Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Immune Checkpoint Inhibitor-Associated Systemic Sclerosis in the Treatment of a Small Cell Lung Cancer Patient with Durvalumab: A Case Report
Received 23 November 2023
Accepted for publication 11 March 2024
Published 18 March 2024 Volume 2024:17 Pages 663—669
DOI https://doi.org/10.2147/CCID.S451386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
De-Hu Li, Xian-Zhi Xiong
Department of Pulmonary and Critical Care Medicine, NHC Key Laboratory of Pulmonary Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Xian-Zhi Xiong, Email [email protected]
Abstract: As one of the key cancer treatment measures, immune-checkpoint inhibitors (ICIs) have revolutionized the treatment landscape of various cancers, including malignancies previously thought to be untreatable. Immune checkpoint inhibitors work by targeting the dysfunctional immune system, to enhance cancer-cell killing by CD8-positive T cells. Despite the beneficial effects of ICIs, these treatments are also linked to a novel class of side effects, termed immune-related adverse events (irAEs). Immune-related adverse events can affect multiple organ systems, such as endocrine, neurological, gastroenteric, dermatologic, ocular, hepatic, renal, and rheumatic ones. While variable in severity, irAEs can be associated with significant morbidity, mortality, cessation of ICI treatment and can be potentially life-threatening sometimes. Among varieties of irAEs, dermatological manifestations are frequently reported, since they can be easily observed. Here, we present a case of a 74-year-old patient with widespread fibrosis of skin, eventually diagnosed as diffuse cutaneous systemic sclerosis after the treatment with durvalumab for small cell lung cancer (SCLC). Prompt recognition and treatment of immune-checkpoint inhibitors-associated systemic sclerosis may help enhance tolerance to ICIs and ensure better performance in treating tumors.
Keywords: systemic sclerosis, durvalumab, small cell lung cancer, immune checkpoint inhibitors, immune-related adverse events
Introduction
Immune-checkpoint inhibitors (ICIs) have become a crucial component of cancer treatment.1 Starting with the approval of anti-cytotoxic T lymphocyte-associated protein 4 (anti-CTLA-4) for advanced-stage melanoma in 2011, immune checkpoint inhibitors (ICIs) have been widely applied in the treatment of a variety of malignancies.2 Instead of CTLA-4 (ipilimumab), ICIs now also include antibodies against programmed cell death 1 (PD-1; nivolumab, pembrolizumab, cemiplimab, sintilimab, camrelizumab, toripalimab, tislelizumab, zimberelimab, prolgolimab, and dostarlimab) and its ligand (PD-L1; atezolizumab, avelumab, and durvalumab).3 Immune checkpoints are receptors expressed on immune cells and allow for the dynamic regulation of immunological homeostasis.1 PD-1 and PD-L1 are expressed on T cells, tumor cells and tumor-infiltrating myeloid cells, respectively.1 T cell exhaustion is caused by the interaction of these two proteins and is characterized by decreased or missing effector function (cytotoxicity or cytokine production), lack of reactivity to stimuli and altered transcriptional and epigenetic states.4,5 On the other hand, CTLA-4 is one type of negative immune regulator constitutively expressed on regulatory T (Treg) cells and upregulated on activated T cells.6 CTLA-4 inhibits T cell activation through various suppressive functions including competition with CD28, regulation of the inhibitory function of Treg cells, such as transendocytosis, and the control of adhesion and motility.6 However, more and more immune-related adverse effects (irAEs), which are unique and accompanied by a delayed onset and prolonged duration, have been reported as a result of this increased use.7 IrAEs can occur in any organ system and 2–16 weeks can be the median onset after the start of therapy.8 Varieties of dermatological manifestations can occur associated with ICIs such as vitiligo, lichenoid dermatitis, psoriasis, bullous pemphigoid, granulomatous diseases, drug rash with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome and Sweet syndrome.9–11 The anti-PD-1 (34–42%) and anti-PD-L1 monotherapy (−20%) are associated with lower rates of cutaneous adverse events than anti-CTLA-4 monotherapy (44–59%), but combination therapy is linked to the highest incidence (59–72%).11,12 However, among these dermatological irAEs, systemic sclerosis induced by the treatment of durvalumab in small cell lung cancer (SCLC) patients has not been reported before. Here, we described a case of durvalumab-associated systemic sclerosis in a patient with SCLC after the treatment with durvalumab.
Case Presentation
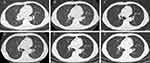
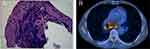
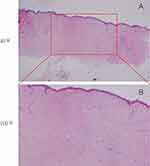
A 74-year-old man was admitted to hospital with cough and chest tightness for one month. He complained of a cough with no obvious cause in the past month, which intensified into paroxysms, coughing up pus sputum, accompanied by chest tightness, shortness of breath, dyspnea, hemoptysis, and sometimes blood in the sputum. He had not had autoimmune diseases in the past, and he denied prior familial history of autoimmune diseases. After the computed tomography (CT) scan of the chest (Figure 1A), lesions biopsy by electronic bronchoscopy (Figure 2A) and positron emission tomography (PET)/CT scan (Figure 2B), he was diagnosed with limited-stage SCLC. The results of laboratory tests, including anti-Scl-70 antibody, anti-centromere antibody, anti-RNA polymerase III antibody, anti-neutrophil cytoplasmic antibody, anti-myeloperoxidase (MPO) antibody, anti-glomerular basement membrane (GBM) antibody, anti-Smith (Sm) antibody, anti-SSA antibody, anti-SSB antibody, anti-dsDNA antibody and anti-histidyl tRNA synthetase (Jo-1) antibody were all negative. Besides, the anti-nuclear antibody titer was 1:100 and the patient’s eosinophils were 0.22*109/L. Due to the lack of surgical opportunity, he received chemotherapy plus immunotherapy. According to the treatment guidelines for SCLC, he underwent five cycles of cisplatin (130 mg/m2) plus etoposide (100 mg/day) in combination with durvalumab (1000 mg/day). After the combination therapy of chemotherapy plus immunotherapy, he was treated with ten cycles of maintenance therapy with durvalumab from July 2021 to April 2022. However, the patient gradually felt slight swelling and tightness of the skin on his trunk and limbs accompanied by skin itching after ten cycles of durvalumab maintenance therapy (Figure 3). Besides, he had difficulty swallowing, but no Raynaud’s phenomenon. Therefore, maintenance therapy with durvalumab was suspended owing to these clinical manifestations. Afterwards, he received routine anti-allergy treatment during his hospitalization, but there was no relief of skin symptoms after the anti-allergy treatment. During hospitalization, according to the opinion of the dermatologist, he underwent a skin biopsy. After skin biopsy of the lesion on the right abdomen, histopathologic examination was reported as systemic sclerosis (Figure 4). At the time of discharge, he was instructed to take oral prednisone acetate tablets 40 mg daily with a weekly dose reduction of 5 mg. One month later, he felt that the skin stiffness of his extremities was better than before, but the skin of neck, chest, back, and abdomen was still stiff. Therefore, he was admitted to the rheumatology department, where he received intravenous hydroprednisone (40mg/d). During hospitalization in rheumatology department, the results of laboratory tests, including erythrocyte sedimentation rate (ESR) was 28mm/h, C-reactive protein (CRP) was 11.4mg/L, decrease in complement C3 and anti-nuclear antibody titer was 1:320. In addition, anti-Scl-70 antibody, anti-centromere antibody, anti-RNA polymerase III antibody, anti-neutrophil cytoplasmic antibody, anti-MPO antibody, anti-GBM antibody, anti-Sm antibody, anti-SSA antibody, anti-SSB antibody, anti-dsDNA antibody and anti-histidyl tRNA synthetase (Jo-1) antibody were all negative. The patient’s eosinophil count was 0.16*109/L. Esophageal manometry suggested that the patient had esophageal motor disorders. The rheumatologist considered that the patient can be diagnosed as diffuse cutaneous systemic sclerosis and the digestive tract had been affected. Therefore, he was advised to take oral mycophenolate mofetil (MMF) dispersible tablets 0.5 g twice daily plus prednisone acetate tablets 20 mg once daily, and the dose was reduced to 15 mg daily after half a month.
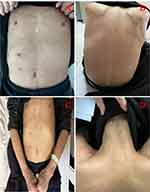 |
Figure 3 Widespread areas of skin sclerosis of the patient. (A) Chest and Abdomen (B) Back (C) Chest, Abdomen and Upper Limbs (D) Neck. |
Fortunately, according to the CT scan (Figure 1D), there was no progression of cancer after the end of immune maintenance treatment. After regular oral prednisone treatment, the patient’s systemic sclerosis is now under control, but the skin of the trunk is still hard and tight, and the patient still has dysphagia. Even though SCLC is an extremely aggressive neuroendocrine tumor, characterized by rapid growth and early metastasis.13 There is no recurrence or metastasis of the primary tumor after the administration of chemotherapy plus immunotherapy during regular follow-up (Figure 1E and F), though this patient had been diagnosed with SCLC for more than two years.
Discussion
Durvalumab is a fully human monoclonal IgG1 k antibody targeting PD-L1, which has been approved by the US Food and Drug Administration (FDA) to treat solid organ malignancies in 2017.14,15 Some cases have been reported where systemic sclerosis or morphea can be induced by atezolizumab, pembrolizumab or nivolumab.16–20 However, systemic sclerosis induced by durvalumab has not been reported before. Systemic sclerosis is an immune-mediated rheumatic disease characterized by fibrosis of the skin and internal organs and vasculopathy.21 Early vasculopathy, innate and adaptive immune system dysfunction, and aberrant connective tissue development are factors in the etiology of systemic sclerosis.22
It is estimated that approximately 11.3% to 24.6% of patients diagnosed with cancer have pre-existing autoimmune disease (AIDs).23 Recent studies have suggested that patients with pre-existing AIDs are more prone to developing irAEs than patients without AIDs in the past. In a systematic review conducted by Abdel-Wahab et al, they found that 75% cancer patients with pre-existing AIDs had exacerbation of pre-existing AIDs, irAEs or both after receiving ICIs treatment.24 Machado et al suggested that cancer patients with gastrointestinal and rheumatologic AIDs had a higher incidence of AIDs flare-ups after ICIs treatment.25 Besides, pre-existing AIDs are risk factors for irAEs.26 In cancer patients, irAEs are more common with combination ICIs therapy than ICIs monotherapy.27 ICIs-induced inflammatory arthritis, myositis, sicca, and vasculitis are classified as rheumatic irAEs.27 New-onset systemic sclerosis induced by the treatment of ICIs accounts for approximately less than 10% of all rheumatic-irAEs (and <1% of all types of irAEs).28,29 There are clinical distinctions between rheumatic irAEs and pre-existing AIDs. Macklin et al suggested that ICIs-associated systemic sclerosis patients differed significantly from primary systemic sclerosis cases in that they exhibited less vascular characteristics and lower seropositivity, including antinuclear and systemic sclerosis-specific antibodies.30 Although one of the three systemic sclerosis-associated antibodies (anti-Scl-70 antibody, anti-centromere antibody, or anti-RNA polymerase III antibody) is positive in 60–80% of pre-existing systemic sclerosis patients, none of the ICIs-systemic sclerosis cases showed positive systemic sclerosis-associated antibodies.31,32
Barbosa et al described two cases of patients developing systemic sclerosis when pembrolizumab therapy was used for treating metastatic melanoma.17 One patient was diagnosed as diffuse systemic sclerosis after 14 cycles of pembrolizumab treatment for metastatic melanoma and the patient had normal levels of anti-nuclear antibody, anti-centromere antibody, anti-ribonucleoprotein antibody, and anti-Scl-70 antibody.17 Afterwards, the patient was advised to take prednisone 1 mg/kg daily and received intravenous immunoglobulin, 0.4 mg/kg daily for 5 days monthly, and mycophenolate mofetil, 1000 mg twice daily.17 However, the patient died of unknown causes (autopsy was declined) after subjective improvement in the skin changes.17 The other patient was diagnosed as limited systemic sclerosis with negative systemic sclerosis-related specific antibodies.17 He was advised to take hydroxychloroquine (200 mg twice daily) by a dermatologist and prednisone (1 mg/kg) by his oncologist and his skin changes improved considerably after immunosuppressant and prednisone treatment.17 Terrier et al reported two cases with underlying limited cutaneous systemic sclerosis who showed a significant increase in skin thickening after pembrolizumab treatment.28 One 49-year-old woman with metastatic non-small cell lung cancer has finally been diagnosed with diffuse cutaneous systemic sclerosis after pembrolizumab treatment.28 The anti-nuclear antibody was positive with a titer of 1/640, but were negative for anti-Scl-70 antibody, anti-centromere antibody, or anti-RNA polymerase III antibody, in this patient.28 She received intravenous cyclophosphamide in combination with prednisone at a dose of 10 mg/day.28 The other patient with pre-existing limited cutaneous systemic sclerosis finally developed diffuse extension of the skin thickening to proximal limbs and the trunk after the pembrolizumab treatment for metastatic non-small cell lung cancer.28 However, the patient improved skin thickening after 6 cycles of cyclophosphamide.28 In our case report, the patient developed diffuse systemic sclerosis after 10 cycles of durvalumab treatment and systemic sclerosis-related specific antibodies including anti-Scl-70 antibody, anti-centromere antibody and anti-RNA polymerase III antibody were all negative. The patient received prednisone (15–40 mg once daily) and mycophenolate mofetil 0.5 g (twice daily). Fortunately, the patient’s skin symptoms got subjective improvement, and he did not experience recurrence as well as metastasis of the primary tumor despite the interruption of durvalumab therapy. Therefore, systemic corticosteroids (0.5–2 mg/kg daily, depending on the severity of symptoms) can be used to treat most irAEs.33,34 However, patients may require immunosuppressive or modulatory therapy, such as azathioprine, mycophenolate mofetil, cyclosporine, and tumor necrosis factor α inhibitors, if the irAEs are not responsive to corticosteroids.33,35 Besides, patients with systemic sclerosis secondary to ICIs treatment in our case and in the cases mentioned above usually lacked systemic sclerosis-associated specific antibodies as well as Raynaud’s phenomenon.
Conclusion
In conclusion, we report a very rare case of systemic sclerosis induced by immunotherapy with durvalumab. It is consistent with recent findings in the literature that patients receiving anti-PD-1 or anti-PD-L1 antibody therapy and experiencing irAEs have better overall response rates, progression-free survival rates, and overall survival.36 Given the cases, we reported on this occasion, we should keep a close eye on the irAEs during the use of ICIs. In the process of treating SCLC with ICIs, clinicians should pay attention to the patient’s irAEs, especially when the patient’s skin appears to be sclerotic, they should be alert to the possibility of ICIs-associated systemic sclerosis. At this time, they should actively take the appropriate treatment measures to alleviate the condition, and to prevent the aggravation of the condition, so as to avoid the negative impact on the ICIs treatment. Early identification and management of irAEs may enhance tolerance to ICIs and improve cancer survival. In addition, further studies will need to be done to develop a better understanding of the mechanisms associated with the systemic sclerosis caused by ICIs therapies.
Consent for Publication
The patient had given written informed consent for the publication of his clinical details and images. Institutional approval is not required for this case study.
Funding
This work was funded by Jointown Caritas Fund of Hubei Red Cross Foundation.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19(4):254–267. doi:10.1038/s41571-022-00600-w
2. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi:10.1146/annurev-pathol-042020-042741
3. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. doi:10.1186/s12943-021-01489-2
4. Blank CU, Haining WN, Held W, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19(11):665–674. doi:10.1038/s41577-019-0221-9
5. Philip M, Fairchild L, Sun L, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545(7655):452–456. doi:10.1038/nature22367
6. Mitsuiki N, Schwab C, Grimbacher B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol Rev. 2019;287(1):33–49. doi:10.1111/imr.12721
7. Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38. doi:10.1038/s41572-020-0160-6
8. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, Phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi:10.1016/S1470-2045(15)70076-8
9. Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi:10.1016/j.ejca.2016.02.010
10. Goldinger SM, Stieger P, Meier B, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. 2016;22(16):4023–4029. doi:10.1158/1078-0432.CCR-15-2872
11. Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. doi:10.1007/s40257-017-0336-3
12. Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41(2):125–128. doi:10.1016/j.currproblcancer.2016.12.001
13. Wang WZ, Shulman A, Amann JM, Carbone DP, Tsichlis PN. Small cell lung cancer: subtypes and therapeutic implications. Semin Cancer Biol. 2022;86(Pt 2):543–554. doi:10.1016/j.semcancer.2022.04.001
14. Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):8. doi:10.1186/s40425-018-0316-z
15. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi:10.1016/S0140-6736(19)32222-6
16. Shenoy N, Esplin B, Barbosa N, Wieland C, Thanarajasingam U, Markovic S. Pembrolizumab induced severe sclerodermoid reaction. Ann Oncol. 2017;28(2):432–433. doi:10.1093/annonc/mdw543
17. Barbosa NS, Wetter DA, Wieland CN, Shenoy NK, Markovic SN, Thanarajasingam U. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92(7):1158–1163. doi:10.1016/j.mayocp.2017.03.016
18. Tjarks BJ, Kerkvliet AM, Jassim AD, Bleeker JS. Scleroderma-like skin changes induced by checkpoint inhibitor therapy. J Cutan Pathol. 2018;45(8):615–618. doi:10.1111/cup.13273
19. Cho M, Nonomura Y, Kaku Y, et al. Scleroderma-like syndrome associated with nivolumab treatment in malignant melanoma. J Dermatol. 2019;46(1):e43–e44. doi:10.1111/1346-8138.14492
20. Grant C, Chalasani V, Uchin JM, Dore A. Atezolizumab-induced scleroderma: a rare complication. BMJ Case Rep. 2021;14(11):e244968. doi:10.1136/bcr-2021-244968
21. Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. doi:10.1016/S0140-6736(17)30933-9
22. Jerjen R, Nikpour M, Krieg T, Denton CP, Saracino AM. Systemic sclerosis in adults. Part I: clinical features and pathogenesis. J Am Acad Dermatol. 2022;87(5):937–954. doi:10.1016/j.jaad.2021.10.065
23. Khan SA, Pruitt SL, Xuan L, Gerber DE. Prevalence of autoimmune disease among patients with lung cancer: implications for immunotherapy treatment options. JAMA Oncol. 2016;2(11):1507–1508. doi:10.1001/jamaoncol.2016.2238
24. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Internal Med. 2018;168(2):121–130. doi:10.7326/M17-2073
25. Pizuorno Machado A, Shatila M, Liu C, et al. Immune-related adverse events after immune checkpoint inhibitor exposure in adult cancer patients with pre-existing autoimmune diseases. J Cancer Res Clin Oncol. 2023;149(9):6341–6350. doi:10.1007/s00432-023-04582-9
26. Yamaguchi A, Saito Y, Okamoto K, et al. Preexisting autoimmune disease is a risk factor for immune-related adverse events: a meta-analysis. Support Care Cancer. 2021;29(12):7747–7753. doi:10.1007/s00520-021-06359-7
27. Reid P, Cappelli LC. Treatment of rheumatic adverse events of cancer immunotherapy. Best Pract Res. 2022;36(4):101805. doi:10.1016/j.berh.2022.101805
28. Terrier B, Humbert S, Preta LH, et al. Risk of scleroderma according to the type of immune checkpoint inhibitors. Autoimmunity Rev. 2020;19(8):102596. doi:10.1016/j.autrev.2020.102596
29. Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Rheumatic syndromes associated with immune checkpoint inhibitors: a single-center cohort of sixty-one patients. Arthritis Rheumatol. 2019;71(3):468–475. doi:10.1002/art.40745
30. Macklin M, Yadav S, Jan R, Reid P. Checkpoint inhibitor-associated scleroderma and scleroderma mimics. Pharmaceuticals. 2023;16(2):1.
31. Mecoli CA, Casciola-Rosen L. An update on autoantibodies in scleroderma. Curr Opin Rheumatol. 2018;30(6):548–553. doi:10.1097/BOR.0000000000000550
32. Salazar GA, Assassi S, Wigley F, et al. Antinuclear antibody-negative systemic sclerosis. Semin Arthritis Rheumatism. 2015;44(6):680–686. doi:10.1016/j.semarthrit.2014.11.006
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi:10.1016/j.ejca.2015.11.016
34. Weber JS, Dummer R, de Pril V, Lebbé C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675–1682. doi:10.1002/cncr.27969
35. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi:10.1016/j.ctrv.2016.02.001
36. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. doi:10.1186/s40425-019-0805-8
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A Case of Hepatocellular Carcinoma Successfully Resumed Atezolizumab and Bevacizumab After Associated Grade 3 Diarrhea and Grade 2 Colitis: Case Report and Literature Review
Fuji T, Arai J, Otoyama Y, Nio Y, Sugiura I, Nakajima Y, Kajiwara A, Ichikawa Y, Uozumi S, Shimozuma Y, Uchikoshi M, Sakaki M, Nozawa H, Momo K, Sasaki T, Yoshida H
OncoTargets and Therapy 2022, 15:1281-1288
Published Date: 25 October 2022
Onychopathy Following Durvalumab Treatment for Extensive-Stage Small-Cell Lung Cancer: A Case Report
Zhang C, Wang K, Zhang H, Liu J, Zheng C, Tao J, Lin L, Zhai L
Clinical, Cosmetic and Investigational Dermatology 2023, 16:2429-2432
Published Date: 5 September 2023