Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Impact of Fixed Combination of Metformin and Pioglitazone on Insulin Resistance of Patients with Type 2 Diabetes: Results of a Randomized Open-Label Study
Authors Sun R, Yuan L, Shen Y, Shen Z, Ding B, Ma J
Received 29 May 2023
Accepted for publication 29 August 2023
Published 20 September 2023 Volume 2023:16 Pages 2911—2919
DOI https://doi.org/10.2147/DMSO.S423322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Rui Sun,* Lu Yuan,* Yun Shen,* Ziyang Shen, Bo Ding, Jianhua Ma
Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Ding; Jianhua Ma, Email [email protected]; [email protected]
Aim: To compare the effect of metformin, a fixed combination of metformin and pioglitazone, or dapagliflozin on insulin resistance in patients with newly diagnosed type 2 diabetes.
Methods: In this 6-week randomized open-label trial, 58 patients were randomly assigned to insulin with metformin, a fixed combination of metformin and pioglitazone, or dapagliflozin for 4 weeks. Hyperinsulinemic euglycemic clamp tests and FreeStyle Libre Pro Sensor were used to evaluate the insulin sensitivity represented by glucose-infusion rate (M value) and glycemic control, respectively. The main outcome was changes in insulin resistance compared with baseline.
Results: The baseline characteristics were well matched among the three groups. When compared to baseline, insulin sensitivity after treatment was significantly improved. Further study revealed that the fixed combination of metformin and pioglitazone provided superior M-value improvement compared with metformin, but not different from dapagliflozin. Moreover, a greater reduction in insulin dose was observed in the fixed combination of metformin and pioglitazone group than the metformin or dapagliflozin group. However, there were no significant differences in the parameters of glycemic control within the groups.
Conclusion: In patients with newly diagnosed type 2 diabetes, a fixed combination of metformin and pioglitazone provided greater improvement in insulin resistance than metformin alone and similar changes in insulin resistance to dapagliflozin.
Keywords: insulin resistance, metformin, pioglitazone, dapagliflozin, type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM), accounting for >90% of patients with diabetes, is a chronic disease characterized by insulin resistance or relative insulin deficiency. Previous epidemiological studies have revealed that the global prevalence of T2DM has significantly increased over the last 4 decades.1 Subjects with diabetes are susceptible to both microvascular and macrovascular complications, which lead to a long-term health damage and enormous economic losses.2 Massive studies have uncovered the fundamental pathophysiological mechanisms of insulin resistance underlying the development of T2DM. Insulin resistance is a complex condition where insulin-dependent cells, such as skeletal muscle cells and adipocytes, respond inappropriately to normal insulin levels, and thus more insulin is needed to maintain normal function.3 Obesity and visceral adiposity are common habitual factors aggravating insulin resistance.4 Progression of insulin resistance worsens the burden on beta cells and contributes to hyperinsulinemia and frank diabetes.5 Moreover, insulin resistance has been demonstrated to be associated with an increased risk of cardiovascular events in patients with diabetes. Therefore, insulin resistance should be ameliorated early for the optimal management of T2DM.6
Among the oral antihyperglycemic drugs, metformin is effective, safe, and inexpensive, as well as able to reduce the risk of cardiovascular events and death. It may reduce fasting and postprandial plasma glucose levels by decreasing hepatic glucose output, suppressing intestinal absorption of glucose and enhancing insulin sensitivity due to improved peripheral glucose utilization.7 Pioglitazone, a PPARγ agonist, has been approved to treat T2DM for more than 20 years.8 Activation of PPARγ may promote transcription of numerous genes in target organs and remodeling of white adipose tissue, which leads to an improvement in insulin sensitivity.9 In comparison with metformin, pioglitazone has a stronger capacity to ameliorate insulin resistance and a unique effect on insulin-mediated glucose uptake.10,11 Recent studies further demonstrated that pioglitazone is the first agent to prevent both progression to diabetes and major cerebrovascular diseases.12 Clinically, both metformin and pioglitazone can be used in monotherapy or combination therapy. Greater efficacy has been obtained in combination therapy due to the different effects of metformin and pioglitazone.13 Moreover, a fixed-dose combination of metformin and pioglitazone provides an effective method for raising the therapeutic compliance of patients on the basis of glycemic control. SGLT2 inhibitors, a relatively new type of oral antidiabetic drug that includes dapagliflozin and empagliflozin, not only diminish reabsorption of glucose in the renal proximal tubule through inhibition of SGLT2 but also indirectly enhance peripheral insulin sensitivity owing to weight loss and elimination of glucose toxicity.14 Additionally, inhibition of SGLT2 has been demonstrated to restore brain insulin action and whole-body metabolism in individuals with prediabetes and obese rats.15,16
Although the aforementioned drugs are promising treatment strategies for insulin resistance, a critical question arises as to which drug benefits patients with T2DM more from reduced insulin resistance and consequent glycemic control. In this study, we aimed to evaluate the effects of metformin, a fixed-dose combination of metformin and pioglitazone (500 mg metformin plus 15 mg pioglitazone), and dapagliflozin on insulin sensitivity and glycemic control in overweight patients with newly diagnosed T2DM.
Methods
Study Participants
The current randomized, open-label study was conducted in Nanjing First Hospital between May 2021 and February 2023. All authors of this paper participated in the recruitment of subjects. Overweight adult patients with newly diagnosed T2DM according to WHO diagnostic criteria (1999) who had received no medication for diabetes were enrolled in this study. Briefly, the inclusion criteria were willingness to participate in this study, age 18–60 years, regular diet and exercise, body mass index (BMI) ≥24 kg/m2 and glycated hemoglobin (HbA1c) >9%. Exclusion criteria were other types of diabetes, insulin allergy or intolerance of metformin, pioglitazone, or dapagliflozin, renal dysfunction (estimated glomerular filtration rate <45 mL/(min/1.73 cm2), severe liver disease or elevated transaminases (2.5-fold the upper limit), history of alcohol dependence or drug abuse in the last 5 years, systemic steroid therapy or other medication influencing cholesterol metabolism in the last 3 months, acute infection or stress state within 1 month prior to the study, pregnancy or lactation, history of diabetic ketoacidosis within the previous year, psychiatric disease, and other serious comorbid conditions, including severe cardiovascular and pulmonary disease, heart failure of New York Heart Association class IV, and tumors. This study was approved by the research ethics board of Nanjing First Hospital, Nanjing Medical University in accordance with the Helsinki Declaration (KY20220314-01) and is registered at ClinicalTrials.gov (identifier NCT05591235). Written informed consent was obtained from all enrolled patients.
Study Design
Prior to randomization, subjects underwent eligibility screening and entered a 1-week run-in period of single insulin therapy with continuous subcutaneous insulin infusion to attain the glycemic target of fasting glucose <7 mmol/L, 2-hour postprandial glucose <11 mmol/L and time in range (TIR) (3.9–10 mmol/L) ≥80% based on 7-point self-monitored blood glucose.17 Then, insulin sensitivity was evaluated using the hyperinsulinemic euglycemic clamp test. Randomization was subsequently performed by random allocation in a 1:1:1 manner using a computer-based system into three groups: group A for insulin with metformin (a minimum daily dose of 1500 mg), group B for insulin with fixed-dose combination of metformin and pioglitazone (two tablets of 500 mg metformin plus 15 mg pioglitazone), and group C for insulin with dapagliflozin (10 mg once a day). During this 4-week time frame, all patients were instructed to change the insulin pump infusion site and provide one profile of 7-point self-monitored blood glucose every 3 days. The insulin doses were titrated every day by physicians to achieve glycemic control. Participants who failed to attain the glycemic target three consecutive times or could not afford the side effects of hypoglycemic medications were excluded from this study. On the last day of week 4, a FreeStyle Libre Pro Sensor (flash continuous glucose monitoring [FGM] system) was inserted to record glycemic data for up to 14 days. At the end of week 5, all antidiabetic agents were stopped and the hyperinsulinemic euglycemic clamp test was performed again for remaining subjects. This study was completed when the FGM system was removed (Figure 1A).
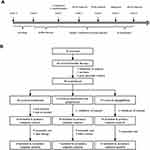 |
Figure 1 Study design (A) and participant flow (B). |
The sample size required was calculated using PASS 21.0.3 (NCSS, Kaysville, Utah, USA). Based on our preliminary experiment, the mean M-values were 5.8, 7.0, and 6.6 for the metformin, fixed-dose combination of metformin and pioglitazone, and dapagliflozin groups, respectively, and the standard deviation was 1.0, 0.95, and 0.9 for the three groups, respectively. When we set the significance level (α) to 0.05 and the desired power of the study (1 − β) to 90%, the minimum number of patients required was 54. Assuming a 20% dropout rate, at least 69 cases needed to be screened.
Hyperinsulinemic Euglycemic Clamp Test
The insulin pump was stopped for 12 hours, and then the hyperinsulinemic euglycemic clamp test was performed according to a published protocol with slight adjustment.18,19 After 12-hour overnight fasting, exogenous primed regular human insulin was infused at a rate of 4 mU/(kg/min) for the first 10 minutes, followed by a continuous insulin infusion at 2 mU/(kg/min) for the remaining 140 minutes in order to totally inhibit endogenous glucose production. The glucose-infusion rate was adjusted to achieve the target glucose level (4.5–5.5 mmol/L) based on the blood glucose measured every 5 minutes. The steady glucose-infusion rate during the last 30 minutes (120–150 min) was used to calculate the glucose-metabolized (M) value (mg/[kg/min]).
Measurements
Demographic data (name, age, and sex) and anthropometric measures (weight, height, and blood pressure) were collected at baseline and the end of study if needed. Fasting venous blood samples were drawn for laboratory measurements. Conventional routine methods were used for determination of fasting glucose and lipid profiles. Serum HbA1c and C-peptide were measured by high-performance liquid chromatography and radioimmunoassay, respectively.
End Points
The primary end point was changes in insulin resistance represented by M value from week 1 to week 5. Additionally, insulin doses and body weight were evaluated over the same time period for the primary end point. Secondary end points were glycemic control assessed by FGM, including TIR, mean glucose level, and mean amplitude of glycemic excursions between week 4 and week 6. All safety end points, including diabetic ketoacidosis, metabolic acidosis, heart failure or myocardial infarction, urinary tract infection, edema, cancer, and death, were also documented throughout this study.
Statistical Analysis
All analyses were performed with SPSS 20 (IBM, USA). Normally distributed continuous variables are presented as means ± SD and skewed data as medians (IQR) or as indicated. Categorical variables are given as numbers and percentages. Differences among three groups were assessed using one-way ANOVA (normally distributed variables), Kruskal–Wallis H test (skewed variables), or x2 test (categorical variables) as appropriate. ANCOVA was used for the analysis of changes in M values, insulin dose, and body weight from baseline to week 5. Two-sided P<0.05 was considered statistically significant.
Results
Participant Disposition
A total of 70 subjects were screened, and 65 consented to participate in the study. Of the 58 participants who completed the therapy, 20 were randomized to the metformin group, 19 to the fixed-dose combination of metformin and pioglitazone group, and 19 to the dapagliflozin group. Dropout rates at the end of this study were 10.0% (two of 20), 5.2% (one of 19), and 5.2% (one of 19) in the metformin group, fixed-dose combination of metformin and pioglitazone group, and the dapagliflozin group, respectively. Detailed reasons for dropouts are shown in Figure 1B.
Baseline Characteristics
As shown in Table 1, age, sex, BMI and prevalence of hypertension were well matched among the three groups (P>0.05). There were no differences in most of the clinical parameters either, including lipid profiles (triglycerides, high–density lipoprotein cholesterol, and low–density lipoprotein cholesterol), metrics of glucose control (fasting plasma glucose and HbA1c), measures of insulin secretion (C-peptide and postprandial C-peptide), and renal and liver function (glomerular filtration rate, alanine transaminase, and aspartate transaminase) among groups (P>0.05). Higher total cholesterol in the dapagliflozin group was observed (P<0.05).
 |
Table 1 Demographic, anthropometric, and disease characteristics of trial patients |
Primary Outcome
Insulin sensitivity improved significantly in all groups (Figure 2A), whereas absolute M values were comparable among the groups after treatment. The rise in M values with the fixed dose combination of metformin and pioglitazone was significantly greater than with metformin with adjustment for baseline M levels, but not different from dapagliflozin (Figure 2A). With the improvement in insulin sensitivity, insulin dose decreased in all groups. The fixed-dose combination of metformin and pioglitazone showed the highest reduction in insulin dose when compared with metformin and dapagliflozin (Figure 2B). However, we did not observe significant changes in body weight among the three groups (Figure 2C).
Secondary Outcomes
As for glycemic parameters, there were no significant differences in mean glucose, TIR, time below range, time above range, low blood glucose index, high blood glucose index, or mean amplitude of glycemic excursions within the three groups with or without medication according to the FGM data (Supplementary Table 1).
Safety Outcomes
No severe adverse events were reported in the current study. Moderate gastrointestinal side effects, such as nausea, diarrhea, and reduced appetite, were observed in four subjects in the metformin group, two in the fixed-dose combination of metformin and pioglitazone group, and one in the dapagliflozin group (P=0.317). None of the patients suffered from heart failure, fracture, edema, or urinary tract infection.
Discussion
Therapies for insulin resistance have stagnated for a number of years due to a lack of knowledge of underlying mechanisms and specific molecular drug targets. Previous studies revealed that increased glucose–fatty acid cycle, O-GlcNAcylation of key enzymes in the hexosamine biosynthesis pathway, ectopic lipid accumulation, and inflammation and endoplasmic reticulum stress associated with insulin resistance.20,21 Therefore, stimulation of fat oxidation and muscle mass and inhibition of hepatic fat synthesis with novel molecular compounds might provide an alternative strategy for insulin resistance.
Consistently with the DEFENCE study by Shigiyama et al, insulin resistance as assessed by homeostatic model assessment of insulin resistance (HOMA-IR) decreased significantly in both dapagliflozin and metformin groups in patients with early-stage T2DM, while the improvement in insulin sensitivity was comparable between the two groups.22 In patients with T2DM receiving stable basal insulin therapy, HOMA-IR was improved with an add-on therapy in combination with pioglitazone and metformin other than metformin alone.23 There have also been studies demonstrating that pioglitazone–metformin combination therapy is associated with significantly decreased levels of HOMA-IR when compared with metformin monotherapy in patients with T2DM.13,24 In contrast, HOMA-IR did not change consistently with dapagliflozin or metformin treatment in a population of overweight women with gestational diabetes pregnancy.25 Several factors may contribute to the inconsistency in these findings, including different disease populations, ethnicity, and sample size. Furthermore, the hyperinsulinemic euglycemic clamp test, the gold-standard method for assessing insulin sensitivity, was performed in this study, which varies from the aforementioned studies.
Among the currently available antidiabetic agents, dapagliflozin exerts hypoglycemic effects independently of insulin secretion and insulin resistance, which significantly decreases HbA1cand BMI when compared with placebo. Monotherapy with dapagliflozin displays a similar reduction in HbA1cand fasting blood glucose with metformin or pioglitazone.26 Given the unique protective effect on macrovascular and renal complications, dapagliflozin is a preferred choice in patients with atherosclerotic cardiovascular disease, heart failure, or moderate chronic kidney disease.27 However, concerns about the risk of lower-limb amputation, euglycemic ketoacidosis, bone fracture, acute renal injury, and Fournier gangrene have also been raised with the extensive application of dapagliflozin.28 Dapagliflozin should be prescribed with caution after balancing the benefits with side effects. Although the indirect insulin-sensitizing action of dapagliflozin mainly relies on the relief of glucose toxicity,28 we observed improved insulin sensitivity with dapagliflozin under tight glycemic control, which indicated a direct role of dapagliflozin in insulin resistance. Possibly, dapagliflozin may exert beneficial roles in insulin sensitivity through attenuating the downregulation of pAkt and increasing GLUT4 protein levels.29,30 Dapagliflozin administration can also decrease the expression level of oxidative stress markers and suppress the secretion of inflammatory cytokines, both of which may improve insulin resistance.31,32 Furthermore, the insulin resistance–lowering effect of dapagliflozin could partly be explained by enhancing fat utilization and browning of adipose tissue, thus inhibiting fat accumulation and hepatic steatosis.33
In comparison with dapagliflozin, pioglitazone is a powerful insulin sensitizer that directly improves insulin sensitivity through activating insulin signaling on muscle cells.34 Although pioglitazone remains available at an accessible price in clinical management, its usage has dramatically declined due to the fear of side effects. It has long been blamed for increased risk of bladder cancer and atypical bone fractures.35 Fluid retention caused by pioglitazone has also restricted its application in patients with congestive heart failure. However, there are several unique advantages of pioglitazone, such as durable responsiveness, reasonable price, low risk of hypoglycemia as monotherapy, flexible combination with additional agents, and little influence of renal function.36 In patients with prediabetes, pioglitazone treatment may delay the development of T2DM with sparse serious adverse events and cardiovascular complications, according to a recent meta-analysis of 4186 participants.37 Emerging evidence has also demonstrated the protective effect of a low dose of pioglitazone on liver steatosis and inflammation independent of glycemic control in patients with T2DM.38 Moreover, pioglitazone can reduce the risk of myocardial infarction and ischemic stroke in primary and secondary prevention without proven direct harm on the myocardium. In vitro studies have confirmed that pioglitazone shows solid benefits on cardiomyocyte electrophysiology, ischemia–reperfusion injury, and cardiac remodeling.39,40 Given the beneficial effects, it might be time to reconsider the status of pioglitazone in therapeutic strategies for T2DM.
Metformin is a very modest insulin sensitizer in muscle and a powerful drug targeting glucose production in liver.41 It has been shown that metformin confers cardiovascular protection in patients with T2DM;42,43 however, there have been studies showing no benefits of metformin on mortality or cardiovascular risk.44–47 Metformin and pioglitazone improve insulin sensitivity through different molecular and organ-specific mechanisms. The synergistic action of metformin and pioglitazone provides a rationale for the combination of both drugs in treatment of insulin resistance. In addition to the benefits over glycemic control, improvements in diabetic dyslipidemia, inflammation, and cardiovascular events have been reported. Therefore, the fixed-dose combination of pioglitazone and metformin serves as a more convenient option for control of T2DM based on the bioequivalence between the fixed-dose combination and coadministration of individual medication.48
Clinically, lean individuals with insulin resistance are not uncommon. Results from a cross-sectional study showed that half the young insulin-resistant adults were lean.49 It was inappropriate to treat these lean but insulin-resistant subjects with metformin or dapagliflozin, which might further exacerbate weight loss. Conversely, a combination of pioglitazone and metformin may counteract adverse events without masking the significant improvement in blood glucose control, as weight gain is a well-known disadvantage of pioglitazone.50,51 Additionally, pioglitazone was also proved to mitigate the dapagliflozin-related weight loss.52 Therefore, a bright future for fixed-dose combination metformin and pioglitazone application can be seen for diabetic patients, especially those with cardiovascular complications, poor lipid control, and low body weight. The failure to find significant changes in body weight in the current study may be explained by the short time frame.
There are several strengths to our study. The gold-standard technique of the hyperinsulinemic euglycemic clamp test was applied to evaluate insulin resistance before and after antidiabetic agent treatment. To avoid the influence of hyperglycemia on insulin sensitivity,53,54 all included participants received a period of intensive therapy in order to maintain optimized glycemic control before assessing insulin sensitivity.
However, some limitations should be addressed. We could not ascertain the effect of long-term exposure of insulin combined with metformin, a fixed-dose combination of metformin and pioglitazone, or dapagliflozin on insulin sensitivity, glycemic control, or weight due to a short duration of intervention. Moreover, the lipid profiles and C-peptide were not reassessed at the end of the trial; therefore, whether the current treatments can improve islet secretion was unclear. Additionally, the data on glycemic control assessed by FGM may be not reliable due to too many patients dropping out. To better explore the effects on glucose fluctuation, it is undoubtedly crucial to include more data on glycemic control assessed by FGM.
In conclusion, a short-term fixed combination of metformin and pioglitazone provides better improvement in insulin sensitivity than metformin alone and similar changes in insulin resistance with dapagliflozin in overweight individuals with T2DM. Further study with a substantial sample and longer follow-up are warranted to confirm the findings.
Abbreviations
BMI, body mass index; TIR, time in range.
Data Sharing
The data are not freely publicly available due to privacy reasons. After publication, the data presented in this study will be available upon request from the corresponding author.
Acknowledgment
This research was supported by the Nanjing Health Science and Technology Development Special Fund Project Plan (YKK20107).
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin North Am. 2021;50(3):337–355. doi:10.1016/j.ecl.2021.05.013
2. Pearson ER. Type 2 diabetes: a multifaceted disease. Diabetologia. 2019;62(7):1107–1112. doi:10.1007/s00125-019-4909-y
3. Yaribeygi H, Farrokhi FR, Butler AE, et al. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. 2019;234(6):8152–8161. doi:10.1002/jcp.27603
4. Ndisang JF, Vannacci A, Rastogi S. Insulin resistance, type 1 and type 2 diabetes, and related complications 2017. J Diabetes Res. 2017;2017:1478294. doi:10.1155/2017/1478294
5. Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci. 2019;20(6):1351. doi:10.3390/ijms20061351
6. Laakso M. Biomarkers for type 2 diabetes. Mol Metab. 2019;27(Suppl 2):S139–s146. doi:10.1016/j.molmet.2019.06.016
7. Tanase DM, Gosav EM, Costea CF, et al. The intricate relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196. doi:10.1155/2020/3920196
8. Nauck MA, Wefers J, Meier JJ. Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol. 2021;9(8):525–544. doi:10.1016/S2213-8587(21)00113-3
9. Mastrototaro L, Roden M. Insulin resistance and insulin sensitizing agents. Metabolism. 2021;125:154892. doi:10.1016/j.metabol.2021.154892
10. Eguchi K, Tomizawa H, Ishikawa J, et al. Comparison of the effects of pioglitazone and metformin on insulin resistance and hormonal markers in patients with impaired glucose tolerance and early diabetes. Hypertens Res. 2007;30(1):23–30. doi:10.1291/hypres.30.23
11. Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49(3):434–441. doi:10.1007/s00125-006-0141-7
12. Inzucchi SE, Viscoli CM, Young LH, et al. Pioglitazone prevents diabetes in patients with insulin resistance and cerebrovascular disease. Diabetes Care. 2016;39(10):1684–1692. doi:10.2337/dc16-0798
13. Perez A, Zhao Z, Jacks R, et al. Efficacy and safety of pioglitazone/metformin fixed-dose combination therapy compared with pioglitazone and metformin monotherapy in treating patients with T2DM. Curr Med Res Opin. 2009;25(12):2915–2923. doi:10.1185/03007990903350011
14. Ferrannini G, Hach T, Crowe S, et al. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1730–1735. doi:10.2337/dc15-0355
15. Ruud J, Steculorum SM, Brüning JC. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat Commun. 2017;8(1):15259. doi:10.1038/ncomms15259
16. Kullmann S, Hummel J, Wagner R, et al. empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: a randomized, double-blind, placebo-controlled, Phase 2 trial. Diabetes Care. 2022;45(2):398–406. doi:10.2337/dc21-1136
17. Nunez Lopez YO, Retnakaran R, Zinman B, et al. Predicting and understanding the response to short-term intensive insulin therapy in people with early type 2 diabetes. Mol Metab. 2019;20:63–78. doi:10.1016/j.molmet.2018.11.003
18. Baldi S, Bonnet F, Laville M, et al. Influence of apolipoproteins on the association between lipids and insulin sensitivity: a cross-sectional analysis of the RISC study. Diabetes Care. 2013;36(12):4125–4131. doi:10.2337/dc13-0682
19. Yang L, Liang H, Liu X, et al. Islet function and insulin sensitivity in latent autoimmune diabetes in adults taking sitagliptin: a randomized trial. J Clin Endocrinol Metab. 2021;106(4):e1529–e1541. doi:10.1210/clinem/dgab026
20. Luukkonen PK, Zhou Y, Sädevirta S, et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64(5):1167–1175. doi:10.1016/j.jhep.2016.01.002
21. Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46(1):15–37. doi:10.4093/dmj.2021.0280
22. Shigiyama F, Kumashiro N, Miyagi M, et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16(1):84. doi:10.1186/s12933-017-0564-0
23. Hanefeld M, Pfützner A, Forst T, et al. Double-blind, randomized, multicentre, and active comparator controlled investigation of the effect of pioglitazone, metformin, and the combination of both on cardiovascular risk in patients with type 2 diabetes receiving stable basal insulin therapy: the PIOCOMB study. Cardiovasc Diabetol. 2011;10:65. doi:10.1186/1475-2840-10-65
24. Kaku K. Efficacy and safety of therapy with metformin plus pioglitazone in the treatment of patients with type 2 diabetes: a double-blind, placebo-controlled, clinical trial. Curr Med Res Opin. 2009;25(5):1111–1119. doi:10.1185/03007990902820816
25. Elkind-Hirsch KE, Seidemann E, Harris R. A randomized trial of dapagliflozin and metformin, alone and combined, in overweight women after gestational diabetes mellitus. Am J Obstet Gynecol MFM. 2020;2(3):100139. doi:10.1016/j.ajogmf.2020.100139
26. Wang Y, Hu X, Liu X, et al. An overview of the effect of sodium glucose cotransporter 2 inhibitor monotherapy on glycemic and other clinical laboratory parameters in type 2 diabetes patients. Ther Clin Risk Manag. 2016;12:1113–1131. doi:10.2147/TCRM.S112236
27. Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 Inhibitors. Circ Res. 2018;122(10):1439–1459. doi:10.1161/CIRCRESAHA.117.311588
28. Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772. doi:10.1161/CIRCULATIONAHA.116.021887
29. El-Sayed N, Mostafa YM, AboGresha NM, et al. Dapagliflozin attenuates diabetic cardiomyopathy through erythropoietin up-regulation of AKT/JAK/MAPK pathways in streptozotocin-induced diabetic rats. Chem Biol Interact. 2021;347:109617. doi:10.1016/j.cbi.2021.109617
30. Joannides CN, Mangiafico SP, Waters MF, et al. Dapagliflozin improves insulin resistance and glucose intolerance in a novel transgenic rat model of chronic glucose overproduction and glucose toxicity. Diabetes Obes Metab. 2017;19(8):1135–1146. doi:10.1111/dom.12923
31. Tsai KF, Chen Y-L, Chiou TT-Y, et al. Emergence of SGLT2 inhibitors as powerful antioxidants in human diseases. Antioxidants. 2021;10(8):1166. doi:10.3390/antiox10081166
32. Zhang E, Zhao Y, Hu H. Impact of sodium glucose cotransporter 2 inhibitors on nonalcoholic fatty liver disease complicated by diabetes mellitus. Hepatol Commun. 2021;5(5):736–748. doi:10.1002/hep4.1611
33. Han T, Fan Y, Gao J, et al. Sodium glucose cotransporter 2 inhibitor dapagliflozin depressed adiposity and ameliorated hepatic steatosis in high-fat diet induced obese mice. Adipocyte. 2021;10(1):446–455. doi:10.1080/21623945.2021.1979277
34. DeFronzo RA, Inzucchi S, Abdul-Ghani M, et al. Pioglitazone: the forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diab Vasc Dis Res. 2019;16(2):133–143. doi:10.1177/1479164118825376
35. Lewis JD, Habel LA, Quesenberry CP, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314(3):265–277. doi:10.1001/jama.2015.7996
36. Einhorn D, Fonseca V. Revisiting the use of Pioglitazone in the treatment of type 2 diabetes. Endocr Pract. 2016;22(11):1343–1346. doi:10.4158/EP161409.CO
37. Ipsen E, Madsen KS, Chi Y, et al. Pioglitazone for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;11(11):Cd013516. doi:10.1002/14651858.CD013516.pub2
38. Della Pepa G, Russo M, Vitale M, et al. Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial. Diabetes Res Clin Pract. 2021;178:108984. doi:10.1016/j.diabres.2021.108984
39. Nesti L, Tricò D, Mengozzi A, et al. Rethinking pioglitazone as a cardioprotective agent: a new perspective on an overlooked drug. Cardiovasc Diabetol. 2021;20(1):109. doi:10.1186/s12933-021-01294-7
40. Lee M, Saver JL, Liao H-W, et al. Pioglitazone for secondary stroke prevention: a systematic review and meta-analysis. Stroke. 2017;48(2):388–393. doi:10.1161/STROKEAHA.116.013977
41. Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321(19):1926–1927. doi:10.1001/jama.2019.3805
42. Zhang K, Yang W, Dai H, et al. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: results from meta-analysis. Diabetes Res Clin Pract. 2020;160:108001. doi:10.1016/j.diabres.2020.108001
43. Han Y, Xie H, Liu Y, et al. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. 2019;18(1):96. doi:10.1186/s12933-019-0900-7
44. Li T, Providencia R, Mu N, et al. Association of metformin monotherapy or combined therapy with cardiovascular risks in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):30. doi:10.1186/s12933-020-01202-5
45. Bromage DI, Godec TR, Pujades-Rodriguez M, et al. Metformin use and cardiovascular outcomes after acute myocardial infarction in patients with type 2 diabetes: a cohort study. Cardiovasc Diabetol. 2019;18(1):168. doi:10.1186/s12933-019-0972-4
46. Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60(9):1620–1629. doi:10.1007/s00125-017-4337-9
47. Lamanna C, Monami M, Marchionni N, et al. Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2011;13(3):221–228. doi:10.1111/j.1463-1326.2010.01349.x
48. Seufert J. A fixed-dose combination of pioglitazone and metformin: a promising alternative in metabolic control. Curr Med Res Opin. 2006;22(Suppl 2):S39–48. doi:10.1185/030079906X121002
49. Šebeková K, Gurecká R, Csongová M, et al. Lean insulin-resistant young adults display increased cardiometabolic risk: a retrospective cross-sectional study. Diabetes Res Clin Pract. 2022;185:109217. doi:10.1016/j.diabres.2022.109217
50. Derosa G, Maffioli P. Thiazolidinediones plus metformin association on body weight in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;91(3):265–270. doi:10.1016/j.diabres.2010.08.001
51. Derosa G, Tinelli C, Maffioli P. Effects of pioglitazone and rosiglitazone combined with metformin on body weight in people with diabetes. Diabetes Obes Metab. 2009;11(12):1091–1099. doi:10.1111/j.1463-1326.2009.01087.x
52. Rosenstock J, Vico M, Wei L, et al. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35(7):1473–1478. doi:10.2337/dc11-1693
53. Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13(6):610–630. doi:10.2337/diacare.13.6.610
54. Luo W, Ai L, Wang B-F, et al. High glucose inhibits myogenesis and induces insulin resistance by down-regulating AKT signaling. Biomed Pharmacother. 2019;120:109498. doi:10.1016/j.biopha.2019.109498
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

