Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Onychopathy Following Durvalumab Treatment for Extensive-Stage Small-Cell Lung Cancer: A Case Report
Authors Zhang C, Wang K, Zhang H, Liu J, Zheng C, Tao J, Lin L, Zhai L
Received 21 June 2023
Accepted for publication 25 August 2023
Published 5 September 2023 Volume 2023:16 Pages 2429—2432
DOI https://doi.org/10.2147/CCID.S415119
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Cuifen Zhang,1,* Ke Wang,1,* Hong Zhang,1 Jingjing Liu,1 Chuangjie Zheng,1 Jiahao Tao,1 Lizhu Lin,2 Linzhu Zhai3
1the First Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 2Cancer Center, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 3Cancer Center, Departments of Radiation Oncology, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Linzhu Zhai, Cancer Center, Departments of Radiation Oncology, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, No. 16 Jichang Road, Baiyun District, Guangzhou, 510405, People’s Republic of China, Tel +86-20-36591419, Email [email protected]
Abstract: Patients treated with immune checkpoint inhibitors (ICIS) are prone to immune related adverse events (irAEs), making it important to pay attention to these adverse events. Herein, we report a case of onychopathy after treatment of extensive small cell lung cancer (ES-SCLC) with durvalumab; this is the first report of onychopathy caused by durvalumab in a patient with lung cancer. The change in the patient’s nails mainly manifested in the form of pigmentation and the thickening of the nails. Antifungal ointment was ineffective, and these changes were unrelated to malnutrition or any other factors. In addition, this case shows that onychopathy may occur within 2 years after treatment, indicating that these patients need long-term follow-up.
Keywords: onychopathy, durvalumab, ES-SCLC, immune checkpoint inhibitors, immune-related adverse events
Introduction
Durvalumab is a selective, high-affinity human IgG1 monoclonal antibody that blocks the binding of programmed death ligand 1 (PD-L1) with programmed death ligand 1 (PD-1) and CD80, allowing T cells to recognize and kill tumor cells.1 It is suitable for unresectable patients with stage 3 non-small cell lung cancer after concurrent radiotherapy and platinum chemotherapy.2,3 The combination of first-line durvalumab and etoposide plus cisplatin or carboplatin (EP) significantly improved the overall survival rate (OS) over EP alone in cases of extensive small cell lung cancer (ES-SCLC).4,5 However, immune checkpoint inhibitors (ICIs), including durvalumab, are often associated with immune-related adverse events (irAEs), including pneumonia, hepatitis, colitis, nephritis, dermatitis, and myositis.6 Although toxicity of ICIs can occur in various organs, only a few studies have evaluated onychopathy after ICI treatment. Here, we report a case of onychopathy after durvalumab treatment for ES-SCLC.
Case Report
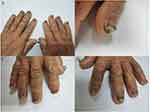
A patient in their 60s presented with a sore throat when drinking water in May 2020. Computed tomography (CT) showed the left tonsil to be swollen. Positron emission tomography (PET-CT) found masses in the left tonsil, bilateral supraclavicular and infraclavicular regions, mediastinum, the left internal mammary region, left hilar hepatogastric space, and retroperitoneal enlarged lymph nodes. They also found multiple bone metastases throughout the body. In June 2020, tonsil and lung biopsies confirmed that the pathology was poorly differentiated neuroendocrine carcinoma, concluded to have originated from the lung. After the patient was diagnosed, they were given two courses of etoposide and cisplatin as first-line treatment combined with durvalumab 1000 mg q3w. A CT scan after two courses of durvalumab indicated stable disease (SD). After a standard six-course treatment, CT showed partial remission (PR). The patient had bone marrow toxicity during the treatment, manifesting as grade III leukopenia, which was improved after injection with granulocyte colony stimulating factor (G-CSF). Then, the patient was treated with three courses of durvalumab alone, followed by palliative radiotherapy for the residual lesions in the left lung. After radiotherapy, the patient continued to receive maintenance therapy with durvalumab (14 courses) until July 2022. On April 2022 (22 months after first administration of durvalumab), the patient presented with an adverse reaction of the fingernails of both hands, albeit with no pain (Figure 1). Antifungal cream was used, but the pigmentation and thickening of the nails did not improve, the patient also experienced skin peeling and scaling on his hands, which improved with lubricating ointment (Figure S1). The patient’s nails were biopsied, and the pathological diagnosis was nail keratinization and fibrous connective tissue of the fingertips and toes, with no tumor involvement. PAS and PASM staining showed no fungal infection (Figures 2 and S2). The patient was still under durvalumab treatment 24 months after the initiation of ICI therapy and showed no sign of metastatic lesion regrowth. The patient’s diet and lifestyle habits did not change during the treatment period.
Discussion
Targeted therapy and immunotherapy are associated with a wide range of dermatologic adverse events (DAEs), such as rashes and peeling of the skin. Dermatologic toxicities include damage to the skin, oral mucosa, hair, and nails.7 Immune checkpoint inhibitors (ICIs) most often induce nonspecific maculopapular eruptions as well as eczema-like or psoriasis-like lesions, lichen-like dermatitis, pruritus, and xerosis. However, there are few reports of onychopathy after ICI treatment.8 Yuriko et al reported two cases of urothelial carcinoma with onychopathy after administration of pembrolizumab, and Khokhar et al reported a case of nail peeling after treatment of malignant melanoma with pembrolizumab.9 These patients’ symptoms, which included redness around the eyes, redness and edema of the lips, erosive skin damage, and nail loss, can be attributed to the autoimmune complications caused by pembrolizumab. We drew this conclusion after excluding psoriasis-like mossy dermatitis and paraneoplastic pemphigus. These two articles mainly describe the skin peeling-off at the edge of the nail and the nail adverse reaction in the form of nail peeling caused by pembrolizumab. Here, however, we mainly report nail toxicity in the form of nail thickening and pigmentation caused by durvalumab. The nail lesion in our case must be differentiated from onychomycosis. First, fungal infection was excluded as a cause of the adverse reaction by pathological examination. More importantly, all 10 of the patient’s fingernails showed pigmentation and thickening, which is very rare in cases of fungal infection. Moreover, antifungal cream could not improve the thickened nails. At the same time, follow-up was conducted on the patient, and it was found that there were no changes in their diet or lifestyle habits during the treatment period. Therefore, it can be determined that the nail changes were unrelated to factors such as malnutrition. These results fully support the diagnosis of immunotoxicity. These findings were not made in previous reports. To our knowledge, this is the first report of onychopathy caused by durvalumab in a patient treated for lung cancer. Onychopathy has not been mentioned in previous large-scale studies such as PACIFIC and CASPIAN clinical trials.10 The mechanism underlying onychopathy remains unconfirmed, but it may involve autoimmune inflammation, which eventually leads to serious damage to nail stromal cells. This case also suggests that onychopathy can occur as late as 2 years after treatment, which indicates that these patients require long-term follow-up. The management of onychopathy caused by ICIs is still unclear, and the potential of hormone therapy needs to be explored further.
Conclusion
This case report indicates that using durvalumab in cancer patients may cause onychopathy. Although we were able to exclude factors such as fungal infection and malnutrition, the underlying mechanism was not confirmed. This mechanism may involve autoimmune inflammation that severely damages the nail stromal cells. We must follow up with these patients for a long period of time to better observe them.
Publication of the details of this case is subject to institutional approval.
Ethical Approval Statement
This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine. (No. K-2023-056).
Ethics Statement
Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported herein, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of the above. All authors took part in drafting, revising, or critically reviewing the article and gave final their approval of the version to be published; all have also agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Beijing Xisike Clinical Oncology Research Foundation (grant no. Y-2020Sciclone/zb/qn-0005), the National Natural Science Foundation of China (grant nos. 81873147 and 82274604), and the “Double first-class” and high-level university construction discipline (Integrated Traditional Chinese and Western Medicine) reserve talent cultivation project of Guangzhou University of Chinese Medicine (grant no. A1-2601-22-415-014).
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937
2. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, Phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi:10.1016/S0140-6736(19)32222-6
3. De Ruysscher D, Ramalingam S, Urbanic J, et al. CheckMate 73L: a Phase 3 study comparing nivolumab plus concurrent chemoradiotherapy followed by nivolumab with or without ipilimumab versus concurrent chemoradiotherapy followed by durvalumab for previously untreated, locally advanced stage III non-small-cell lung cancer. Clin Lung Cancer. 2022;23(3):e264–e268. doi:10.1016/j.cllc.2021.07.005
4. Hotta K, Nishio M, Saito H, et al. First-line durvalumab plus platinum-etoposide in extensive-stage small-cell lung cancer: CASPIAN Japan subgroup analysis. Int J Clin Oncol. 2021;26(6):1073–1082. doi:10.1007/s10147-021-01899-8
5. Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi:10.1016/S1470-2045(20)30539-8
6. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi:10.1001/jamaoncol.2018.3923
7. Lacouture M, Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol. 2018;19(Suppl 1):31–39. doi:10.1007/s40257-018-0384-3
8. Mathieu L, Shah S, Pai-Scherf L, et al. FDA approval summary: atezolizumab and durvalumab in combination with platinum-based chemotherapy in extensive stage small cell lung cancer. Oncologist. 2021;26(5):433–438. doi:10.1002/onco.13752
9. Sugihara Y, Kawai T, Yamada D, Furuyama C, Sato Y, Kume H. Onychomadesis following pembrolizumab treatment for metastatic urothelial carcinoma: a report of two cases. Eur J Cancer. 2020;126:91–92. doi:10.1016/j.ejca.2019.12.009
10. Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–1311. doi:10.1200/JCO.21.01308
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.