oncol. 2022;12:884372. doi:10.3389/fonc.2022.884372
16. Chen HH, Chiu NT, Su WC, Guo HR, Lee BF. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology. 2012;264(2):559–566. doi:10.1148/radiol.12111148
17. Lim R, Eaton A, Lee NY, et al. 18F-FDG PET/CT metabolic tumor volume and total lesion glycolysis predict outcome in oropharyngeal squamous cell carcinoma. J Nucl Med. 2012;53(10):1506–1513. doi:10.2967/jnumed.111.101402
18. Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. 2018;7(3):235–260. doi:10.1159/000488035
19. Huo L, Li N, Wu H, et al. Performance evaluation of a new high-sensitivity time-of-flight clinical PET/CT system. EJNMMI Phys. 2018;5(1):29. doi:10.1186/s40658-018-0229-4
20. Davison J, Mercier G, Russo G, Subramaniam RM. PET-based primary tumor volumetric parameters and survival of patients with non-small cell lung carcinoma. AJR Am J Roentgenol. 2013;200(3):635–640. doi:10.2214/AJR.12.9138
21. Boellaard R, O’Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37(1):181–200. doi:10.1007/s00259-009-1297-4
22. Hwang SH, Lee JW, Cho HJ, Kim KS, Choi GH, Yun M. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with very early and early hepatocellular carcinoma. Clin Nucl Med. 2017;42(1):34–39. doi:10.1097/RLU.0000000000001449
23. Castello A, Rimassa L, Personeni N, Pressiani T, Smiroldo V, Lopci E. Metabolic switch in hepatocellular carcinoma patients treated with sorafenib: a proof-of-concept trial. Mol Imaging Biol. 2020;22(5):1446–1454. doi:10.1007/s11307-020-01489-6
24. Takeuchi S, Rohren EM, Abdel-Wahab R, et al. Refining prognosis in patients with hepatocellular carcinoma through incorporation of metabolic imaging biomarkers. Eur J Nucl Med Mol Imaging. 2017;44(6):969–978. doi:10.1007/s00259-016-3583-2
25. Ito K, Schöder H, Teng R, et al. Prognostic value of baseline metabolic tumor volume measured on (18) F-fluorodeoxyglucosepositron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging. 2019;46(4):930–939. doi:10.1007/s00259-018-4211-0
26. Seban RD, Mezquita L, Berenbaum A, et al. Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur J Nucl Med Mol Imaging. 2020;47(5):1147–1157. doi:10.1007/s00259-019-04615-x
27. Larimer BM, Wehrenberg-Klee E, Dubois F, et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 2017;77(9):2318–2327. doi:10.1158/0008-5472.CAN-16-3346
28. Hurkmans DP, Basak EA, Schepers N, et al. Granzyme B is correlated with clinical outcome after PD-1 blockade in patients with stage IV non-small-cell lung cancer. J Immunother Cancer. 2020;8(1):e000586. doi:10.1136/jitc-2020-000586
29. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
30. Lee PC, Chao Y, Chen MH, et al. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers. 2020;12(1). doi:10.3390/cancers12010182
31. Choi WM, Lee D, Shim JH, et al. Effectiveness and safety of nivolumab in child-pugh B patients with hepatocellular carcinoma: a real-world cohort study. Cancers. 2020;12(7):1968. doi:10.3390/cancers12071968
32. Kuo HY, Chiang NJ, Chuang CH, et al. Impact of immune checkpoint inhibitors with or without a combination of tyrosine kinase inhibitors on organ-specific efficacy and macrovascular invasion in advanced hepatocellular carcinoma. Oncol Res Treat. 2020;43(5):211–220. doi:10.1159/000505933
33. Harding JJ, Abu-Zeinah G, Chou JF, et al. Frequency, morbidity, and mortality of bone metastases in advanced hepatocellular carcinoma. J Natl Compr Canc Netw. 2018;16(1):50–58. doi:10.6004/jnccn.2017.7024
34. Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44(2):213–221.
35. Torizuka T, Tamaki N, Inokuma T, et al. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med. 1995;36(10):1811–1817.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.