Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Long-Term Treatment with Trazodone Once-A-Day (TzOAD) in Patients with MDD: An Observational, Prospective Study
Authors Shrashimirova M, Tyanev I, Cubała WJ , Wichniak A, Vodickova-Borzova C, Ruggieri A, Bonelli A, Lipone P, Comandini A, Cattaneo A
Received 11 January 2023
Accepted for publication 27 April 2023
Published 12 May 2023 Volume 2023:19 Pages 1181—1193
DOI https://doi.org/10.2147/NDT.S399948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Milena Shrashimirova,1,* Ivan Tyanev,2,* Wiesław J Cubała,3,* Adam Wichniak,4,* Claudia Vodickova-Borzova,5,* Alessandro Ruggieri,6,* Annalisa Bonelli,6,* Paola Lipone,6,* Alessandro Comandini,6,* Agnese Cattaneo6,*
1Diagnostic Consultative Center 14, Hospital VITA, Sofia, Bulgaria; 2Multiprofile Hospital for Active Treatment, Medical Clinic, Targovishte, Bulgaria; 3Department of Psychiatry, Medical University of Gdańsk, Gdańsk, Poland; 4Third Department of Psychiatry and Sleep Disorders Center, Institute of Psychiatry and Neurology, Warsaw, Poland; 5Psychiatry and Neurology, Brain-Soultherapy.s.r.o, Kladno, Czech Republic; 6Global Medical Department, Angelini Pharma S.p.A, Rome, Italy
*These authors contributed equally to this work
Correspondence: Alessandro Ruggieri, Global Medical Department, Angelini Pharma S.p.A, Viale Amelia 70, Rome, 00181, Italy, Tel +390691045309, Email [email protected]
Purpose: This was an observational, prospective, single-group, multicentre, international study aimed to describe the clinical response, functional impairment, and quality of life (QoL) of patients suffering from major depressive disorder (MDD) and in treatment with Trazodone Once-A-Day (TzOAD) monotherapy, over a 24-week period.
Patients and Methods: A total of 200 patients with a diagnosis of MDD who had been treated with TzOAD monotherapy were enrolled from 26 sites across 3 European countries (Bulgaria, Czech Republic, and Poland), including psychiatric private practices, and outpatient departments from general and psychiatric hospitals. Study assessments were completed by physicians and patients during routine visits within the normal practice of care.
Results: Clinical response was assessed by Clinical Global Impressions – Improvement (CGI-I) responders’ percentage at 24 (± 4) weeks. The majority of patients (86.5%) reported an improvement on the CGI-I compared to baseline. Results of the study confirm the well-known safety and tolerability of TzOAD, as well as its effectiveness on depressive symptoms, such as improvement in QoL, sleep quality, and overall functioning accompanied by favourable adherence and low drop-out rate.
Conclusion: To our knowledge, this is the first observational, long-term study in patients suffering from MDD, conducted with TzOAD. The improvement observed in clinical response, overall functioning, depressive symptoms, and QoL along the 24 weeks (+4) maintenance period and the very good retention rate, suggest that TzOAD may represent an effective and well tolerated treatment option for patients suffering from MDD.
Keywords: major depressive disorder, trazodone, patient-reported outcome, real-world evidence, effectiveness, long-term follow-up
Introduction
Major depressive disorder (MDD) is a severe psychiatric disorder that affects approximately 280 million people and is considered the largest contributor to disability worldwide.1
Many antidepressant classes are currently available for the management of MDD with different mechanisms of action and safety and tolerability profiles. Among the current antidepressant medications, selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatments due to their improved safety and tolerability. These drugs have several therapeutic limitations, however, including a remission rate ≤50%,2 a slow onset of action,3 and variable efficacy against symptom clusters associated with MDD.4 Furthermore, side effects such as sexual dysfunction, weight gain, insomnia, and anxiety are commonly observed with SSRIs and SNRIs treatment.5–8
Sexual dysfunction, weight gain, and tiredness are perceived as the most troublesome side effects by depressed patients and are recognized among those most likely to encourage non-compliance and treatment discontinuation.9
Trazodone hydrochloride is a triazolopyridine derivative, and it is the first serotonin receptor antagonist and reuptake inhibitor (SARI) developed for the treatment of depression.10 This drug is currently approved and marketed in several countries for the treatment of adults with MDD, with or without anxiety. Relating to its pharmacological actions in humans, trazodone is defined as an up-to date, multimodal11 and multifunctional drug with dose-dependent activity.12
Trazodone minimises the stimulation of post-synaptic receptors and can reduce some of the adverse events (AEs) often associated with SSRI and SNRI therapy, including insomnia, sexual dysfunction, and anxiety.13–16
Since its approval, the efficacy and favourable tolerability profile of trazodone have been confirmed by several pharmacological and clinical studies. In clinical trials, trazodone was clearly demonstrated as being as effective as other antidepressants, like tricyclic antidepressants (TCAs),17,18 SSRIs,19–23 and SNRIs24,25 in the management of depressive disorders.
The Trazodone Once-A-Day (TzOAD) formulation releases the active ingredient over 24 hours, which can improve treatment adherence and provide an effective antidepressant dosing (300 mg/day) in a single administration. The pharmacokinetic profile of TzOAD is characterised by a slow increase of plasma level with a single low and delayed peak followed by a slow decline in plasma concentration, resulting in a reduction of associated AEs like sedation or hypotension.26 By reducing the peak plasma concentration, higher doses of TzOAD would be better tolerated by patients who would more easily reach the target antidepressant dose of 300 mg/day.27
TzOAD is available in 150 and 300 mg bisectable tablets; this feature allows a proper titration up to the daily dosage of 150/300 mg, with 3-day increments.
Observing functional symptoms and QoL in depressed patients during long-term antidepressant therapy has gained increasing importance because it provides a more comprehensive picture of a patient’s health status. In terms of treatment outcomes, the return to usual levels of functioning in daily activities (ie, family life, social life and work/school) and to premorbid QoL are just as important to patients as the resolution of depressive symptoms.28 Additionally, functional impairment and a lower QoL are associated with an elevated risk for the recurrence of a major depressive episode.29,30
The aim of the present observational, prospective study was to assess the long-term clinical response, functional impairment, and QoL in outpatients with MDD who demonstrated an initial positive response to acute treatment with TzOAD monotherapy for up to 24 weeks.
Materials and Methods
Study Design and Participants
This was an observational, prospective, single-group, multicentre, international study conducted from 23 June 2020 to 16 November 2021. The aim was to describe the clinical response, functional impairment, and QoL of outpatients suffering from MDD who were treated with TzOAD monotherapy over a 24-week period.
The study population was adult outpatients aged ≥ 18 years with MDD according to the 5th edition of the Diagnostic and Statistical Manual of Mental Disorder (DSM-5) or International Classification of Diseases, 10th Revision (ICD-10), who presented with an episode of MDD, were judged as responders to acute monotherapy with TzOAD after a minimum of 6 weeks, and were eligible to continue treatment with TzOAD monotherapy. Exclusion criteria included any diagnosis of psychotic disorders, dysthymic or adjustment disorders, mental retardation, or other mental disorders; patients at serious risk of suicide; and patients with substance abuse. In total, 200 patients were enrolled in the study from 26 sites in 3 European countries: Bulgaria, Czech Republic, and Poland. All 200 patients were included in the safety population: seven patients were excluded from the efficacy population since they did not have a post-baseline Clinical Global Impressions – Improvement (CGI-I) evaluation or they did not fulfil the inclusion criteria (n=193) (Figure 1).
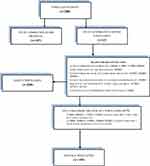 |
Figure 1 Disposition of patients. |
Data were collected during routine clinical visits at baseline (T0, end of acute treatment phase), week 12 (±3), week 18 (±3), and 24 weeks (±4) post-baseline. This study was conducted in accordance with the Declaration of Helsinki, Ethical Principles for Medical Research involving Human Patients, and all applicable Good Clinical Practice (GCP) and Good Pharmacoepidemiology Practice (GPP) principles. Review and approval from the appropriate local independent ethics committee (IEC)/institutional review board (IRB)/regulatory authorities (RAs) was obtained according to local regulations, as well as written informed consent from patients. In Bulgaria review was undertaken by the Central Ethics Committee for Clinical Trials, in Czech Republic review was undertaken by the Central State Institute of Drug Control ethics, and in Poland this was undertaken by four local ethics committees: Bioethics Committee at the Regional Medical Chamber in Gdansk, Bioethics Committee at Wielkopolska Medical Chamber, Bioethics Committee at Medical University, and Bioethics Committee at Institute of Psychiatry and Neurology.
Study Outcomes
The research question of this study was to describe the effect of the long-term TzOAD treatment on the clinical response, functional impairment, and QoL of outpatients with MDD. The primary objective was to assess clinical response, expressed as CGI-I responder percentage at 24 (±4) weeks.31 The CGI-I scale is a standardised rating tool used to assess patients’ clinical progress in terms of global improvement or change of illness from the initiation of a treatment that is completed by the physician using a 7-point scale.
The secondary objectives included examining the following: clinical response (expressed as Clinical Global Impressions – Improvement (CGI-I) responders’ percentage at 12 (± 3) and 18 (+ 3) weeks by the CGI-I scale); functional impairment (measured by the Sheehan Disability Scale [SDS], reported by the patient); QoL (assessed by the EQ-5D-5L, reported by the patient) over the 24-week period; as well as the discontinuation rate, safety, including sleep quality, tolerability, and changes in concomitant treatments over the 24-week observation period. Sleep quality was reported through PRO by the patient. Patients were asked if any change in sleep quality occurred and if so to specify if “Not restful sleep” or “Restful sleep”.
AEs were spontaneously reported by the patient or observed by the physician and were recorded according to local regulations.
Statistical Analysis
The safety population consisted of all patients who took at least 1 dose of the study medication. The efficacy population consisted of all patients who took at least 1 dose of study medication, had the baseline assessment, and at least 1 post-baseline evaluation of CGI-I during the observation period. The primary objective was evaluated in the efficacy population. Missing values for CGI-I were replaced by last observation carried forward (LOCF) values. Drug tolerability, AEs, and adverse drug reactions (ADRs) were analysed for all patients who took at least 1 dose of study medication during the observation period (safety population). In order to verify whether a statistically significant change in the primary parameter was observed between 12 vs 18 weeks, 12 vs 24 weeks and 18 vs 24 weeks, the CGI-I variable was made dichotomous by grouping positive responses (ie 1-Very much improved, 2-Much improved and 3-Minimally improved) vs the negative responses (ie, 4-No change plus 5-Minimally worse) followed by a McNemar test. In addition, a Repeated Measures Analysis of Variance was applied to the total score of the Quality-of-Life questionnaire.
Results
Study Population
The majority of the study population (safety population) was female (69%), the mean age was 53.5 years, 76% of patients were aged <65 years, and 99.5% of patients were predominantly white (see Table 1). The mean duration of TzOAD treatment during the study observation period was 167.4 days (range 18–209 days). Among the patients who reported past medical history (n=24), the most commonly reported past medical events were cholecystectomy (n=5) and cerebrovascular accident (n=3). Hypertension was the most commonly reported event (n=51) among the patients reporting ongoing medical events (n=79). Only 2.5% of patients in the safety population reported a history of alcohol abuse, none of which were ongoing at baseline. The majority of patients in the safety population (76%) reported a history of previous MDD episodes, and one-fifth reported they had previously been hospitalised due to their MDD. Within the efficacy population, in terms of severity at baseline, approximately three-quarters (76%) of patients experienced a recurrent episode of MDD, and a majority (79.3%) reported previous moderate episodes, with 12.4% experiencing a severe episode and 8.3% a mild episode.
 |
Table 1 Demographic Characteristics at the Baseline Visit – Safety Population |
Within the safety population, 135 patients reported previous medications, including beta-blocking agents, calcium channel blockers, antiepileptics, psycholeptics, and psychoanaleptics. Additionally, 70 patients reported concomitant medications, the most common being agents acting on the renin-angiotensin system (n=37).
Effectiveness/Clinical Response
Improvement in clinical response, as assessed by the CGI-I, was observed over the 24 weeks (±4), for the majority of patients (86.5%) (see Table 2). Approximately one-third of patients (34.2%) had a very much improved condition, and slightly more than one-third (37.8%) reported a much-improved condition. Only 11.9% of patients had no change in score, and very few patients (1.6%) had a minimally worse report for their condition than at baseline.
 |
Table 2 CGI-I Distribution Over Time – Efficacy Population |
In terms of severity, at baseline a majority of the patients (79.3%) experienced a moderate episode, whereas some patients experienced either a severe episode (12.4%) or a mild episode (8.3%). At week 12 (±3), the majority of patients reported a minimally, much, or very much improved CGI-I score (80.8%). Only 16.6% of patients reported no change, and very few patients reported a minimally worse change (2.6%). At week 18 (±3), most patients reported a minimally, much, or very much improved CGI-I score. A lower percentage of patients reported no change (13.5%) or a minimally worse change (1.0%) compared to week 12. The McNemar’s test showed significant differences between 12 and 18 weeks (p = 0.0490) and between 12 and 24 weeks (p = 0.0192) but no differences between 18 and 24 weeks (p = 0.7539). Applying the Bonferroni correction for multiple comparisons, none of the previous comparisons were statistically significant anymore, obtaining p = 0.147, p = 0.06 and p = 2.26, respectively.
Quality of Life and Functional Impairment
Clinically relevant improvements were observed in measures of QoL from baseline to week 24, with patients reporting milder depressive symptoms and improved mobility, self-care, daily activities, and pain or discomfort over the 24-week period. At 24 weeks, less than half of patients (48.2%) reported some level of anxiety or depression, compared to the majority (94.8%) observed at baseline. Similarly, those reporting they were not anxious or depressed at all significantly improved at week 24 (51.9%) compared to baseline (5.2%). Sustained improvements in patient reported health-related QoL were observed over the 24-week period, with the mean EQ-5D-5L score increasing from 60.96 at baseline to 82.14 at week 24 (see Table 3). The clinically meaningful improvement in the QoL scale was also confirmed by a statistically significant difference at each timepoint versus the baseline value by the Repeated Measures Analysis of Variance also after applying the Bonferroni correction for multiple comparison (p < 0.0001 for each contrast).
 |
Table 3 EQ-5D-5L Distribution of Time – Efficacy Population |
Similar improvements were observed in functional impairment. At baseline, the mean total SDS scores indicated MDD had a more significant impact on functional impairment (13.51 units) than it did at week 24 (mean score 6.21 units). The scores regarding functional impairment more than halved during the 24-week period.
Mean responses to each individual SDS item contributing to the total SDS score also consistently improved from baseline to week 24 (Table 4). At each of the 3 timepoints, the mean response value was higher on the second SDS item, indicating that MDD symptoms had disrupted social life more than work/school or family/home life. While this scored the highest of the individual items, mean responses over time for social life did decrease at each time point, demonstrating an improvement from baseline (4.62 units) to week 24 (2.11 units). Mean responses to item 4 (Days Lost) showed improvement from baseline (1.79 days, range 0–7) to week 24 (0.27 days, range 0–4). The mean number of days reported as unproductive also improved with time, decreasing from 2.9 days unproductive at baseline to 0.6 days unproductive at week 24.
 |
Table 4 SDS Distribution Over Time – Efficacy Population (EP) |
Improvements were also observed in other endpoints, including sleep quality (see Table 5). However, there was a large amount of missing data for this assessment, with 60, 127, and 149 patients missing information on changes in sleep quality at weeks 12, 18, and 24, respectively. Despite this, among the patients who responded to the question, the majority described a positive change in terms of more restful sleep, at each time point.
 |
Table 5 Change in Sleep Quality Over Time – Efficacy Population |
Safety
Fifty-nine AEs were reported in the study (Table 6) among 40 patients (20% of the total safety population). Of these 59 reported AEs, 31 (occurring in 29 patients) were judged by the investigator as being related to TzOAD (52.5%). Only 1 serious event was reported: major depression that was judged not to be related to the medication. This event resulted in hospitalisation or prolongation of hospitalisation, and the patient recovered. There was no change to therapy, and the severity was determined to be moderate for this event. Overall, 14 events were classified as moderately severe (including the 1 serious event), and 18 were classified as mildly severe. Twenty-seven events did not have information on their severity.
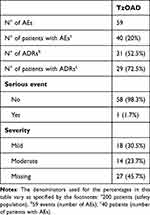 |
Table 6 Summary of AEs – Safety Population |
Discussion
To our knowledge, this is the first observational study to assess the long-term clinical response of TzOAD in patients with MDD. Data were collected from a large population (n=200) across 26 sites in 3 European countries (Bulgaria, Czech Republic, and Poland). Sustained improvements in clinical response, functional impairment, depressive symptoms, and health-related QoL were observed in patients with MDD treated with TzOAD in routine appointments over a period of 24 (±4) weeks.
Results of this study confirmed the well-known favourable safety and tolerability profile of TzOAD; accompanied by a very low discontinuation rate observed (6.5%) and low number of ADRs reported. It also demonstrated the effectiveness of TzOAD on core depressive symptoms. With 91.7% of patients enrolled in this study diagnosed with moderate or severe depression, and the majority reporting previous episodes of MDD (76.2%), the results demonstrate that 86.5% of patients in treatment with TzOAD reported an improvement in their CGI-I score (minimally improved, much improved, or very much improved). The results from this study also show that TzOAD has a positive effect on QoL, as assessed by the EQ-5D-5L, which showed a general positive improvement in patient’s self-rated health over the treatment period. Functional impairment also improved, as self-reported scores on the SDS demonstrate that the impact of MDD was more severe at baseline compared to the end of the treatment period. Additionally, an improvement in sleep quality was also reported by 68.4% of patients.
The observation of symptoms regarded as important to patients with MDD, such as functional symptoms and QoL, as well as the use of patient-reported outcome measures to assess these symptoms, supports the importance of the patient perspective. Accounting for patients’ perspectives is crucial: it provides a more comprehensive picture of patient’s health status and could help to reduce the risk of reoccurrence of an MDD episode.
The study was subject to the limitations foreseen by observational investigations, such as being essentially descriptive and did not include any hypotheses to be tested. Therefore, we were not able to define the efficacy of TzOAD from a statistical point of view, nevertheless clinically meaningful findings about the long-term treatment of TzOAD were observed in this study. Sample sizes were driven by the precision expected for the primary outcome, and an appropriate sample size was enrolled to assess the clinical effectiveness of TzOAD as long-term therapy. It was not possible to gather the physician and patient information at all assessment windows, and therefore the number of reports for secondary objectives were lower at week 18 and 24 than at baseline and week 12. Data quality was dependent on the data documentation available in the medical records. Since physicians reported patient/treatment history it was expected that the variables related to these outcomes were likely to be well captured in the patient medical record. Additionally, site personnel were asked to make every effort to collect missing information.
The data collection process relied on physician reports and patient self-report. It must be noted that while physicians and patients are best suited to make judgements about treatment effectiveness, clinical response, and patient QoL, this also relies on the interpretation of individuals and the bias that can impact self-report.
The current study found that 86.5% of the patients in the efficacy population had an improved CGI-I score (either minimally, much, or very much improved). QoL, assessed by the EQ-5D-5L scale (self-care, usual activities, pain/discomfort, anxiety/depression), was found to have improved, as indicated by mean levels of patients’ self-rated health over the maintenance treatment period (mean total value increasing from 71.14 at week 12 to 82.14 at week 24). Quality of sleep was found to have improved during the study among the majority of patients, with 68.4% reporting an improvement in sleep quality already at week 12.
Furthermore, TzOAD confirmed its well-known safety profile during the approximately 24-week study period, as only one SAE was reported (the single SAE event was major depression and was judged not to be related to a medication). Treatment adherence to TzOAD was also very good, as only 13 patients out of 200 (6.5%) failed to complete the full study period.
Conclusion
The results of this study indicate that TzOAD was effective as long-term treatment (approximately 6 months) in patients with MDD who responded to TzOAD as monotherapy during a 6- to 8-week acute treatment phase. Improvements were observed in clinical response, overall functioning, depressive symptoms, and QoL. This confirms that MDD patients can benefit from the well-tolerated TzOAD treatment not only in the acute phase, but also in the long term. Results suggest that TzOAD may represent an effective and well tolerated maintenance therapy for patients suffering from MDD.
Abbreviations
ADR, adverse drug reaction; AE, adverse event; CGI-I, Clinical Global Impressions – Improvement; DSM-5, 5th edition of the Diagnostic and Statistical Manual of Mental Disorder; GCP, Good Clinical Practice; GPP, Good Pharmacoepidemiology Practice; ICD-10, International Classification of Diseases, 10th Revision; IEC, independent ethics committee; IRB, institutional review board; LOCF, last observation carried forward; MMD, major depressive disorder; QoL, quality of life; RA, regulatory authority; SARI, serotonin receptor antagonist and reuptake inhibitor; SD, standard deviation; SDS, Sheehan Disability Scale; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, serotonin reuptake inhibitor; T0, baseline; TCA, tricyclic antidepressant; TzOAD, Trazodone Once-A-Day.
Acknowledgments
We would like to acknowledge all of the study sites that participated in this study.
Bulgarian Sites:
BU101 – Hristo Kozhuharov, Diagnostic Consultative Centre Mladost – M OOD, Varna
BU102 – Georgi Panov, University Multiprofile Hospital for Active Treatment, Stara Zagora
BU103 – Toni Donchev, Medical Center Intermedica OOD, Sofia
BU104 – Sibila Dimitrova, Medical Center Hera EOOD, Sofia
BU105 – Emil Grashnov, Center for Mental Health, Sofia
BU106 – Neli Todorova, State Psychiatric Hospital, Tsarev Brod
BU107 – Milena Strashimirova, Diagnostic - Consultative Center (DDC) - XIV – EOOD, Sofia
BU108 – Dancho Dilkov, Outpatient clinic for group practice for specialized medical help- Psihea OOD, Sofia
BU109 – Maya Stoimenova-Popova, Group practice for Outpatient specialized psychiatric help Vitalis, Sofia
BU110 – Ivan Tyanev, Multiprofile Hospital for Active Treatment (MHAT), Targovishte
BU111 – Petar Valkanov, Multiprofile Hospital for Active Treatment Sv. Ivan Rilski, Razgrad
BU112 – Krasimira Marcheva, Medical Center Excelsior OOD, Sofia
BU113 – Vihra Milanova, Multiprofile Hospital for Active Treatment Aleksandrovska EAD, Sofia
BU114 – Kalina Savova, Medical center Vip Clinic OOD, Vidin
BU115 – Temenuzhka Dechkova-Novakova, Mental Health Center, Ruse
Czech Republic sites:
CZ201 – Klaudia Vodickova-Borzova, Brain-soultherapy s.r.o., Kladno
CZ202 – Zdenek Solle, Clintrial s.r.o., Prague
CZ203 – Jan Hanka, Narodni ustav dusevniho zdravi (NÚDZ), Klecany
CZ204 - Michaela Klabusayova, Privatni psychiatricka ambulance, Brno
CZ206 – Simona Papezova, Poliklinika Prosek a.s., Prague
CZ207 – Marta Holanova, Soukroma Psychiatricka Ambulance, Brno
CZ208 – Jiri Masopust, NeuropsychiatrieHK s.r.o., Hradec Kralove
CZ209 – Martin Anders, PRAGTIS s.r.o., Prague
CZ210 – Sylva Rackova, Psychiatrická ambulance Liliová, Plzeň
Polish sites:
PO302 – Filip Rybakowski, Filip Rybakowski Indywidualna Specjalistyczna Praktyka Lekarska, Pzonan
PO303 – Wiesław J. Cubała, Indywidualna Specjalistyczna Praktyka Lekarska Wieslaw Jerzy Cubala, Lublin
PO304 – Adam Wichniak, Instytut Psychiatrii i Neurologii, Warszawa
PO305 – Napoleon Waszkiewicz, Samodzielny Publiczny Psychiatryczny Zaklad Opieki Zdrowotnej im. S. Deresza, Choroszcz
Also we would like to thank to Maria Teresa Rosignoli, Giorgio Di Loreto, Elisa Quarchioni, Raffaella Fallone of Angelini Pharma S.p.A. for all the collaboration during the study preparation and during the statistic analysis of the study.
Disclosure
Wiesław Jerzy Cubała has had grants from Acadia, Alkermes, Allergan, Angelini, Auspex Pharmaceuticals, BMS, Celon, Cephalon, Cortexyme, Ferrier, Forest Laboratories, GedeonRichter, GWPharmaceuticals, HMNC Brain Health, IntraCellular Therapies, Janssen, KCR, Lilly, Lundbeck, Minerva, MSD, NIH, Novartis, Orion, Otsuka, Perception Neuroscience, Sanofi, Servier. Wiesław Jerzy Cubała reports Honoraria from: Adamed, Angelini, AstraZeneca, BMS, Celon, GSK, Janssen, KRKA, Lekam, Lundbeck, Minerva, NeuroCog, Novartis, Orion, Pfizer, Polfa Tarchomin, Sanofi, Servier, Zentiva. Involvement in Advisory boards: Angelini, Celon (terminated), Douglas Pharmaceuticals, Janssen, MSD, Novartis, Sanofi. Adam Wichnaik has received research support from Angelini, Servier and Lekam; he has served on speakers’ bureaus for Adamed, Angelini, Biofarm, Egis, G.L. Pharma, Janssen, Krka, Lekam, Lundbeck, PolfaTarchomin, Sanofi, Servier; he has received personal feeds from Aflofarm, Bausch, Chiesi, Gedeon Richter, Polpharma, Stada; and he has served as a consultant for Adamed, Angelini, Elmiko, Janssen, Lekam, Lundbeck. Claudia Vodickova-Borzova reports grants from Clinical study, outside the submitted work. Alessandro Ruggieri, Paola Lipone, Alessandro Comandini, and Agnese Cattaneo are full-time employees of Angelini Pharma S.p.A. Annalisa Bonelli was a full-time employee of Angelini Pharma S.p.A. at the time of study conduction. Prof Ivan Tyanev declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Prof Milena Shrashimirova declare that they have no competing interests.
References
1. World Health Organization. Depression; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/depression.
2. Machado M, Iskedjian M, Ruiz I, Einarson TR. Remission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trials. CMRO. 2006;22(9):1825–1837.
3. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi:10.1176/appi.ajp.163.1.28
4. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. JCP. 1999;60(4):18118.
5. Clayton A, Keller A, McGarvey EL. Burden of phase-specific sexual dysfunction with SSRIs. JAD. 2006;91(1):27–32.
6. Uguz F, Sahingoz M, Gungor B, Aksoy F, Askin R. Weight gain and associated factors in patients using newer antidepressant drugs. Gen Hosp Psychiatry. 2015;37(1):46–48. doi:10.1016/j.genhosppsych.2014.10.011
7. Thompson C. Onset of action of antidepressants: results of different analyses. Hum Psychopharmacol. 2002;17(S1):S27–S32. doi:10.1002/hup.386
8. Fava M. Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry. 2004;65(Suppl 16):27–32.
9. Ashton AK, Jamerson BD, Weinstein WL, Wagoner C. Antidepressant-related adverse effects impacting treatment compliance: results of a patient survey. Curr Ther Res Clin Exp. 2005;66(2):96–106. doi:10.1016/j.curtheres.2005.04.006
10. Stahl SM. Essential Psychopharmacology of Depression and Bipolar Disorder. Cambridge University Press; 2000.
11. Saltiel PF, Silvershein DI. Major depressive disorder: mechanism-based prescribing for personalized medicine. Neuropsychiatr Dis Treat. 2015;11:875. doi:10.2147/NDT.S73261
12. Stahl SM. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr. 2009;14(10):536–546. doi:10.1017/S1092852900024020
13. Clayton AH, Pradko JF, Croft HA, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002;63(4):357–366. doi:10.4088/JCP.v63n0414
14. Thase ME. Treatment issues related to sleep and depression. J Clin Psychiatry. 2000;61:46–50.
15. Fava M, Hoog SL, Judge RA, Kopp JB, Nilsson ME, Gonzales JS. Acute efficacy of fluoxetine versus sertraline and paroxetine in major depressive disorder including effects of baseline insomnia. J Clin Psychopharmacol. 2002;22(2):137–147. doi:10.1097/00004714-200204000-00006
16. Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60(12):1350–1355. doi:10.1016/j.biopsych.2006.06.015
17. Goldberg H, Finnerty R. A double-blind study of trazodone. Psychopharmacol Bull. 1980;16(3):47–49.
18. Patten SB. The comparative efficacy of trazodone and imipramine in the treatment of depression. CMAJ. 1992;146(7):1177.
19. Beasley CM, Dornseif BE, Pultz JA, Bosomworth JC, Sayler M. Fluoxetine versus trazodone: efficacy and activating-sedating effects. J Clin Psychiatry. 1991;52(7):294–299.
20. Falk WE, Rosenbaum JF, Otto MW, Zusky PM, Weilburg JB, Nixon RA. Fluoxetine versus trazodone in depressed geriatric patients. Topics Geriatr. 1989;2(4):208–214.
21. Kasper S, Olivieri L, Di Loreto G, Dionisio P. A comparative, randomised, double-blind study of trazodone prolonged-release and paroxetine in the treatment of patients with major depressive disorder. CMRO. 2005;21(8):1139–1146.
22. Namiki M, Eij IM, Minemoto H, et al. A clinical Phase III study of SME3110 (fluvoxamine maleate) in depressed patients at the department of internal medicine. A double-blind, comparative study with trazodone hydrochloride. Clin Ther. 1996;12(4):651–677.
23. Munizza C, Olivieri L, Loreto GD, Dionisio P. A comparative, randomized, double-blind study of trazodone prolonged-release and sertraline in the treatment of major depressive disorder. Curr med res opin. 2006;22(9):1703–1713. doi:10.1185/030079906X121039
24. Cunningham LA, Borison RL, Carman JS, et al. A comparison of venlafaxine, trazodone, and placebo in major depression. J Clin Psychopharmacol. 1994;14(2):99–106. doi:10.1097/00004714-199404000-00003
25. Fagiolini A, Albert U, Ferrando L, et al. A randomized, double-blind study comparing the efficacy and safety of trazodone once-A-day and venlafaxine extended-release for the treatment of patients with major depressive disorder. Int Clin Psychopharmacol. 2020;35(3):137. doi:10.1097/YIC.0000000000000304
26. Fagiolini A, Comandini A, Dell’Osso MC, Kasper S. Rediscovering trazodone for the treatment of major depressive disorder. CNS Drugs. 2012;26(12):1033–1049. doi:10.1007/s40263-012-0010-5
27. Hidalgo RB. Trazodone extended release for major depressive disorder. Curr Psychiatr. 2010;9(12):76.
28. Langlieb AM, Guico-Pabia CJ. Beyond symptomatic improvement: assessing real-world outcomes in patients with major depressive disorder. Prim Care Companion CNS Disord. 2010;12(2):26705.
29. Solomon DA, Leon AC, Endicott J, et al. Psychosocial impairment and recurrence of major depression. Compr Psychiatry. 2004;45(6):423–430. doi:10.1016/j.comppsych.2004.07.002
30. Sotsky SM, Glass DR, Shea MT, et al. Patient predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH Treatment of Depression Collaborative Research Program. Focus. 2006;148(2):997.
31. Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976.
32. Feng YS, Kohlman T, Janssen MF, Bucholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30:647–673. doi:10.1007/s11136-020-02688-y
33. Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717–1727. doi:10.1007/s11136-012-0322-4
34. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L)0. Qual Life Res. 2011;20(10):1727–1736. doi:10.1007/s11136-011-9903-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
