Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Safety and Efficacy of Drug-Eluting Bead Transarterial Chemoembolization Combined with Lenvatinib and Anti-PD-1 Antibodies for Unresectable Hepatocellular Carcinoma: A Retrospective Analysis
Authors Wu SJ, Ruan DD, Wu QY, Tang Y, Zhang JH, Cai SL, Zhou YF, Luo JW , Fang ZT
Received 15 February 2023
Accepted for publication 20 May 2023
Published 2 June 2023 Volume 2023:10 Pages 807—820
DOI https://doi.org/10.2147/JHC.S408819
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Manal Hassan
Shao-Jie Wu,1,2,* Dan-Dan Ruan,1,* Qiu-Yan Wu,1,* Yi Tang,1,2 Jian-Hui Zhang,1 Sen-Lin Cai,1,2 Yan-Feng Zhou,1,2 Jie-Wei Luo,1,3 Zhu-Ting Fang1,2
1Fujian Provincial Hospital, Shengli Clinical Medical College of Fujian Medical University, Fuzhou, People’s Republic of China; 2Department of Interventional Radiology, Fujian Provincial Hospital, Fuzhou, People’s Republic of China; 3Department of Traditional Chinese Medicine, Fujian provincial hospital, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie-Wei Luo; Zhu-Ting Fang, Email [email protected]; [email protected]
Background: Drug-eluting bead transarterial chemoembolization (DEB-TACE) has good efficacy in the treatment of unresectable hepatocellular carcinoma (uHCC), with a relatively high objective response rate (ORR) compared to conventional transarterial chemoembolization (cTACE). This study aimed to evaluate the safety and medium-term clinical efficacy of DEB-TACE combined with lenvatinib (LEN) plus PD-1 inhibitors as a triple therapy for the treatment of uHCC.
Methods: Data of patients with uHCC who received triple therapy of DEB-TACE combined with LEN plus PD-1 inhibitors from January 2019 to June 2021 were analyzed retrospectively. The study endpoints were ORR, progression-free survival (PFS), and treatment-related adverse events based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST).
Results: Thirty-five patients were included in this study, with a median follow-up period of 15 months. The median cycle of DEB-TACE was 1, while that of all forms of TACE procedures per patient was 2. The median administration time of LEN was 7 months, and the median number of PD-1 inhibitor treatment was 4 cycles. The ORR based on mRECIST was 82.9%, disease control rate was 91.4%, and the median time to response was 7 weeks. Among these, the ORR of Barcelona Clinic Liver Cancer (BCLC) stage A reached 100%, while that of BCLC stages B and C reached 84.6% and 78.9%, respectively. The median PFS was 9 months; the mOS was not reached. Fourteen patients (40%) successfully underwent downstaging conversion and surgical resection, 32 patients (91.4%) experienced treatment-related adverse events, and no grade 5-related adverse reactions occurred.
Conclusion: DEB-TACE combined with LEN and PD-1 inhibitors has a high ORR and surgical conversion rate in the treatment of uHCC tumors, and the toxicity and side effects were tolerable.
Keywords: unresectable hepatocellular carcinoma, transarterial chemoembolization, drug-eluting bead, lenvatinib, PD-1 inhibitor, combined therapy
Background
Hepatocellular carcinoma (HCC) is one of the most common cancers, and surgery is an important treatment option for patients with liver cancer to achieve long-term survival. However, the onset of liver cancer is insidious, and most cases are often in the middle or late stages when diagnosed, thus, surgery no longer remains an option; additionally, the 5-year survival rate is less than 20%.1 Transcatheter arterial chemoembolization (TACE) and systemic therapy are standard treatment methods for unresectable hepatocellular carcinoma (uHCC).
TACE is currently a common local therapy for the non-surgical treatment of HCC. According to clinical practice guidelines, TACE is recommended as the preferred treatment for Barcelona Clinic Liver Cancer (BCLC) stage B HCC, as well as a treatment option for some stage C HCCs in some Asian countries.2,3 Conventional TACE (cTACE) uses iodine oil as a drug carrier to load and release anticancer drugs and gelatin sponge or other embolic materials to block the blood supply to the target tumor. The systemic toxicities of cTACE cannot be ignored in liver cancers with heavy tumor loads due to the dual side effects of cytotoxicity of chemotherapeutic drugs and ischemia caused by embolization. Unlike cTACE, drug-eluting beads TACE (DEB-TACE) can increase the intensity and duration of tumor ischemic necrosis and can deliver a large number of chemotherapeutic drugs to the tumor in a controlled and sustained manner. For HCCs with high tumor loads, DEB-TACE may result in a higher objective response rate (ORR) and fewer systemic side effects compared to cTACE.4,5
Lenvatinib (LEN) is a first-line regimen for the systemic treatment of liver cancer, and in a Phase 3, multinational, randomized, non-inferiority trial (REFLECT), LEN demonstrated higher ORR and progression-free survival (PFS) than sorafenib, and the overall survival (OS) was not lower than that of sorafenib. The TACTICS-L study showed that TACE combined with LEN for the treatment of uHCC had an ORR of 79% in some selected BCLC stage B liver cancers, with a complete remission (CR) rate of 53.2%.6 In a previously reported TACTICS study, the ORR of the TACE alone group was 61.8%, and the CR rate was 27.6%.7 This study preliminarily suggested the therapeutic value of LEN combined with TACE in some patients with uHCC.
Immune checkpoint inhibitors (ICIs) offer hope for the treatment of advanced HCC as a new therapeutic approach. The IMbrave150 and ORIENT-32 Phase III trials have shown that combined treatment with ICIs and anti-angiogenic drugs is effective for uHCC.8,9 However, the Checkmate-459 (Nivolumab vs sorafenib) and Keynote-240 (Pembrolizumab vs placebo) studies revealed negative results.10,11 The initiation of an immune response requires antigen-presenting cells to take up and process tumor antigens. HCC tumor cell nests and the surrounding matrix lack lymphocyte infiltration, making it difficult to produce effective anti-tumor immunity. TACE treatment destroys the local tumor and releases large amounts of tumor antigens, thereby initiating the tumor’s immune response cycle.12,13 This finding provides a theoretical basis for the treatment of TACE combined with ICIs.
To date, the efficacy and safety of TACE combined with LEN and ICIs for uHCC has been confirmed by our center in related studies.14,15 However, DEB-TACE combined with LEN and ICIs has been less studied. Therefore, we conducted this retrospective study to evaluate the efficacy and safety of DEB-TACE combined with LEN and anti-PD-1 antibodies (PD-1 and ICIs) in the treatment of uHCC, with the aim of exploring more effective treatments for uHCC.
Methods
Study Design and Patient Selection
The clinical data of patients with uHCC who received triple therapy with DEB-TACE combined with LEN and PD-1 inhibitors in our hospital from January 2019 to June 2021 were collected for retrospective analysis. The inclusion criteria were: (1) patients with primary liver cancer clinically diagnosed according to the American Association for the Study of Liver Diseases guidelines or with HCC confirmed through pathological histological examination;3 (2) patients with BCLC stage A, B, or C who were unsuitable for surgical resection; (3) age 18–77 years; (4) liver function Child–Pugh grade A or B (≤7 points) and Eastern Cooperative Oncology Group Performance Status (ECOG-PS) score 0–1; (5) at least one reproducibly measured lesion according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria;16 and (6) DEB-TACE as the first TACE treatment, with LEN administered for ≥1 month, and PD-1 inhibitors administered at least once. Both LEN and PD-1 inhibitors were used within one month before or after the first DEB-TACE treatment, defined as a combination therapy of DEB-TACE+LEN+PD-1 (DEB-TACE-L-P). The exclusion criteria were: (1) previous or current history of other cancers; (2) known allergy to tyrosine kinase inhibitors (TKIs) or PD-1 inhibitors; (3) history of human immunodeficiency virus infection, immunodeficiency, or history of organ allografts; (4) any active autoimmune disease or history of autoimmune disease; (5) history of radiation therapy, radiofrequency ablation, microwave ablation, particle implantation, systemic therapy, or other treatments; and (6) incomplete data. All procedures were performed in accordance to the tenets of the Declaration of Helsinki and this clinical retrospective analysis was approved by the Ethics Committee of Fujian Provincial Hospital, and informed consent was obtained from all patients for their data to be used for research purposes.
Baseline Information Collection
The patients’ previous medical records and original records of relevant examinations were accessed through an electronic medical record system. The basic patient information included age, sex, height, weight, ECOG score, BCLC stage, prevalent etiology, history of cirrhosis, Child–Pugh score, pathology, laboratory tests, and abdominal contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI).
TACE Procedure
The first TACE procedure was DEB-TACE. The drug-loaded microspheres used for DEB-TACE were 100–300-μm diameter CalliSpheres® drug-loaded embolic microspheres (Hengrui Medical, Suzhou, China). Each vial of drug-loaded microspheres was loaded with 60 mg of epirubicin (Hisun Pfizer Pharmaceuticals, Fuyang, China) according to the standard formulation method.17
TACE treatment procedure: Seldinger’s method was used to puncture the right or left femoral arteries, and a 5F RH catheter was used to perform routine coeliac trunk and hepatic artery arteriography. Based on the location and size of the tumor and whether the tumor staining was intact, angiography was performed on the suspected extrahepatic blood supply arteries or parasitic blood supply arteries, such as the phrenic artery, superior mesenteric artery, left gastric artery, intercostal artery, right renal artery, and internal mammary artery, to identify the blood supply arteries of all tumors.18 A 2.8F microcatheter (Renegade Hi Flo, Boston Scientific Corp, USA) was superselectively inserted into the tumor blood supply artery, and oxaliplatin (150 mg) was infused for 20 min. The DEB-TACE procedure utilized the CalliSpheres® drug-loaded embolic microspheres as the embolic material, and preconfigured drug-eluting beads were slowly injected (1 mL/min) into the tumor vasculature. The cTACE procedure utilized ultra-liquefied iodine oil (Guerbet, Paris, France) as the embolic material, and a 20–40 mg emulsified suspension of the super-liquefied iodine oil and epirubicin (Hisun Pfizer Pharmaceuticals, Fuyang, China) was slowly injected into the tumor vessel, with no more than 20 mL of iodine oil used per session.
Embolization endpoint determination: Embolization was stopped when the contrast agent was retained in the blood supply artery for 3–5 cardiac cycles. After a 5-minute pause, the angiogram was performed again. If embolization was incomplete, sequential embolization with 300–500-μm diameter Hepasphere® (Beijing Meriton Medical Devices Co., Ltd., China) and gelatin sponge pellets (Alicon, Guangzhou, China) was performed until the end point of embolization was reached.19
TACE repetition: According to the patient’s fitness status and liver function score, on-demand TACE treatment was performed in patients with liver cancer who had surviving lesions on review-enhanced MRI or CT. For TACE again, DEB-TACE or cTACE was taken according to the patient’s own wishes.
Lenvatinib and PD-1 Inhibitor Administration
All patients received oral lenvatinib (Eisai, Tokyo, Japan) for at least one month at a dose of 12 mg orally in those weighing > 60 kg or 8 mg once a day in those weighing < 60 kg, and the interval from the first DEB-TACE was less than one month. All patients received at least one cycle of immunotherapy with PD-1 inhibitors, including Sintilimab 200 mg (Innovent Biologics, Suzhou, China), Tislelizumab 200 mg (BeiGene, Shanghai, China), Camrelizumab 200 mg (Hengrui Pharma, Lianyungang, China), Pembrolizumab 200 mg (Merck Sharp & Dohme, New Jersey, USA), and Toripalimab 240 mg (Zhonghe Biomedical Technology, Suzhou, China), which were administered intravenously every 3 weeks, and the interval between first use and first DEB-TACE was not more than 1 month.
Follow-Up
Follow-up imaging and case data were collected from patients to determine the timing and mode of tumor progression, and the survival status and time to death data of each patient were obtained through electronic medical records and telephone follow-ups. All patients were followed-up regularly every 4–8 weeks after the initial treatment. Each follow-up included a detailed medical history, physical examination, hematological and biochemical examination, abdominal CT or MRI enhancement examination, chest CT, and other imaging examinations, if clinically necessary. The last follow-up ended on April 30, 2022.
During the follow-up process, if the tumor was downstaged and met the surgical indications, the patient received radical surgical resection after a multidisciplinary team discussion and patient’s will. If there was tumor progression, intolerable toxic side effects, or changes in regimen, the triple therapy regimen was discontinued. Follow-up treatment was chosen based on the opinions of the multidisciplinary team and the patient’s wishes. The scheme included second-line targeted drugs, ICIs, hepatic artery infusion chemotherapy (HAIC), radiotherapy, ablation, and the best supportive therapy.
Assessments and Outcomes
PFS was defined as the time from the initiation of any combination therapy regimen to the objective progression of the tumor or death from any cause. OS was defined as the time from the initiation of any combination therapy regimen to all-cause death. For patients who were alive at the last contact, their OS was censored on the date of the last contact. Tumor response was assessed using the mRECIST criteria, which classifies tumor responses as CR, partial remission (PR), stable disease (SD), and progressive disease (PD). The ORR was defined as the percentage of patients with best tumor response of CR and PR. Disease control rate (DCR) was defined as the percentage of patients with best tumor response of CR, PR, and SD. Time to response (TTR) was defined as the time from initiation of either triple therapy to the first meeting of the PR or CR criteria. Treatment-related adverse events (TRAEs) were recorded and assessed according to the Common Terminology Criteria for Adverse Events version 5.0.
Statistical Analyses
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software (Version 26, SPSS, Inc., Chicago, IL, USA). Baseline analysis of the demographics and relevant characteristics of the selected cases was performed, and descriptive statistical analysis and list description were used. The measurement data were statistically described as mean ± standard deviation or median (interquartile range). The counting data were statistically described using frequency (composition ratio). Survival rates were calculated using the life table method. OS and PFS were analyzed using the Kaplan–Meier method. P-values < 0.05 were considered statistically significant. Safety and tolerability were analyzed using descriptive statistics. The adverse events that occurred in this study were described in a list, including the adverse events that occurred during treatment and the treatment-related adverse events.
Results
Study Population
Between January 2019 and June 2021, 73 patients were treated with DEB-TACE at our center. Three patients had incomplete follow-up information, 2 patients were referred to other hospitals during treatment, 7 patients’ first TACE was not DEB-TACE, and 5 patients were treated with HAIC or simultaneous ablation during DEB-TACE. The remaining 56 patients were included in this study. A total of 21 patients used or combined with anti-angiogenic drugs except for LEN or with TKIs. Eleven patients either did not use anti-angiogenic drugs and ICIs within the specified time or did not use them at all. The remaining 35 patients received the DEB-TACE-L-P triple therapy method, as defined above (Figure 1).
Thirty-five patients were eligible for the triple therapy, including 32 male patients and 3 female patients, with a median age of 58 years (range 31–79 years). Thirty-one patients were combined with hepatitis B (88.6%), and 19 patients with alpha-fetoprotein (AFP) > 400 ng/mL (54.3%). Three patients with BCLC stage A, 13 with BCLC stage B, and 19 with BCLC stage C. The median size of the baseline target lesions was 9.0 cm (range: 3–19.1 cm). The tumors were located in one lobe in 20 patients, and bilobar tumors were present in 15 patients. There were 19 cases of macrovascular invasion and 3 cases of extrahepatic metastasis. The three patients with extrahepatic metastasis had macrovascular invasion. The general characteristics of the patients included in this study are shown in Table 1.
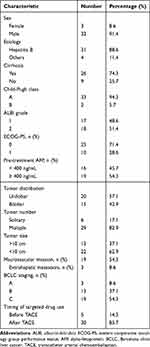 |
Table 1 Baseline Characteristics in 35 Patients |
The median cycle of DEB-TACE was 1 (range, 1–4), and the median cycle of all forms of TACE procedures per patient was 2 (range, 1–7). Five patients received LEN within 1 month before the first TACE treatment, and 30 patients received LEN within 1 month after the first TACE. The median administration time for LEN was 7 months (range, 3–17). PD-1 immunotherapy was performed within 1 month after TACE, and the median number of treatment was four cycles (range, 1–15 cycles). The PD-1s used were Sintilimab (200 mg, n = 13), Tislelizumab 200 mg (200 mg, n = 6), Camrelizumab 200 mg (200 mg, n = 9), Pembrolizumab 200 mg (200 mg, n = 4), and Toripalimab 240 mg (200 mg, n = 3).
Survival and Tumor Response
All patients underwent at least two imaging evaluations after treatment and were followed up for 8–35 months, with a median follow-up of 15 months. Twenty-four patients were alive at the last follow-up date. Thirteen patients were still receiving LEN, while 11 received immunotherapy, including seven of the 11 patients that were receiving both. LEN was discontinued in 11 patients due to side effects (n = 3), disease progression (n = 3), and CR after surgical resection (n = 5). Thirteen patients discontinued the PD-1 therapy due to side effects (n = 3), disease progression (n = 4), and CR after surgical resection (n = 6). The follow-up treatments for disease progression included ablation (n = 2), stereotactic intensity-modulated radiotherapy (n = 2), and changes to second-line systemic drugs (n = 5). Overall, the median PFS was 9 months (Figure 2), and median overall survival (mOS) was not achieved.
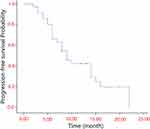 |
Figure 2 Kaplan-Meier analyses of progression-free survival according to treatment group. |
According to the mRECIST criteria, 12 patients achieved CR (34.3%) and 17 patients achieved PR (48.6%); therefore, the ORR was 82.9%. The median time to response was 7 weeks (range, 4–30 weeks). The efficacy in the three patients was evaluated as SD (8.6%), resulting in a DCR of 91.4% (Table 2). The target lesions of patients treated with triple therapy were reduced by varying degrees. The three patients with PD efficacy evaluations were assessed as progressive due to the appearance of new lesions, of which two cases showed reductions of more than 30% in the diameter of the target lesions. The waterfall map of the optimal change in the target lesion diameter is shown in Figure 3A. Of the three patients with uHCC in BCLC stage A, two (66.7%) and one (33.3%) patients achieved CR and PR, respectively, with an ORR of 100%. Of the 13 patients with uHCC in BCLC stage B, 3 (23.1%) and 8 (61.5%) patients achieved CR and PR, respectively, with an ORR of 84.6%. Of the 19 patients with uHCC in BCLC stage C, 7 (36.8%) and 8 (42.1%) patients achieved CR and PR, respectively, with an ORR of 78.9% (Table 3, Figure 3B).
 |
Table 2 Radiological Responses According to the mRECIST Criteria and Clinical Efficacy |
 |
Table 3 Tumor Responses According to the mRECIST Criteria for Different BCLC Stage |
Safety
Thirty-two out of 35 patients (91.4%) experienced any grade of adverse reactions (TRAEs) during the treatment period. The most frequent adverse reactions were increased glutamic oxaloacetic transaminase (71.4%), increased glutamic-pyruvic transaminase (68.6%), hypoalbuminemia (48.6%), increased bilirubin (42.9%), and hypertension (48.6%), followed by thyroid dysfunction, fever, fatigue, and digestive system-related symptoms such as nausea, vomiting, abdominal pain, and diarrhea. The incidence of grade ≥ 3 TRAEs was 22.9% (8/35) (Table 4). Three patients (3/35) had grade 4 TRAEs (Immune-related liver injury (n = 2) and upper gastrointestinal bleeding (n = 1)). The PD-1 inhibitors used in patients with Immune-related liver injury were Sintilimab (n = 1) and Camrelizumab (n = 1), and they showed recovery of liver function after discontinuation of PD-1 inhibitors, treatment with corticosteroids and liver supportive therapy. The PD-1 inhibitor used in the patient with gastrointestinal bleeding was Camrelizumab (n = 1).This patient has concurrent cutaneous capillary malformation hyperplasia, and gastroscopy confirmed severe ulcer bleeding and intragastric mucosa with similar abnormal capillary malformation changes. After discontinuing PD-1 inhibitor, endoscopic hemostasis and proton pump inhibitor treatment, his condition improved. As of the follow-up date, no patient had grade 5 TRAEs. There were 6 cases of drug reduction, interruption, or withdrawal due to LEN adverse reactions and 3 cases of interruption or withdrawal of drugs due to adverse reactions to ICIs. During the follow-up period, there was no treatment-related death, and all TRAEs were assessed as mild-to-moderate and manageable.
 |
Table 4 Treatment-Related Adverse Events [n (%)] |
Resection Conversion After Triple Therapy
After triple therapy, 14 patients (14/35, 40%) were downstaged and underwent radical surgical resection (1 patient with BCLC stage A, 3 with BCLC stage B, and 10 with BCLC stage C). Among them, five patients had no tumor recurrence on continuous review after surgical resection and continued to use LEN and PD-1 for at least six months. Three patients met the criteria for surgical resection but refused surgical resection and continued triple therapy. The median interval between the initiation of triple therapy and resection was 4 months (rang 3–10 months). One patient developed intrahepatic recurrence 4 months post-operatively, one developed pulmonary metastasis 7 months post-operatively, and one developed humeral metastasis 8 months post-operatively.
Discussion
According to our study, the triple treatment led to a significant ORR (82.9%) and DCR (91.4%) with tolerable side effects. The ORR of BCLC stage A reached 100%, whereas the ORR of BCLC stages B and C reached 84.6% and 78.9%, respectively. The target lesions of patients who received triple therapy were reduced by varying degrees. The three patients with efficacy evaluations of PD were assessed as progressive due to the appearance of new lesions rather than enlargement of the target lesions. In addition, the DEB-TACE-L-P triple therapy was effective quickly and led to a fast tumor response time. The median time to response was 7 weeks (range, 4–30 weeks). Therefore, the median follow-up period in our trial was 15 months (range, 8–35 months), median PFS was 9 months, and the median OS was not met. The conversion rate to liver resection was 40.0% (14/35), and three patients refused resection even though they met the criteria for surgical resection. The DEB-TACE-L-P triplet regimen had manageable side effects, with an incidence of grade ≥ 3 TRAEs of 22.9% (8/35) and grade 4 TRAEs of 8.6% (3/35), no grade 5-related adverse reactions, and no treatment-related death.
TACE is the guideline-recommended standard treatment for intermediate-stage HCC and is associated with a high tumor response for uHCC. TACE injected chemotherapeutic drugs and embolic materials into the tumor blood supply artery, which induces tumor ischemic necrosis by mechanical embolization, kills tumor cells and induces apoptosis by drug toxicity.2,20 Traditional TACE is a hepatic artery embolization therapy based on lipiodol-loaded chemotherapeutic drugs, and this method has been proven to confer survival benefits. The literature reports that the one-year OS of unresectable liver cancer ranges from 57%–82%. A retrospective study that included 10,108 cases receiving cTACE showed that the median OS was 19.4 months, and the 1-, 2-, 3-, and 5-year OS values were 70.3%, 51.8%, 40.4%, and 32.4%, respectively.21 An ideal TACE regimen should allow for maximum and sustained chemotherapeutic drug concentrations in the tumor and achieve minimal systemic chemotherapeutic drug exposure while blocking the tumor vasculature.
DEB-TACE is a new TACE technique developed in the last decade. This technique embolizes tumor blood vessels through drug-loaded microspheres loaded with chemotherapeutic drugs and mechanically blocks tumor vessels while releasing chemotherapeutic drugs locally and continuously, thereby prolonging the duration of drug-tumor interactions and reducing systemic toxic side effects. The results of some prospective randomized controlled trials22 and meta-analysis23,24 showed that there was no statistical difference between DEB-TACE and cTACE treatment in terms of ORR and OS, but DEB-TACE showed some advantages in some specific patient populations.4,17,25 The results of the PRECISION V study26 showed that the DEB-TACE group demonstrated higher rates of complete response, objective response, and disease control compared with the cTACE group (27% vs 22%, 52% vs 44%, and 63% vs 52%, respectively), but the difference was not statistically significant and the result was negative. However, patients with Child-Pugh B, ECOG 1, bilobar disease, and recurrent disease showed a significant increase in objective response (52.4% VS 34.7%, P = 0.038) compared to cTACE.5,25,26 The study of Myeong Jun Song et al4 included patients with BCLC stage A/B and evaluated the response to tumor treatment according to mRECIST criteria, the results showed that the ORR of DEB-TACE and cTACE treatment was 81.5% and 49.4%, respectively. In a study that evaluated resected livers from liver transplant patients, it was found that 77% of tumors treated with DEB-TACE had complete necrosis, whereas only 27% of the blank embolization group achieved complete necrosis (P = 0.043).27 In addition, DEB-TACE reduced the incidence of severe liver injury toxicity (P < 0.001) and doxorubicin-related side effects (P = 0.0001) relative to cTACE.19,26 At present, the outcome of most studies showed that DEB-TACE was not able to improve ORR and OS as compared to cTACE in general HCC population, but some subgroups of patients may have higher ORR and may lead to higher resection conversion rate, which requires further exploration to identify suitable potential beneficiaries.
In recent years, the development of TKIs and ICIs has provided more options for systemic treatment of uHCC. Currently, the first-line treatment options for progressive liver cancer include sorafenib, lenvatinib, and atezolizumab combined with bevacizumab. The second-line treatment options include regorafenib, cabozantinib, Pembrolizumab, Nivolumab, and ramucirumab. Sorafenib is a classic representative TKIs, and in the SHARP trial study, sorafenib prolonged the OS of uHCC patients by approximately 3 months compared to that by placebo.26 The REFLECT trial revealed that LEN treatment led to an OS of 13.6 months, which was non-inferior to sorafenib (OS of 12.3 months). However, the ORR of TKIs therapy alone was relatively low. In the aforementioned study, the ORR of sorafenib alone was only 2%, and the ORR of LEN was relatively high, reaching 24.1%.6 Similar to TKIs, although PD-1 immunotherapy has shown good efficacy in the treatment of HCC in most studies, the efficiency rate is < 20% when used alone, and the ORR of ICIs monotherapy for progressive liver cancer ranges from 14–20% according to the literature.8,11,12,28–30 In recent years, with the development of TKIs and ICIs research, an increasing number of clinical trials have confirmed that the combination of the two can lead to higher ORR and survival benefits than monotherapy. Atezolizumab combined with bevacizumab is recommended as a first-line regimen in the National Comprehensive Cancer Network, European Society for Medical Oncology, American Society of Clinical Oncology, Chinese Society Of Clinical Oncology, and other guidelines due to its higher ORR of 33.2% (mRECIST) and median PFS of 6.8 months in the IMbrave150 trial.8 The HIMALAYA study was the first phase III clinical study to use cytotoxic T lymphocyte-associated antigen 4 on the basis of ICIs and compared with the targeted drug sorafenib, and the related study was published in NEJM Evidence,31 with an mOS of 16.43 months, a 3-year OS rate of 30.7%, and an ORR of 20.1%. The Chinese Society of Clinical Oncology diagnosis and treatment guidelines for primary hepatocellular carcinoma 2022 has included durvalumab + tremelimumab (Class I expert recommendation, Class 1A evidence) in the guidelines. In the 2022 V1 version of the National Comprehensive Cancer Network guidelines, durvalumab (Class 2A evidence) has also been added as a first-line treatment option for advanced hepatocellular carcinoma. Similarly, a combination regimen of Pembrolizumab combined with LEN in the KEYNOTE-524 study had a mOS of 22.0 months, mPFS of 8.6 months, DCR of 88%, and ORR of 46% for unresectable liver cancer.30 However, in another LEN plus pembrolizumab study, LEAP-002’s primary study endpoints OS and PFS did not meet the expected targets, with no statistically significant differences in outcomes (mOS: 21.2 months vs 19 months, HR = 0.840, 95% CI: 0.708–0.997, P = 0.0227; PFS, HR = 0.867, 95% CI: 0.734–1.024, P = 0.0466), but ORR was 26.1% in the combination group and 17.5% in the targeted group.32 Analysis of subgroups showed that Asian populations with a background of viral hepatitis B appeared to benefit more from the combination of LEN and pembrolizumab (HR = 0.75).32 In addition, the recent RESCUE trial33 demonstrated that apatinib combined with Camrelizumab showed good efficacy and safety in the treatment of advanced HCC, with an ORR of 46%, DCR of 79%, mPFS of 6.4 months, 1-year OS rate of 75%, and mOS of 20.3 months in the first-line treatment group, thus providing a new treatment option for uHCC. The combination of drugs seems to achieve higher ORR than monotherapy, but the surgical conversion rate is still relatively low in most studies.
Most patients with liver cancer in China are in the middle or late stages at first diagnosis and have a low chance of primary surgical resection. Unresectable liver cancer is often accompanied by a large tumor load and poor prognostic outcomes, with an age-standardized five-year survival rate of approximately 14.1%, a value that is much lower than that reported in other countries. TACE and systemic therapy are the standard treatments for unresectable liver cancer; however, monotherapy has certain limitations, and an increasing number of local therapy combined with systematic therapy modalities are being used to treat initial uHCC. Previous studies14,28,34 have shown that uHCC patients treated with TACE combined with LEN and PD-1 inhibitors had a reported PFS of 11.4–13.3 months and OS of 23.6–24.0 months. An interim analysis of a prospective, multicenter cohort study was presented at the 2022 European Society for Medical Oncology Annual Meeting. To evaluate the safety and efficacy of LEN combined with TACE and PD-1 inhibitors (Len-TAP) versus TACE alone in the conversion resection for initially uHCC, 71 patients were enrolled in both groups, the ORR based on mRECIST criteria of Len-TAP group and TACE group was 78.9% and 16.9%, respectively (P < 0.001). The Len-TAP group had a higher conversion resection rate compared to the TACE group (50.7% vs 15.5% P < 0001).35 A study conducted a retrospective analysis of the conversion rate of potentially resectable advanced HCC with LEN + PD-1 combined with interventional therapy, with no specific restriction on which PD-1 was used in the design, and HAIC/TACE/HAIC+TACE were uniformly identified as interventional therapy and included in the analysis, and also achieved a positive conversion rate (40.5%).36 Compared to the results of previous studies, the tumor load in our study was higher (the maximum tumor size was 12.3 ±4.8 cm, and with a considerable proportion of > 3 intrahepatic tumors, bilobar tumor distributions, portal vein infiltration, and extrahepatic metastasis), and even so, the ORR reached 82.9%, the mPFS was 9 months, and 40% of patients were successfully downstaged for surgical resection, which was similar to the medium-term efficacy of the previous study. Other local treatment modalities HAIC or stereotactic body radiotherapy (SBRT) combined with systemic therapy are also effective. Two studies presented at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting evaluated the efficacy and safety of HAIC combined with sintilimab (S) and bevacizumab biosimilar (IBI305), and HAIC combined with apatinib and camrelizumab (TRIPLET study) in patients with CNLC stage IIb – IIIb and BCLC stage C hepatocellular carcinoma, respectively. The results showed that 20 of the 30 subjects enrolled in the HAIC-S-IBI305 study were evaluated as PR according to mRECIST criteria, with an ORR of 66.7%.37 The 29 participants enrolled in the TRIPLET study had an ORR of 70.96% and 87.10% based on RECIST v1.1 and mRECIST, respectively, and an mPFS of 10.80 months based on mRECIST.38 A single-arm, Phase II study investigating the safety and assessing the efficacy of SBRT combined with sintilimab in patients with oligometastatic hepatocellular carcinoma was reported at the 2022 ASCO Annual Meeting. The study enrolled 25 subjects with a median follow-up time of 16.9 months, and results assessed based on RECIST v1.1 criteria showed that mPFS was not achieved and ORR was 96% (24/25, CR in 18 cases; PR in 6 cases).39 These studies once again proved that local therapy combined with systemic therapy can be an effective treatment for uHCC, significantly improving ORR and surgical conversion rates.
The triple therapy we studied may be a better treatment option for patients with advanced liver cancer than simple local therapy or simple systematic therapy, and its efficacy may be attributed to the following reasons: (1) TACE causes tumor ischemia and hypoxia by mechanically blocking the tumor blood supply, while TACE damages tumor DNA and induces cell apoptosis by gathering high concentration of chemotherapy drugs inside the tumor, so as to kill the tumor.4,27 TACE destroys the tumor antigens released by tumor cells, which facilitates the recognition of antigen-presenting cells, activates the immune response, and promotes the activation of T cells and the progress of immune cycle. (2) Compared to the transient perfusion chemotherapy of cTACE, the drug-loaded microspheres of DEB-TACE can slowly release chemotherapeutic drugs to maintain a high blood concentration in the tumor, reduce the systemic side effects caused by the circulation of chemotherapeutic drugs, and prolong their duration of action on tumor tissues.5,17 However, TACE-induced hypoxia increases vascular endothelial growth factor (VEGF) and PD-L1 expression thus promotes tumor growth.40,41 (3) LEN is a multi-kinase inhibitor with anti-proliferative and anti-angiogenic activities that block the VEGF credit pathway and modulate the tumor immune microenvironment, thereby enhancing the immune response of PD-1 inhibitors in HCC.29,42 (4) PD-1 inhibitors block the binding of PD-1 to PD-L1 and counteract the upregulation of PDL-1 expression in tumor tissues induced by hypoxia after TACE.43 Therefore, the combination of DEB-TACE, LEN, and PD-1 inhibitors may bring about synergistic anti-tumor activity, complement each other mechanistically, and help improve the clinical outcomes of patients with unresectable HCC.
In terms of adverse reactions, the toxic and side effects of the triple therapy regimen in this study were tolerable and manageable, and no new or unexpected treatment-related adverse reactions were found. These results are generally consistent with the previously reported occurrence of adverse reactions observed when using these treatments alone or in combination.6,9–11,33 The most common TRAEs were mainly transient adverse reactions associated with DEB-TACE, such as elevated glutamic pyruvic transaminase, elevated glutamic oxaloacetic transaminase, elevated bilirubin, and low protein levels; however, most of these were mild and could be improved in the short-term with symptomatic treatment. Adverse reactions associated with LEN and PD-1 treatment can be alleviated by drug-dose reduction, temporary interruption, or discontinuation.
This study has some limitations. First, the types of PD-1 inhibitors used in this study varied, and different PD-1 inhibitors may have different effects on treatment outcomes. Second, this was a retrospective study with a relatively short follow-up period, and the treatment regimen was chosen based on the attending physician’s and patient’s preferences, which inevitably leads to selection bias. Third, this study was a single-arm and single-center study; thus, it lacks the validation of a corresponding large-sample multicenter randomized controlled study during the same period. Fourth, the sample size of this study was limited and the results of the subgroup analysis should be interpreted with caution. Further randomized trials are necessary to verify our findings.
In conclusion, DEB-TACE combined with LEN and PD-1 inhibitors in the treatment of uHCC has a high tumor response and controllable toxic side effects. Patients with unresectable liver cancer may be able to obtain better long-term benefits from triple therapy, although this needs to be further confirmed with longer follow-up and prospective randomized controlled trials with large samples.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Ethics Approval and Consent to Participate
All procedures were performed in accordance to the tenets of the Declaration of Helsinki and this clinical retrospective analysis was approved by the Ethics Committee of Fujian Provincial Hospital and reported anonymously. All participants signed informed consent documentation.
Acknowledgments
We thank the patient for cooperating with our investigation and acknowledge all participants for their valuable contributions to this article.
Consent for Publication
Not applicable. This manuscript does not include information or images that could lead to the identification of study participants.
Funding
This work was supported by Fujian Province Natural Science Fund Project (2020J011096, 2021J02053, 2020J011064), Startup Fund for scientific research, Fujian Medical University (2020QH1163), the Special Research Foundation of Fujian Provincial Department of Finance (No. 2020-822, 2021-848, 2021-917), and the Fujian Province Medical Innovation Foundation (No. 2021CXB001).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Zhou J, Sun H, Wang Z, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720.
3. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
4. Song MJ, Chun HJ, Song DS, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57(6):1244–1250.
5. Liapi E, Geschwind JF. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovasc Intervent Radiol. 2011;34(1):37–49.
6. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173.
7. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501.
8. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–1905.
9. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2-3 study. Lancet Oncol. 2021;22(7):977–990.
10. Yau T, Park J-W, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
11. Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: a Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
12. Ochoa de Olza M, Navarro Rodrigo B, Zimmermann S, Coukos G. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development. Lancet Oncol. 2020;21(9):e419–e430.
13. Rizvi S, Wang J, El-Khoueiry AB. Liver Cancer Immunity. Hepatology. 2021;73(Suppl 1):86–103.
14. Wu JY, Yin ZY, Bai YN, et al. Lenvatinib Combined with Anti-PD-1 Antibodies Plus Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma: a Multicenter Retrospective Study. J Hepatocell Carcinoma. 2021;8:1233–1240.
15. Wu JY, Wu JY, Li YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for neoadjuvant treatment of resectable hepatocellular carcinoma with high risk of recurrence: a multicenter retrospective study. Front Oncol. 2022;12:985380.
16. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
17. Liu Y, Huang W, He M, et al. Efficacy and Safety of CalliSpheres((R)) Drug-Eluting Beads Transarterial Chemoembolization in Barcelona Clinic Liver Cancer Stage C Patients. Oncol Res. 2019;27(5):565–573.
18. Miyayama S, Yamashiro M, Sugimori N, Ikeda R, Okimura K, Sakuragawa N. Outcomes of Patients with Hepatocellular Carcinoma Treated with Conventional Transarterial Chemoembolization Using Guidance Software. J Vasc Interv Radiol. 2019;30(1):10–18.
19. Lencioni R, de Baere T, Burrel M, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35(5):980–985.
20. Kishore SA, Bajwa R, Madoff DC. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers. 2020;12(4):346.
21. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116.
22. Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255–264.
23. Gao S, Yang Z, Zheng Z, et al. Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60(124):813–820.
24. Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016;48(6):571–577.
25. Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5(9):1100–1108.
26. Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52.
27. Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21(3):327–332.
28. Liu J, Li Z, Zhang W, et al. Comprehensive Treatment of Trans-Arterial Chemoembolization Plus Lenvatinib Followed by Camrelizumab for Advanced Hepatocellular Carcinoma Patients. Front Pharmacol. 2021;12:709060.
29. Hack SP, Zhu AX, Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: challenges and Opportunities. Front Immunol. 2020;11:598877.
30. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38(26):2960–2970.
31. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evidence. 2022;1(8):EVIDoa2100070.
32. Finn RS, Kudo M, Merle P, et al. LBA34 Primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33:S1401.
33. Xu J, Shen J, Gu S, et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): a Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res. 2021;27(4):1003–1011.
34. Cao F, Yang Y, Si T, et al. The Efficacy of TACE Combined With Lenvatinib Plus Sintilimab in Unresectable Hepatocellular Carcinoma: a Multicenter Retrospective Study. Front Oncol. 2021;11:783480.
35. Xiaoyun Z, Zhu X, Feng X, et al. 715P The safety and efficacy of lenvatinib combined with TACE and PD-1 inhibitors (Len-TAP) versus TACE alone in the conversion resection for initially unresectable hepatocellular carcinoma: interim results from a multicenter prospective cohort study. Ann Oncol. 2022;33:S870.
36. Song TQ, Lang MR, Lu W, et al. Conversion of initially unresectable hepatocellular carcinoma (HCC) with triple-combination therapy (lenvatinib, anti-PD-1 antibodies, and transarterial therapy): a retrospective analysis. J Clin Oncol. 2022;40(4_suppl):413–413.
37. Liu DM, Mu H, Liu CF, et al. Hepatic artery infusion chemotherapy (HAIC) combined with sintilimab and bevacizumab biosimilar (IBI305) for initial unresectable hepatocellular carcinoma (HCC): a prospective, single-arm phase II trial. J Clin Oncol. 2022;40(16_suppl):4073–4073.
38. Gu YK, Zhang TQ, Zuo MX, et al. Hepatic artery infusion chemotherapy (HAIC) combined with apatinib and camrelizumab for hepatocellular carcinoma (HCC) in BCLC stage c: a prospective, single-arm, phase II trial (TRIPLET study). J Clin Oncol. 2022;40(16_suppl):4106–4106.
39. Chen YX, Yang P, Du SS, et al. A phase II study of stereotactic body radiotherapy (SBRT) combined with sintilimab in patients with recurrent or oligometastatic hepatocellular carcinoma (HCC). J Clin Oncol. 2022;40(16_suppl):4071–4071.
40. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529.
41. Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790.
42. Kudo M, New Treatment A. Option for Intermediate-Stage Hepatocellular Carcinoma with High Tumor Burden: initial Lenvatinib Therapy with Subsequent Selective TACE. Liver Cancer. 2019;8(5):299–311.
43. Ribas A. Releasing the Brakes on Cancer Immunotherapy. N Engl J Med. 2015;373(16):1490–1492.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


