Back to Journals » Cancer Management and Research » Volume 12
Independent Risk Factors of Early Recurrence After Curative Resection for Perihilar Cholangiocarcinoma: Adjuvant Chemotherapy May Be Beneficial in Early Recurrence Subgroup
Authors Zhao J, Zhang W, Zhang J, Chen YT, Ma WJ , Liu SY, Li FY , Song B
Received 28 October 2020
Accepted for publication 3 December 2020
Published 22 December 2020 Volume 2020:12 Pages 13111—13123
DOI https://doi.org/10.2147/CMAR.S289094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Jian Zhao,1,2,* Wei Zhang,1,2,* Jun Zhang,1 Yun-Tian Chen,1 Wen-Jie Ma,3 Si-Yun Liu,4 Fu-Yu Li,3,* Bin Song1,*
1Department of Radiology, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, People’s Republic of China; 2Department of Radiology, Armed Police Force Hospital of Sichuan, Leshan 614000, Sichuan, People’s Republic of China; 3Department of Biliary Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, People’s Republic of China; 4GE Healthcare (China), Beijing 100176, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Song Email [email protected]
Purpose: In current clinical practice, early recurrence (ER) is not commonly discussed in perihilar cholangiocarcinoma (pCCA), and its risk factors for this disease have not been well clarified. We carried out this study to analyze the risk factors contributing to ER and explored the prognostic factors after curative resection for pCCA.
Patients and Methods: A total of 335 consecutive pCCA patients were retrospectively analyzed. Risk factors contributing to ER were evaluated using univariate and multivariate logistic regression analyses. Prognostic factors of the ER group were determined by univariate and multivariate Cox regression models. The overall survival (OS) rate was calculated using the Kaplan–Meier method. The Log rank test was used for OS comparison.
Results: Of the 335 cases, 258 patients (77.0%) developed tumor recurrence, 136 patients (40.6%) developed ER, and 122 patients (36.4%) developed late recurrence (LR) postoperatively. The median OS of the ER and LR groups was 15 months and 36 months, respectively (P< 0.001). The multivariate analysis revealed that poor pathological differentiation (P=0.006; moderate vs well, odds ratio [OR]=2.162, 95% confidence interval [CI] 0.753– 6.208, P=0.152; poor vs well, OR=4.839, 95% CI 1.544– 15.170, P=0.007), perineural invasion (OR=4.797, 95% CI 1.586– 14.510, P=0.005), and high levels of preoperative carbohydrate antigen 19– 9 (CA19-9) (OR=2.205, 95% CI 1.208– 4.026, P=0.010) were independent risk factors of developing ER after resection. Adjuvant chemotherapy (HR=0.383, 95% CI 0.154– 0.953, P=0.039) remained as the independent protective factor of OS in patients with ER.
Conclusion: It is recommended that patients with poorly differentiated tumors, presence of perineural invasion, and high levels of preoperative CA19-9 receive closer follow-up and adjuvant chemotherapy following surgery.
Keywords: perihilar cholangiocarcinoma, adjuvant chemotherapy, overall survival, curative-intent resection, early recurrence
Introduction
Cholangiocarcinoma (CCA) is a challenging biliary tract tumor that is often difficult to diagnose and treat.1–3 According to different tumor characteristics, CCA can be divided into 3 groups: intrahepatic cholangiocarcinoma (iCCA), perihilar cholangiocarcinoma (pCCA), and distal cholangiocarcinoma (dCCA).4 pCCA represents the most common type of biliary tract cancer and is often characterized in terms of pathology by aggressive biological behavior.4,5 Identification of driver alterations, earlier diagnosis of cancer, monitoring of treatment response, and exploration of resistance mechanisms are crucial to cancer management.6 Radical resection and neoadjuvant therapy followed by liver transplantation are the only potentially curative treatments for patients with pCCA.7–9 However, even with curative resection, the outcome is dismal, with the 5-year overall survival (OS) and disease-free survival (DFS) remaining unsatisfactory.10–13 These disappointing outcomes are primarily attributable to the high rates of recurrence.7,14,15
Recently, the term “early recurrence (ER)” has become widespread in the discourse surrounding various tumors.16–20 In current clinical practice, the term “ER” is rarely used in relation to pCCA, and its risk factors have not been well clarified. Surgical resection followed by ER has an important negative impact on the survival of patients.21 A thorough understanding of recurrence may improve therapeutic strategies and surgical outcomes. We believe that pre-, intra-, and postoperative clinicopathological variables are indivisible, that all have profound effects on prognosis, and thus should be analyzed simultaneously. The ability of adjuvant therapy to improve OS has been extensively debated. According to the current prevailing opinion, patients with margin-positive and node-positive disease appear to enjoy survival benefits from adjuvant therapy.22 Recently, a Phase 3 BILCAP trial conducted by Primrose and colleagues suggested that adjuvant capecitabine could improve OS for biliary tract cancer compared with observation following surgery.23 However, whether patients at a high risk of ER could benefit from adjuvant therapy remains unknown.
To explore the risk factors of ER and to inform the selection of patients who may benefit most from adjuvant chemotherapy, we retrospectively analyzed data from a high-volume center with a close postoperative follow-up approach.
Patients and Methods
Patient Demographic and Treatment Policy
In this study, no specific personal information was disclosed, and we ensured that anonymity was maintained. The ethics committee of West China Hospital, Sichuan University approved this research (2020–850) and waived the requirement for informed consent because we only collected clinical data and prognosis of patients retrospectively and did not interfere with treatment. Organs involved in this study were donated voluntarily with written informed consent, and related procedures were conducted in accordance with the Declaration of Istanbul. This study was performed as a retrospective study and conducted in accordance with the Declaration of Helsinki.
A total of 377 patients with pCCA who underwent R0 or R1 resection at the West China Hospital between January 1, 2010, and December 31, 2018, were recruited. Only those with histologically confirmed pCCA were included. The exclusion criteria were as follows: (1) patients that underwent palliative surgery; (2) patients with peritoneal dissemination or liver metastasis; (3) patients that were lost to follow-up after discharge; (4) patients who suffered postoperative mortality within 90 days; (5) patients who had received previous anticancer treatments; and (6) patients with a history of other malignancies.
pCCA was defined as cholangiocarcinoma involving hepatic duct bifurcation (lower boundary: the site of cystic duct origin; upper boundary: the second-order branches of the intrahepatic bile ducts). Patients with suspected pCCA were preoperatively assessed by a multidisciplinary team (MDT) comprising surgeons, physicians, medical oncologists, and radiologists. Preoperative examinations included B-ultrasonic examination, computed tomography (CT), magnetic resonance imaging/cholangiopancreatography (MRI/MRCP), endoscopic retrograde cholangiography (ERCP), and/or positron emission tomography (PET).
The operative technique consisted of complete dissection of the hilar structures, skeletonization of the hepatoduodenal ligament, and removal of all fatty and nerve tissue surrounding the common hepatic artery, main portal vein, and bile duct.24 Lymph nodes of the hepatoduodenal ligament, the proper hepatic artery, and the posterior surface of the head of the pancreas were routinely dissected and retrieved. Hemihepatectomy with resection of the caudate lobe was performed routinely except for Bismuth type I.25 Roux-en-Y hepaticojejunostomy was conducted. Resection was guided by intraoperative frozen-section histology examination and intraoperative ultrasound. R0 was defined as no macroscopic or microscopic residual tumor, and R1 was defined as microscopically positive residual tumor. Recurrence was confirmed based on cytological and/or radiological examinations. According to our previous study, ER was defined as relapse within 12 months after surgery.26 The study cohort was classified into the three groups: the ER group (≤12 months before recurrence), the LR group (>12 months before recurrence), and the no recurrence (NR) group.
Adjuvant Gemcitabine-Based Chemotherapy
According to the patients’ wishes and financial situation, adjuvant gemcitabine-based chemotherapy was administered to 25 patients (7.5%) who mainly received resection after 2016. The patients were scheduled to receive 6 cycles of gemcitabine-based chemotherapy. The chemotherapy regimen was as follows: gemcitabine at a dose of 800 mg/m2 biweekly and S1 orally twice daily at a dose of 60 mg/m2/day on days 1 through 28 of a 42-day cycle. When adverse effects were observed, dose modifications and delays were implemented by the attending doctors. Treatment was discontinued upon completion of the regimen, in cases of tumor recurrence or unacceptable toxic effects, or at the request of the patient or clinician.
Clinicopathological Evaluation
All laboratory indicators were examined within 1 week before surgery. Tumor markers were tested, and were controlled in cases of biliary tract inflammation. Routine histopathological workup was conducted for all resected pCCA cases by the Department of Pathology. Perioperative clinicopathological data, including clinical presentation, laboratory data and tumor markers, surgery-associated data, and pathological findings were extracted from the medical records of all patients. Clinicopathological findings were compared among the 3 groups. Tumors were staged in accordance with the American Joint Committee on Cancer (AJCC) tumor–node–metastasis (TNM) classification, 8th edition.27 The postoperative complications group included patients with ≥ Clavien–Dindo grade III complications requiring medical intervention. Patients without complications or with grade I or grade II complications were classified as the no complications group.
Postoperative Follow‑up
Patients were followed up until January 2020. Outpatient follow-up was conducted every 2–3 months for the first year after surgery, and every 3–6 months thereafter. At each visit, patients underwent liver function assessment, measurement of tumor markers, and B-ultrasonic, computed tomography (CT), and/or magnetic resonance imaging (MRI) examination.25 All patients analyzed in this study had a follow-up of at least 1 year (excluding cases of patient death). The sites of recurrence were sorted as intrahepatic, lymph node, local (anastomotic stoma), and distal based on radiographic findings. OS was computed as the interval between the date of surgery and the date of death or last follow-up.
Statistical Analysis
The primary end point of the current study was the ER rate, and the secondary study outcome was the OS rate. Numerical data are presented as means with standard deviations, medians with the interquartile Ranges (IQR), or ranges, and were compared using the student’s t-test, Mann–Whitney U-test, or Kruskal–Wallis H-test, as appropriate. Categorical variables are reported as whole numbers and percentages, and were compared using the chi-squared (χ2) test or Fisher’s exact test, as appropriate. Pairwise comparison of categorical variables was performed by partitions of the χ2 method.
The cutoff value of total bilirubin (TBIL), indirect bilirubin (IBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), aspartate amino transferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), and carcinoembryonic antigen (CEA) were 142.4 µmol/L, 128.9 µmol/L, 16.4 µmol/L, 98 IU/L, 80 IU/L, 328 IU/L, 337 IU/L, and 3.0 ng/mL, which were their respective medians. The cutoff value of carbohydrate antigen 19–9 (CA19-9) was 1000 U/mL, which is the upper limit of the threshold level in our institute. Factors with P values <0.10 in the univariate analysis were included in the multivariate logistic regression analysis to identify independent predictors contributing to ER and LR. Survival analysis was estimated using the Kaplan–Meier method and compared using the Log rank test.
Additionally, 1-, 3-, and 5-year survival rates (YSRs) were calculated. Variables are presented as the odds ratio (OR) or hazard ratio (HR) with 95% confidence interval (95% CI). Statistical analyses were performed using SPSS (version 22, IBM Corp., Armonk, NY, USA), and Medcalc (version 15.2.2, http://www.medcalc.org). Two-tailed P values <0.05 were considered to indicate a statistically significant difference. Threshold levels of significance were adjusted for multiple comparisons by Bonferroni correction.
Results
Clinicopathological Characteristics of the Patients
A total of 377 consecutive patients who underwent radical surgery for pCCA were identified. Twenty-five patients were lost to follow-up since discharge and were censored. Seventeen (4.5%) patients that suffered surgery-related mortality (within 90 days) were also excluded. Finally, 335 patients were included in the present research. The median follow-up period of these pCCA patients was 51 months. The median OS and median recurrence-free survival (RFS) of 32 months (range, 4–117) and 15 months (range, 1–117), respectively. The majority of patients were male (n=183, 54.6%), and the median age was 61 years (range, 20–82). The median postoperative hospital stay was 17 days (range, 8–50). A major hepatectomy was performed in 267 (79.7%) patients, and the operation type of each group is described in Table 1. Most patients (n=278, 83.0%) underwent a caudate lobe resection. The remaining 57 (17.0%) patients received out-hepatic bile duct resection without hepatectomy. Eighteen (5.4%) patients had partial pancreatectomy in addition to hepatectomy, with 289 (86.3%) patients having negative margins of the resected specimen (R0) and 46 (13.7%) patients having R1 margins (microscopic residual). The proportion of each surgery was the same across groups (P=0.921). The intraoperative blood loss was also similar among the 3 groups (P=0.646). Adjuvant chemotherapy (gemcitabine-based) was identical among the 3 groups (P=0.349). Fifty-seven patients had postoperative complications (Clavien–Dindo >IIIa), including infection (n=37), hypohepatia (n=4), biliary fistula (n=11), postoperative bleeding (n=12), stress ulcer (n=6), deep venous thrombosis (n=2), and hepatic encephalopathy (n=1). Twelve patients had more than one type of complication. No differences in postoperative complications were observed between the 3 groups (P=0.364).
 |
Table 1 Comparison of Clinicopathological Characteristics of Patients in the ER, LR, and NR Groups |
In terms of recurrence, 136 patients (40.6%) developed ER, 122 patients (36.4%) developed LR, and 77 patients (23.0%) had no recurrence (NR) during the follow-up period. The most common type of recurrence was local recurrence in both the ER (45.0%) and LR (37.0%) groups. In the ER group, intrahepatic metastasis, lymph node metastasis, local recurrence, and distal metastasis occurred in 52, 38, 61, and 15 patients, respectively, and 30 patients had more than one type of recurrence. In the LR group, 33, 43, 45, and 18 patients had intrahepatic metastasis, lymph node metastasis, local recurrence, and distal metastasis, respectively, and 22 patients had more than one type of recurrence.
Comparison of Clinicopathological Characteristics, and Operative and Postoperative Data Between the ER, LR, and NR Groups
Table 1 shows the comparison of the patients’ clinicopathological characteristics, along with operative and postoperative data between the ER, LR, and NR groups; Table 2 displays the pairwise comparison. Most of the laboratory data showed no significant differences (TBIL [P=0.052]; DBIL [P=0.058]; ALT [P=0.302]; AST [P=0.695]; ALP [P=0.481]; GGT [P=0.808]). There was an overall difference in the serum IBIL level (P=0.034); however, there were no differences observed between the groups by pairwise comparison (Table 2). The serum levels of CEA and CA19-9 of the ER group were higher than those of the NR group. The serum CA19-9 level of the LR group was also significantly higher than that of the NR group; however, this was not the case for the serum CEA level. There was no notable difference in the serum levels of CEA and CA19-9 between the ER and LR groups.
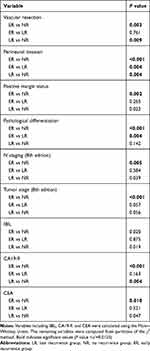 |
Table 2 Pairwise Comparison of Clinicopathological Factors of Patients in the ER, LR, and NR Groups |
The following factors were equivalent between the 3 groups: gender (P=0.177), age (P=0.640), maximum diameter of the tumor (P=0.281), cholelithiasis (P=0.881), preoperative biliary drainage (P=0.300), caudate lobe resection (P=0.343), number of harvested lymph nodes (P=0.087), adjuvant chemotherapy (P=0.349), Bismuth type (P=0.907), T staging (8th edition, P=0.121), and M staging (8th edition, P=0.286). However, several factors were different across the 3 groups, including vascular invasion (P=0.009), perineural invasion (P<0.001), positive margin status (P=0.008), pathological differentiation of the tumor (P<0.001), N staging (8th edition, P=0.014), and TNM stage (8th edition, P=0.001). In the pairwise comparisons (Table 2), all of these factors differed significantly between the ER group and the NR group. Yet, only a few factors (vascular invasion, P=0.009; perineural invasion, P=0.004) of the LR group were significantly different from those of the NR group. Compared to the LR group, the ER group had a higher proportion of perineural invasion (P=0.004) and lower differentiation (P=0.004).
Factors Associated with Early Recurrence
Of the 258 patients who developed recurrence after curative resection of pCCA, ER was observed in 136 patients. By univariate logistic regression analysis (Table S1), 7 variables were identified as potential risk factors contributing to ER (P<0.10): perineural invasion, positive margin status, pathological differentiation, vascular invasion, N stage, and high levels of preoperative CA19-9 and CEA. Multivariate logistic regression was carried out to identify the independent risk factors of ER. It indicated that high levels of preoperative CA19-9 (OR=2.205, 95% CI 1.208–4.026, P=0.010), perineural invasion (OR=4.797, 95% CI 1.586–14.510, P=0.005), and pathological differentiation (total P=0.006; moderate vs well, OR=2.162, 95% CI 0.753–6.208, P=0.152; poor vs well, OR=4.839, 95% CI 1.544–15.170, P=0.007) were independent determinants of ER in patients with pCCA after curative resection (Table 3).
 |
Table 3 Multivariate Logistic Analysis of Predictors of Early Recurrence for Patients with Perihilar Cholangiocarcinoma |
Predictors of OS in the Patients with Early and Late Recurrence
We investigated potential predictors of OS in patients with ER (Table S2). The univariate analysis demonstrated that patients with positive margin status, high levels of ALP, presence of cholelithiasis, and vascular invasion had poorer OS outcomes, whereas patients that received adjuvant chemotherapy had significantly better OS outcomes. In the multivariate analysis, adjuvant chemotherapy (HR=0.383, 95% CI 0.154–0.953, P=0.039) remained as the independent factor of OS in patients with ER (Table S3).
Additionally, we explored potential predictors of OS in patients with LR. The following potential predictors were identified by univariate analysis: age ≥ 60 years, fluke infection, poor pathological differentiation, and high levels of preoperative ALT and AST (Table S4). In the multivariate analysis, age ≥60 years (HR=0.646, 95% CI 0.424–0.983, P=0.042) and poor pathological differentiation (total P=0.035; moderate vs well, HR=0.749, 95% CI 0.381–1.471, P=0.401; poor vs well, HR=1.695, 95% CI 0.747–3.844, P=0.207) were identified as independent factors of OS in patients with LR (Table S5).
Prognostic Outcomes
The survival curves of the ER, LR, and NR groups are depicted in Figure 1A (P<0.001). The median OS of the ER and LR groups were 15 months and 36 months, respectively. Patients in the ER group did not survive 5 years, and had 1- and 3-YSRs of 63.8% and 8.4%, respectively. Patients in the LR group had 1-, 3-, and 5-YSRs of 100.0%, 49.9%, and 11.5%, respectively. In the whole cohort, there were OS differences based on the independent risk factors of ER. Patients with well-differentiated tumors had 1-, 3-, and 5-YSRs of 97.1%, 68.4%, and 47.2%, respectively. However, the 1-, 3-, and 5-YSRs of patients with moderately differentiated tumors decreased significantly to 85.5%, 44.7%, and 20.3%, respectively. Furthermore, patients with poorly differentiated tumors had 1-, 3-, and 5-YSRs of 77.3%, 21.4%, and 7.1%, respectively (P<0.001, Figure 1B). Patients with perineural invasion tumors had 1-, 3-, and 5-YSRs of 83.8%, 38.1%, and 16.9%, respectively, while patients without perineural invasion tumors had 1-, 3-, and 5-YSRs of 95.1%, 75.9%, 49.0%, respectively (P<0.001, Figure 1C). Patients with high levels of preoperative CA19-9 had 1-, 3-, and 5-YSRs of 82.4%, 32.2%, and 13.3%, respectively, while patients with low levels of preoperative CA19-9 had 1-, 3-, and 5-YSRs of 85.9%, 46.1%, and 23.2%, respectively (P=0.002, Figure 1D).
In the ER group, the median OS of patients was 11 and 16 months for those with positive resection margins and those with negative resection margins, respectively (P=0.022, Figure 2A). Patients with low levels of preoperative ALP had a median OS of 16 months, while the high-level subgroup had a median OS of 13 months (P=0.028, Figure 2B). In the positive vascular invasion subgroup, the median OS was 13 months; however, those without vascular invasion had a median OS of 16 months (P=0.046, Figure 2C). Adjuvant chemotherapy had a significantly protective effect on OS: the median OS was 33 months in the adjuvant chemotherapy subgroup and 15 months in the non–adjuvant chemotherapy subgroup (P=0.011, Figure 2D).
In the LR group, the median OS of patients with low levels of preoperative ALT was 41 months, while it was 34 months for those with high levels of preoperative ALT (P=0.016, Figure 3A). Patients with low levels of preoperative AST had a median OS of 40 months, while the high-level subgroup had a median OS of 34 months (P=0.030, Figure 3B). In patients with fluke infection, the median OS was 24 months, while those without fluke infection had a median OS of 38 months (P=0.013, Figure 3C). For tumor differentiation, the OS was 39, 42, 32 for the well-differentiated, moderately differentiated, and poorly differentiated subgroups, respectively, indicating a significant OS difference based on tumor differentiation (P=0.007, Figure 3D). In the paired comparison, patients with poorly differentiated tumors had worse outcomes than those with moderately differentiated tumors (P=0.002, Bonferroni corrected <0.05/6, Figure 3D).
Discussion
Perihilar cholangiocarcinoma (pCCA) currently remains a challenge for hepatobiliary surgeons and oncologists.7 Specifically, there is an ongoing debate regarding the optimal treatment strategy for pCCA after curative resection.28 At present, there is little evidence supporting the usefulness of adjuvant chemotherapy in patients with pCCA following resection.29–31 According to the expert consensus statement, post-resection chemoradiation should be offered to patients who exhibit high-risk features on surgical pathology.32 One meta-analysis found that adjuvant chemotherapy did not significantly improve OS compared with surgery alone.30 However, adjuvant chemotherapy may improve outcomes in node-positive pCCA patients.22,33 Kim et al28 demonstrated that chemoradiotherapy appears to be an appropriate treatment after complete resection for iCCA and pCCA. Also, a propensity-matched study found that adjuvant therapy was associated with improved survival in resected pCCA with positive resection margins.31 Patients with R1 resection and node-positive disease receiving adjuvant chemotherapy after surgery for biliary tract cancers were confirmed to have an OS advantage.34 Thus, the selection of patients before chemotherapy is key to ensuring the efficacy of treatment. We found that patients with a high-risk of ER may be suitable candidates for chemotherapy.
In this analysis, perineural invasion, pathological differentiation, and high levels of preoperative CA19-9 were identified as independent risk factors for ER of tumors after resection. Our results are consistent with previous studies.26,35 In a study analyzing extrahepatic bile duct cancer, age > 75 years, perineural invasion, and high levels of preoperative serum CA19-9 were confirmed to be independent predictors of ER,36 which was similar to our results. Another study showed that preoperative serum CA19-9 level >150 U/mL was associated with ER and lymph node metastasis.37 High levels of CA19-9 may be associated with preoperative micrometastasis. In other tumors, high levels of preoperative serum CA19-9 were also demonstrated to be an independent predictor of ER.38–41
In a randomized Phase III study, gemcitabine and oxaliplatin chemotherapy did not provide evidence of superiority compared to surveillance in resected biliary tract cancer.42 However, in the present study, gemcitabine-based adjuvant therapy was demonstrated to be an independent protective factor of OS in patients with ER. In pancreatic ductal adenocarcinoma, adjuvant chemotherapy was also found to be associated with a reduced risk of ER.43 We recommend that patients with high-risk factors of ER should undergo adjuvant chemotherapy to improve long-term outcomes. Consequently, patients with perineural invasion, poorly differentiated tumors, and high levels of preoperative CA19-9 may be candidates for adjuvant chemotherapy. According to the American Society of Clinical Oncology (ASCO) Clinical Practice Guidelines, hilar cholangiocarcinoma with R1 resections should receive adjuvant chemoradiation therapy.44 This new suggestion increased the range of subject selection. In our cohort, capecitabine was not used to treat pCCA. However, adjuvant capecitabine was shown to improve OS compared with observation following biliary tract cancer surgery.23 The ability of capecitabine to significantly improve the survival of patients with risk factors of ER is noteworthy and warrants further study.
In the survival analysis of the LR subgroup, age and tumor differentiation were identified as independent factors associated with OS. To the best of our knowledge, this was the first study to explore the prognostic risk factors of patients with resected pCCA who developed LR. We found that ER and LR were associated with different prognoses and risk factors after curative resection of pCCA. Similarly, a previous study also demonstrated that ER and LR were also associated with different prognoses and risk factors after curative resection of iCCA.16 In contrast to ER, age ≥60 years and poor pathological differentiation were independent factors of OS in patients with LR. We suggest that more attention should be given to patients under 60 years of age and those with poorly differentiated tumors, even if they are not experienced recurrence within the first year after surgery.
Although CCA is characterized by a high frequency of genetic and molecular aberrations and extreme heterogeneity, advances in genomic sequencing offer hope for novel treatment strategies. Implementation of precision medicine, innovative therapeutic approaches, and personalized therapy may improve clinical outcomes in this highly aggressive disease. In recent years, immunotherapy has shown encouraging results in some tumors and has become increasingly popular.3 In our opinion, the creation of a suitable immunocompetent scheme for CCA could play a prominent role in the next 5 years. Immunotherapy combined with chemotherapy (camrelizumab plus GEMOX [a gemcitabine and oxaliplatin regimen]) has been investigated and shown promising antitumor efficacy in biliary tract cancer.45 Research focused on reported results for specific biliary tract subsites and high-risk patient subgroups is another avenue for future investigation.
Our study has several limitations that should be noted. First, the population that received adjuvant gemcitabine-based chemotherapy was small, and so the result should be interpreted with caution. However, most of the current research also involves a limited number of pCCA patients that received postoperative chemotherapy. In the next phase of research, specifically designed randomized controlled trials are needed to verify the effectiveness of the scheme. Second, although the current cohort involved patients who suffered exclusively from pCCA, the severity of illness could have differed between patients, which might have introduced bias. Third, this study was conducted in a single institute, and thus further multi-institutional and multinational studies with longer follow-up periods are required for more solid conclusions. Moreover, our study was limited by its retrospective nature, and selection bias might have been present in the diagnosis and treatment. Lastly, multigene mutational profiling of pCCA was not explored in this study, but gene mutation has been reported to be associated with ER.46 We intend to explore this issue in upcoming studies.
Conclusion
In summary, ER and LR are associated with distinct prognoses after radical resection for pCCA. We recommend that patients with poorly differentiated tumors, presence of perineural invasion, and high levels of preoperative CA19-9 receive closer follow-up and adjuvant chemotherapy after surgery for pCCA. This study may provide a reference for future screenings of patients suitable for postoperative chemotherapy and close follow-up. Randomized controlled trials can also be designed according to the results of this study.
Author Contributions
All authors made a significant contribution to the work reported, including in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of these areas. All authors also took part in drafting, revising, and critically reviewing the article, and gave final approval of the version to be published. All authors have agreed on the journal to which the article be submitted, and agree to be accountable for all aspects of the work.
Jian Zhao and Wei Zhang contributed equally to this work and should be considered co-first authors.
Funding
There is no funding to report.
Disclosure
Si-Yun Liu is an employee of GE Healthcare (China). The authors report no other potential conflicts of interest for this work.
References
1. Esnaola NF, Meyer JE, Karachristos A, Maranki JL, Camp ER, Denlinger CS. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122(9):1349–1369. doi:10.1002/cncr.29692.
2. Forner A, Vidili G, Rengo M, Bujanda L, Ponz‐Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39(S1):98–107. doi:10.1111/liv.14086.
3. Massa A, Varamo C, Vita F, et al. Evolution of the experimental models of cholangiocarcinoma. Cancers (Basel). 2020;12(8):2308. doi:10.3390/cancers12082308.
4. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. doi:10.1038/nrclinonc.2017.157.
5. Anderson B, Doyle MBM. Surgical considerations of hilar cholangiocarcinoma. Surg Oncol Clin N Am. 2019;28(4):601–617. doi:10.1016/j.soc.2019.06.003.
6. Rizzo A, Ricci AD, Tavolari S, Brandi G. Circulating tumor DNA in biliary tract cancer: current evidence and future perspectives. Cancer Genom Proteom. 2020;17(5):441–452. doi:10.21873/cgp.20203
7. Cillo U, Fondevila C, Donadon M, et al. Surgery for cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):143–155. doi:10.1111/liv.14089.
8. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi:10.1053/j.gastro.2013.10.013.
9. Serrablo A, Tejedor L. Outcome of surgical resection in klatskin tumors. World J Gastrointest Oncol. 2013;5(7):147–158. doi:10.4251/wjgo.v5.i7.147.
10. Li H, Qin Y, Cui Y, Chen H, Hao X, Li Q. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: a Chinese experience. Dig Surg. 2011;28(3):226–231. doi:10.1159/000327361.
11. Kimura N, Young AL, Toyoki Y, et al. Radical operation for hilar cholangiocarcinoma in comparable Eastern and Western centers: outcome analysis and prognostic factors. Surgery. 2017;162(3):500–514. doi:10.1016/j.surg.2017.03.017.
12. Ahn DH, Bekaii-Saab T. Biliary cancer: intrahepatic cholangiocarcinoma vs. extrahepatic cholangiocarcinoma vs. gallbladder cancers: classification and therapeutic implications. J Gastrointest Oncol. 2017;8(2):293–301. doi:10.21037/jgo.2016.10.01.
13. Yao D, Kunam VK, Li X. A review of the clinical diagnosis and therapy of cholangiocarcinoma. J Int Med Res. 2014;42(1):3–16. doi:10.1177/0300060513505488.
14. Komaya K, Ebata T, Yokoyama Y, et al. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery. 2018;163(4):732–738. doi:10.1016/j.surg.2017.08.011.
15. Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik TM. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3(1):18–34. doi:10.3978/j.issn.2304-3881.2014.02.05.
16. Zhang XF, Beal EW, Bagante F, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018;105(7):848–856. doi:10.1002/bjs.10676.
17. Kyang LS, Alzahrani NA, Alshahrani MS, Rahman MK, Liauw W, Morris DL. Early recurrence in peritoneal metastasis of appendiceal neoplasm: survival and prognostic factors. Eur J Surg Oncol. 2019;45(12):2392–2397. doi:10.1016/j.ejso.2019.06.015.
18. Lee S, Kang TW, Song KD, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2019. doi:10.1097/SLA.0000000000003268.
19. Wakatsuki K, Matsumoto S, Migita K, et al. Risk factors and risk scores for predicting early recurrence after curative gastrectomy in patients with stage iii gastric cancer. J Gastrointest Surg. 2020;24(8):1758–1769. doi:10.1007/s11605-019-04327-5.
20. Lin J, Peng J, Zhao Y, et al. Early recurrence in patients undergoing curative resection of colorectal liver oligometastases: identification of its clinical characteristics, risk factors, and prognosis. J Cancer Res Clin Oncol. 2018;144(2):359–369. doi:10.1007/s00432-017-2538-8.
21. Kobayashi T, Aikata H, Kobayashi T, Ohdan H, Arihiro K, Chayama K. Patients with early recurrence of hepatocellular carcinoma have poor prognosis. Hepatobiliary Pancreat Dis. 2017;16(3):279–288. doi:10.1016/s1499-3872(16)60181-9.
22. Mizuno T, Ebata T, Yokoyama Y, et al. Adjuvant gemcitabine monotherapy for resectable perihilar cholangiocarcinoma with lymph node involvement: a propensity score matching analysis. Surg Today. 2017;47(2):182–192. doi:10.1007/s00595-016-1354-0.
23. Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi:10.1016/S1470-2045(18)30915-X.
24. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. doi:10.1016/S0140-6736(13)61903-0.
25. Zhao J, Zhang W, Zhang J, et al. Survival analysis of patients with stage T2a and T2b perihilar cholangiocarcinoma treated with radical resection. BMC Cancer. 2020;20(1):849. doi:10.1186/s12885-020-07357-4.
26. Hu HJ, Jin YW, Shrestha A, et al. Predictive factors of early recurrence after R0 resection of hilar cholangiocarcinoma: a single institution experience in China. Cancer Med. 2019;8(4):1567–1575. doi:10.1002/cam4.2052.
27. Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25(4):845–847. doi:10.1245/s10434-017-6025-x.
28. Kim YS, Oh SY, Go SI, et al. The role of adjuvant therapy after R0 resection for patients with intrahepatic and perihilar cholangiocarcinomas. Cancer Chemother Pharmacol. 2017;79(1):99–106. doi:10.1007/s00280-016-3206-4.
29. Murakami Y, Uemura K, Sudo T, et al. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg. 2009;250(6):950–956. doi:10.1097/sla.0b013e3181b0fc8b.
30. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(16):1934–1940. doi:10.1200/JCO.2011.40.5381.
31. Nassour I, Mokdad AA, Porembka MR, et al. Adjuvant therapy is associated with improved survival in resected perihilar cholangiocarcinoma: a propensity matched study. Ann Surg Oncol. 2018;25(5):1193–1201. doi:10.1245/s10434-018-6388-7.
32. Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):691–699. doi:10.1111/hpb.12450.
33. Seita K, Ebata T, Mizuno T, et al. Phase 2 trial of adjuvant chemotherapy with S - 1 for node-positive biliary tract cancer (N-SOG 09). Ann Surg Oncol. 2020;27(7):2348–2356. doi:10.1245/s10434-020-08355-3.
34. McNamara MG, Walter T, Horgan AM, et al. Outcome of adjuvant therapy in biliary tract cancers. Am J Clin Oncol. 2015;38(4):382–387. doi:10.1097/COC.0b013e31829e19fb.
35. Zhang XF, Beal EW, Chakedis J, et al. Defining early recurrence of hilar cholangiocarcinoma after curative-intent surgery: a multi-institutional study from the us extrahepatic biliary malignancy consortium. World J Surg. 2018;42(9):2919–2929. doi:10.1007/s00268-018-4530-0.
36. Akita M, Ajiki T, Ueno K, et al. Predictors of postoperative early recurrence of extrahepatic bile duct cancer. Surg Today. 2020;50(4):344–351. doi:10.1007/s00595-019-01880-z.
37. Wang JK, Hu HJ, Shrestha A, et al. Can preoperative and postoperative CA19-9 levels predict survival and early recurrence in patients with resectable hilar cholangiocarcinoma? Oncotarget. 2017;8(28):45335–45344. doi:10.18632/oncotarget.17336.
38. Nishio K, Kimura K, Amano R, et al. Preoperative predictors for early recurrence of resectable pancreatic cancer. World J Surg Oncol. 2017;15(1):16. doi:10.1186/s12957-016-1078-z.
39. Ikuta S, Sonoda T, Aihara T, Yamanaka N. A combination of platelet-to-lymphocyte ratio and carbohydrate antigen 19-9 predict early recurrence after resection of pancreatic ductal adenocarcinoma. Ann Transl Med. 2019;7(18):461. doi:10.21037/atm.2019.08.35.
40. Kurahara H, Maemura K, Mataki Y, et al. A therapeutic strategy for resectable pancreatic cancer based on risk factors of early recurrence. Pancreas. 2018;47(6):753–758. doi:10.1097/MPA.0000000000001066.
41. Suzuki S, Shimoda M, Shimazaki J, et al. Predictive early recurrence factors of preoperative clinicophysiological findings in pancreatic cancer. Eur Surg Res. 2018;59(5–6):329–338. doi:10.1159/000494382.
42. Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol. 2019;37(8):658–667. doi:10.1200/JCO.18.00050.
43. Groot VP, Gemenetzis G, Blair AB, et al. Defining and predicting early recurrence in 957 patients with resected pancreatic ductal adenocarcinoma. Ann Surg. 2019;269(6):1154–1162. doi:10.1097/SLA.0000000000002734.
44. Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37(12):1015–1027. doi:10.1200/JCO.18.02178.
45. Chen X, Wu X, Wu H, et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, Phase II trial. J Immunother Cancer. 2020;8(2):e001240. doi:10.1136/jitc-2020-001240.
46. Conci S, Ruzzenente A, Simbolo M, et al. Multigene mutational profiling of biliary tract cancer is related to the pattern of recurrence in surgically resected patients. Updates Surg. 2020;72(1):119–128. doi:10.1007/s13304-020-00718-5.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



