Back to Journals » ImmunoTargets and Therapy » Volume 9
Idiopathic CD4 Lymphocytopenia: Current Insights
Authors Vijayakumar S , Viswanathan S , Aghoram R
Received 10 February 2020
Accepted for publication 10 April 2020
Published 14 May 2020 Volume 2020:9 Pages 79—93
DOI https://doi.org/10.2147/ITT.S214139
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Michael Shurin
Saravanakumari Vijayakumar,1 Stalin Viswanathan,2 Rajeswari Aghoram3
1Pathology, Sri Lakshmi Narayana Institute of Medical Sciences, Pondicherry 605502, India; 2General Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry 605006, India; 3Department of Neurology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry 605006, India
Correspondence: Rajeswari Aghoram
Department of Neurology, Jawaharlal Institute of Postgraduate Medical Education and Research, D. Nagar, Pondicherry 605006, India
Tel +91 413 229 7285
Email [email protected]
Abstract: Idiopathic CD4 lymphocytopenia is a condition characterized by low CD4 counts. It is rare and most of the information about this illness comes from case reports. Presentation is usually in the 4th decade of life with opportunistic infections, autoimmune disease or neoplasia. The pathophysiology of this condition is not well understood. Management revolves around treatment of the presenting condition and close follow-up of these patients. This review presents a narrative summary of the current literature on idiopathic CD4 lymphocytopenia.
Keywords: CD4 T cell lymphocytopenia, opportunistic infections, neoplasia, autoimmune disease
Introduction
Idiopathic CD4 lymphocytopenia (ICL) was accepted as a disease entity in 1992. The Centers for Disease Control gave the criteria for ICL as (1) CD4 T-lymphocyte depletion (absolute CD4 T-lymphocyte level <300 cells/mL or <20% of total lymphocytes at a minimum of two separate time points at least 6 weeks apart); (2) no serological evidence of HIV infection; (3) the absence of any defined immunodeficiency or therapy associated with depressed levels of CD4 T-cells.1 Since then, despite many reports, the condition remains poorly understood.
Epidemiology
In 1993, Smith et al reviewed 230,179 cases in the AIDS reporting system and identified 47 individuals with ICL.2 Busch et al were able to identify only 5 donors with low CD4 counts from 2030 blood donors.3 Bofill et al studied 676 subjects and found 7 subjects (1.4%) with low CD4 counts.4 Thus, ICL appears to be a rare condition. We performed a PUBMED search using the MESH term of idiopathic CD4 lymphocytopenia and restricted the results to case reports. A compilation of various clinical and demographic features of ICL is in Table 1.2,5-8 The disease appears to present in the 4th decade with no definite sex preponderance. Opportunistic infections (OI) seem to be the most common presenting feature.
 |
Table 1 Demographic and Clinical Features – A Summary of Case Reports, Case Series and Cohorts of ICL |
Pathogenesis
Normal CD4 T Cell Physiology
The committed lymphoid precursors from the bone marrow are recruited to the thymus by the chemokine ligands CCL21 and CCL25 and develop into T cell progenitors.9 These progenitor cells (CD34+CD38+CD62L+) enter through the corticomedullary junction and its migration to the subcapsular cortex is mediated by CCL25 and C-X-C motif chemokine ligand 12 (CXCL12).11 Engagement of a Notch receptor on the surface of the progenitor cell leads to commitment to T cell lineage.11 Following this, CD1 is expressed, resulting in a pro-T cell that is triple-negative: CD3-CD4-CD8-.11
Further differentiation progresses through various stages (Figure 1). Thymocytes with low avidity interactions with self-peptide major histocompatibility antigen undergo positive selection and differentiate into CD4 or CD8 single-positive (SP) thymocytes.10 CD4 SP thymocytes undergo negative selection in the medulla by their interaction with medullary thymic epithelial cells which express tissue-restricted self-antigens.9 CD40 and CD40 ligand interaction is the master regulator for negative selection.9
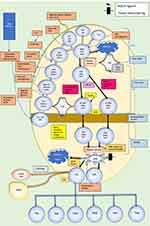 |
Figure 1 CD4+Thymocyte development. Following the migration of lymphoid precursors to the thymus, four stages of double-negative development lead to a double positive thymocyte. Loss of signalling components Lck, SLP-76, and LAT-1 results in a block at this stage of T cell development. Exposure to self-antigens presented by the cortical thymus epithelial cells (cTECs) enables positive selection. Negative selection occurs in the medulla. α, β, ε, γ, δ, and ζ denote chains of the CD3-TCR complex. Those marked with * indicate reported genetic/immunologic defects in idiopathic CD4 lymphocytopenia. Data from Zhang and Davila9 and Weitkamp et al.23Abbreviations: CD, cluster of differentiation; SLP76, SH2-domain-containing leukocyte protein of 76 kDa; ZAP70, zeta-chain-associated protein kinase; Lck, lymphocyte-specific protein-tyrosine kinase; LAT-1, linker for activation of T cells-1; TCR, T cell receptor; NK T, natural killer T cell; Treg, regulatory T cell; DP, dual positive; DN, dual negative; SP, single positive; IL, interleukin; MHC, major histocompatibility complex; V(J)D, variable, diversity and joining regions of T cell receptor; TH, T helper cell; Costim, Costimulatory; ThPOK, T-helper-inducing POZ/Krueppel-like factor; mTEC, medullary thymic epithelial cells; VAV, guanine nucleotide exchange factor; Nur77, orphan nuclear receptor 77; S1P, sphingosine-1 phosphate; JAK3, janus kinase 3; RAG, recombination activating gene; CCL, chemokine ligand; CXCL, C-X-C motif chemokine ligand; CCR, C-C chemokine receptor. |
Sphingosine-1 phosphate mediates egress of these lymphocytes into the circulation.10 Activation (Figure 2) occurs due to the interaction of T Cell Receptor (TCR) of the naïve CD4 cell with MHC class II of antigen-presenting cells, resulting in different subsets– TH1, TH2, TH17, TH22, TH9, and iTreg.9 A supramolecular activation complex or immunological synapse is formed,11 enabling CD3 to bind with zeta associated protein kinase 70 (ZAP70).12 ZAP70 in turn results in activation of downstream pathways such as MAPK (mitogen-activated protein kinase), IL-2 inducible T cell kinase (ITK), and phosphoinositide 3 kinase (PI3-K) which play a role in T cell activation and maintenance.11,12
Interferon γ (IFN-γ), IL-2, and lymphotoxin-α (LTA) are produced by the TH1 cells. IL-2 is necessary for memory of CD4 cells.13 TH1 cells are important for organ-specific immunity and intracellular pathogens like mycobacteria.13 TH2 produces IL-4, IL-5, IL-9, IL-10, IL-13, and IL-25 and has an important role to play in allergic reactions, helminthic infections, and airway hypersensitivity.13 TH17 cells produce IL-17, IL-21, IL-22, and IL-23. They mediate immune responses to extracellular bacteria and fungi and are also implicated in rheumatoid arthritis, psoriasis, type 1 diabetes mellitus, multiple sclerosis, and Sjogren syndrome.9 IL-22 is the most important cytokine produced by TH22. These cells migrate to the skin and may be involved in diseases such as atopic eczema and psoriasis. TH9 cells producing IL-9, have a role in autoimmune and allergic diseases, and infections.9 Following interaction with stromal cells, the CD4+ thymocytes develop into Tregs.9 They constitute 5–10% of peripheral CD4+T cells and express CD4+/CD25+.14 Treg cells have a role in maintaining self-tolerance and regulation of immune responses.13
Termination of T-cell mediated inflammation can be produced by loss of antigen, cell death (apoptosis and necroptosis), inhibitory molecules (CTLA 4), and metabolic factors.15,16 Some of the T cells survive to continue as memory T cells.15,16
Immunological Defects Seen in ICL
The immunological defects reported in ICL are summarised below.
Reduced Naïve T Cells
Recent thymic emigrants (RTE) influence the rate of infections and deaths in patients with renal transplants.17 The immunophenotype of the naïve RTE is CD45RA+CD45RO− CCR7+CD62L+. In a study by Bugault et al, CD45RA+ CD62L+CD4+ T cell population in patients with ICL was reduced, while CD45RA−CD62L− effector memory cells were increased.18 In another study of 20 patients with ICL, the expansion of terminally differentiated effector memory cells and contraction of the naïve T cells was observed.19 CD45RA+CD31+ are thymus-derived naive T cells, while CD45RA+CD31− are peripherally expanded naive T cells. Levels of the latter are reduced in patients with ICL.19 In a study by Kovacs et al, patients with ICL had a lower percentage of naïve cells in the rectosigmoid mucosa compared to effector and central memory cells.20 A higher proportion of Tregs (CD25+FoxP3+CD4+) is also seen in ICL.18 Enhanced CD45RO expression with reduced CD45RA expression (lower naïve T cell) and increase in γδ-TCR cells was reported in a 9-year old boy with ICL and was suggestive of a maturation defect.21
Chronic TCR Stimulation
Lee et al observed that CD4 cells from patients with HIV and ICL showed greater levels of activation and levels of lipopolysaccharide, a marker for microbial translocation, was also similar between the two groups.22 The dual-specificity phosphatases (DUSP) family downregulate TCR signalling. Overexpression of DUSP4 reflects T cell activation strength and the cumulative number of cell cycles. Their overexpression in CD4 cells of patients with ICL suggests chronic TCR stimulation. In vitro TCR signalling improved with silencing of DUSP4.19
Reduced TCR Repertoire
Multipotent double-negative T cells differentiate into αβ and γδ cell populations based upon the surface TCR expression and DNA rearrangements.9 γδTCR is expressed by 2–5% of T lymphocytes in the thymus and peripheral blood.23 The analysis of αβ and γδ type TCRs in three patients of ICL showed that their repertoire was severely affected, though it was not related to the severity of infections.24 In a study by Sheikh et al, γδ CD4 T cells were found in higher proportions in ICL.25
Proposed Reasons for Reduced CD4 Counts in ICL
Several hypotheses proposed to explain the selective depletion of CD4 cells, are discussed in the following sections.
Increased Apoptosis in ICL
Fas (CD95), a member of TNF family trimerizes with the Fas ligand leading to thymocyte apoptosis during negative selection and during peripheral deletion of activated T cells.26,27 Low levels of Fas are seen in quiescent peripheral mononuclear blood cells.28 In a study of eight patients with ICL and clinical immune deficiency, seven had accelerated apoptosis of CD4 cells.28 Increased activated T cell markers–CD25, CD69, and Fas/CD95+ was seen in a patient with ICL compared with normal controls, resulting in more spontaneous and Fas-induced apoptosis.29
Genetic Defects in ICL
Uncoordinated 119 (Unc119) is a transport adaptor protein essential for Lck activation.12 Lck reduction and Unc119 mutations lead to severe combined immune deficiency phenotypes.30 In a study of three subjects with ICL and impaired Lck activity, one patient had a heterozygous missense mutation in the Unc119 gene that resulted in an inability of Unc119 to interact with Lck.12 Homozygosity mapping revealed a mutation in the first exon of the ITK gene in another patient with ICL.31 Recombination activation gene (RAG) encodes proteins that regulate recombination in the β chain of the TCR.9 Two novel heterozygous missense mutations in the RAG1 gene were found in a young girl with ICL.32 STK4 is necessary for T cell migration and has both proapoptotic and antiapoptotic functions.33 An STK4 mutation was seen in a patient with ICL who presented with EBV-related MALT lymphoma.34 MAGT1, a magnesium transporter gene mediates magnesium flux following TCR activation. Mutations lead to impaired downstream signalling events.29 Characteristically, they present with CD4 lymphopenia, EBV infection and neoplasia. This condition is called XMEN syndrome and closely mimics ICL.35
Cytokine Dysregulation
IL-7 is a cytokine produced by non-hematopoietic cells that binds to its receptor CD127 on CD4 cells.36 This leads to phosphorylation of janus kinases (JAK) that activate the signal transducer and activator of transcription 5 (STAT5) pathway.36 It also activates the src kinases pathway that regulate TCR signaling, and the PI3-K pathway that regulates protein kinase B (Akt) involved in cell cycle progression. IL-7 is thus critical for survival (JAK3/STAT5/Bcl2), cell cycle progression (p38 MAP kinase/CD25),37 and reduced apoptosis of T cells.36,38
In ICL, CD127 receptor expression was lower and was associated with reduced responsiveness to IL-7.38 IL-7 release is increased following depletion of CD4 cells and levels correlate with T cell consumption.37 However, this is insufficient to restore CD4 counts.18 Reduced upregulation of IL-7 induced genes, lower STAT-5 phosphorylation in response to IL-7, and reduced signalling in response to IL-2 were seen in patients with ICL.38 Both IL-2 and IL-7 control CD4 pool size.18 IL-7 and IL-2 responses were reduced and STAT5 activation responses impaired in a study of 15 patients with ICL from France.18 IL-2 and IL-7 induce CXCR418 that enables migration of the cell along the gradient of the CXCL12.39 CXCR expression on the T cells was reduced and low levels of CXCR correlated with lymphopenia in patients with ICL. CXCR4 expression increased with IL-2 therapy.7,18
Putative Viral Aetiology of ICL
A virus has been suggested to be the cause of ICL. In a study of seronegative haemophiliacs, five patients were noted to have persistent lymphocytopenia fulfilling criteria for ICL. This was attributed to cirrhosis due to chronic hepatitis C infection in these patients.40 When peripheral blood mononuclear cells from a patient with ICL were co-cultured with HUT78 T-lymphoblastoid cells, an acute cytopathic effect was seen. Those surviving the cytopathic effect showed an intracisternal retroviral particle that reacted with antibodies of sera from ICL patients.41 However, to date, no definite virus has been isolated from patients with ICL.
Sequestration of CD4 Cells in ICL
In rectosigmoid endoscopies of 12 patients with ICL, reduced CD4 lymphocytes with normal functional indicators of enterocyte turnover (intestinal fatty acid-binding protein and inflammatory biomarkers) were observed.20 In this study, patients with ICL had a higher percentage of DN T cells when compared to controls, but TH1 and TH17 cell subsets were normal. This suggested tissue depletion of CD4+ and not entrapment of CD4+ cells in the mucosa.20 This contrasted with the observation by Griffiths et al.42 In three cases of erythroderma, one each due to cutaneous T cell lymphoma, atopic dermatitis, and psoriasis, they found CD4+ counts were markedly increased in the skin with high CD4:CD8 ratios and simultaneous peripheral blood CD4 lymphocytopenia.42 In these patients, resolution of the erythroderma resulted in normalization of CD4 counts. They proposed erythroderma be considered an exclusion criterion for ICL.42
Immune Senescence
In normal individuals, 80% of CD4+ cells express CD28.43 Immune senescence is associated with CD28 loss due to chronic stimulation. Defective TCR responses and telomere shortening were observed in a T cell subset of ICL patients.19 CXCR expression is reduced following TCR stimulation which was observed in patients with ICL.19 CD27− and CD28− costimulatory molecules, and CD57+ and KLRG-1+ are markers of T cell senescence. These were also higher in patients with ICL when compared to healthy subjects.19
HLA-DR and Ki-67 suggestive of activation and cell cycle turnover, respectively, were both increased in CD4 cells of patients with ICL.5 Proportion of Tregs cells was higher and the naïve T cells were lower in these patients.5 Increased activation of CD4 lymphocytes may result in depletion.22 Some patients also have also been reported with CD19 B-cell deficiency, CD8 T-cell deficiency, or CD3−CD16+CD56+ NK cell deficiency.7 The percentage of transitional B cells (CD10+/CD27−) was higher and had a strong inverse relationship with CD4+ counts in a cohort of 25 patients with ICL. This suggested that either CD4 and/or IL-7 had an impact on B cell maturation as well.44
Other Causes
Isgro et al evaluated the bone marrow of five patients with ICL and found a reduction in lymphoid precursor cells, suggesting reduced clonogenic potential of the bone marrow.45 In a patient with cryptococcal meningitis and ICL, Salit et al demonstrated anti CD4+ T cell antibodies in greater concentration than controls.46
In summary, diminished precursors (reduced clonogenic potential), accelerated apoptosis (Fas/Fas ligand), reduced chemotaxis (reduced CXCR expression), poor response to TCR stimulation, impairment of activation (low expression of Lck, MAGT1 defect), defective cytokine production (TNF-α and IFN-Ƴ), elevated IL-17, dysregulation of IL-7 and its downstream targets, others mutations involving RAG1, UNC119, ITK, STK4, and CD45, cytotoxic antibodies to T cells, and sequestration all can contribute to reduced CD4 T cell counts.12,27,30,45-48
Clinical Features
Clinical manifestations arise due to immune deficiency, disparate infections, neoplastic and autoimmune conditions. The presentation is diverse – the patient may be asymptomatic or may have florid infection(s) that ends fatally. The presentations can differ despite similar CD4 counts. Long disease-free intervals have been reported with low CD4 counts.49
The most common organ-systems involved are the skin, central nervous system, and lungs. Less commonly the musculoskeletal system, lymphoreticular system, gastrointestinal tract, hematopoietic system, eyes, ears, and the genitalia and reproductive system involvement have been described.
Infections
Patients develop both opportunistic and non-opportunistic infections. Table 2 provides a summary of the reported infectious agents. Most patients with ICL have had at least one OI. OI in ICL are related to mycobacteria (M. avium-Intercellulare (MAC), M. tuberculosis, M. genavense M. mucogencium, M. kansasii), viruses (Human Herpes Viruses, John-Cunningham virus (JCV), Human Papilloma Virus (HPV)), fungi (Cryptococci, Candida, Histoplasma, Blastomyces, Pneumocystis) and protozoa (Leishmania and Toxoplasma).27 In a follow-up study of 39 patients by Zonios et al, the commonest infections were due to cryptococci, HPV, and nontuberculous mycobacteria.5 Varicella zoster virus (VZV), HPV and cytomegalovirus (CMV) were the most common viral infections reported from another study.6 Leishmaniasis is the most common parasitic infection in ICL.49 Single or multiple infections may coexist in a single patient as it did in a patient with vertebral osteomyelitis (Acinetobacter species, M. abscessus, and Mucormycosis).50 Multiorgan involvement has been reported in infections due to Cryptococcus, CMV, VZV, Blastomyces, and MAC.49,51
 |
Table 2 Infections Reported in ICL |
In our review of 164 cases, we found 89 (52%) had skin manifestations. Most were due to infections. The symptoms varied and included pruritus, erythroderma,42 vesicles, warts, verrucous papules and plaques,79 condylomata,80 and mycetoma (Exophiala jeanselmei and M. chelonae).52 Infection of the skin included viral, bacterial, fungal and parasitic infections like herpes zoster, HPV,80 molluscum contagiosum,59 Stenotrophomonas maltophilia,53 MAC,51 Candida albicans, tinea corporis,60 tinea pedis,68 cryptococci,8 Aureobasidium species, Alternaria species, and Hortaea werneckii, leishmaniasis.49 Premalignant lesions (vulval intraepithelial neoplasia, Bowen disease) and malignancies (e.g., Kaposi sarcoma,61 squamous cells carcinoma and basal cell carcinoma) have been reported.60,61,81 Signs of autoimmune disease (e.g. dermatomyositis,82 vitiligo,83 and psoriasis84) have been described. Often these patients have had a previous history of skin diseases such as atopic dermatitis, psoriasis,42 herpes zoster and recurrent staphylococcal furunculosis.85 Skin abscesses have also been reported.61,69
Neurological manifestations involve the meninges, the cerebrum, brainstem, cerebellum, cranial and peripheral nerves. The commonest reported neurological disorder was cryptococcosis, followed by progressive multifocal leukoencephalopathy (PMLE). Zonios et al, studied 53 patients with ICL and cryptococcosis and found many with other underlying diseases like cirrhosis, diabetes, tuberculosis, and sarcoidosis.70 They presented with headache and fever. Other symptoms were diplopia, confusion, imbalance, dizziness, photophobia, limb weakness, seizures and slurred speech.70,86 Disseminated cryptococcosis involving lung, bone, bone marrow, lymph node, muscle, and blood has been reported.70 Eighteen patients of PMLE with ICL were identified in 2017 and most presented with cognitive decline and progressive focal deficits.62 Other causes of neurological infections were bacterial (Nocardia abscessus, N. farcinia and N. asteroids),71 viral (JCV)8 mycobacterial (MAC), fungal (cryptococci),8 and parasitic (toxoplasmosis)6 organisms. Guillain-Barre syndrome and sensorimotor polyneuropathy have also been reported in ICL.87
Respiratory symptoms included ear pain, productive cough, and breathlessness. Manifestations reported include nasal polypoid tumors,88 otitis media,53 laryngeal papillomas,63 reticulonodular infiltrates,89 pneumonia, bronchiectasis,53 nodules, masses,90 cysts, mediastinal lymphadenopathy,75 pleural effusion,91 and empyema.92 Organisms comprised of mycobacteria (M. tuberculosis, MAC), bacteria (Nocardia,71 H. infleunzae), viruses (VZV, CMV, HPV, and EBV) and fungi (Pneumocystis jirovecii,75 cryptococcosis, mucormycosis, aspergillosis, istoplasmosis72). According to Ahmad et al, Pneumocystis jirovecci was the most common respiratory pathogen.6
VZV and CMV-related retinitis have been reported.6,64,65 “Floaters” and diminution of vision were the clinical symptoms. Encephalitozoon cuniculi causing intraocular infection was mentioned in another report of ICL.76 Anemia due to pure red cell aplasia (human parvovirus19) and myelodysplastic syndrome has been described in ICL.93,94 Gastrointestinal manifestations include symptoms arising due to candida or CMV-related esophagitis, gastric cancer,61 enteritis (Salmonella and Shigella), colitis and hepatic abscess.6 Isolated renal mucormycosis was seen in a 17-year-old girl with ICL that required both surgery and medical therapy.73
Toxoplasma-related myositis has been reported from France.77 Septic arthritis due to M. genavense has been described.56 Vertebral osteomyelitis with triple infections has been previously mentioned.50 Rib osteomyelitis and vertebral spondylitis due to crytptococci have been described in a patient with ICL.74
Autoimmune Manifestations
Zonios et al found autoimmune diseases in 9 (23.1%) patients of ICL.5 The various disorders include systemic lupus erythematosus, Anti-phospholipid antibody syndrome, psoriasis, vitiligo, Graves’ disease, autoimmune thyroiditis, ulcerative colitis, immune thrombocytopenia, autoimmune hemolytic anemia.5,7 Sjogren’s disease, sarcoidosis, and psoriasis were the most common immune-mediated conditions according to Ahmad et al.6 Raynaud disease, thrombotic thrombocytopenic purpura, alopecia areata, and Waldenström hypergammaglobulinemia have also been reported.6 In a study of 80 patients with primary Sjogren’s syndrome, CD4+ counts were lower in patients who were seropositive for anti-SSA antibodies.95 Dermatomyositis has been described in conjunction with ICL.82 ICL has followed systemic vasculitis in a 65-year-old woman.96 A 57-year-old man with ICL and giant cell arteritis has been reported.97
Neoplasia
Malignant disorders include those involving the skin, lymphoreticular system, lung, stomach,61 liver,66 central nervous system, orbit and nasal cavity,88 testes, prostate and bladder, vulva, and the cervix.6 Lymphadenopathy and splenomegaly are seen in lymphoreticular malignancies.64 A case of invasive vulvar carcinoma was reported from The Netherlands.98 Angiocentric nasal T-cell lymphoma, Burkitt’s lymphoma, EBV-related diffuse large B-cell lymphoma, orbital diffuse large-cell B lymphoma, primary effusion lymphoma, central nervous system lymphoma, and cutaneous T-cell non-Hodgkin’s lymphoma have all been described.49,85,88,66 Anaplastic astrocytoma, testicular, prostate, and bladder cancer, acute and chronic lymphoblastic leukemia, and non-small cell lung carcinoma are the other malignancies reported.6 Leiomyoma has been described in a Turkish adolescent.31 Another young male from Japan developed monoclonal gammopathy of unknown significance and MALT lymphoma of the stomach.99
Differential Diagnosis
Table 3 summarises the list of differentials to be considered. Patients with OI with HIV-negative status must be evaluated for ICL. It should also be considered in the setting of autoimmune diseases and some malignancies particularly of the skin and lymphoreticular system. An extensive infective, hematologic, and autoimmune workup is needed to prove the diagnosis of ICL.54 The most important differential is HIV infection. However, in HIV there is a progressive decline in CD4+ counts with higher CD8+ counts and immunoglobulin levels than in ICL. Certain infections like tuberculosis and cryptococcosis may cause reduced CD4+ counts that recover with therapy. Betancourt et al have reported low CD4 counts like ICL in 6 subjects with chronic CMV or EBV infections. The CD4 counts of all these subjects improved with anti-viral therapy.100 Autoimmune diseases to be considered are Sjogren’s syndrome and sarcoidosis. Increased incidence of opportunistic infections such as cryptococcosis has been reported in sarcoidosis also.54 MonoMAC syndrome is an inherited bone marrow failure syndrome due to GATA2 mutation. It is characterized by monocytopenia and MAC infections that can mimic ICL.101,102 Drugs such as corticosteroids and cyclophosphamide cause a higher lymphocyte depletion than methotrexate and azathioprine.55 Physiological factors such as pregnancy and circadian rhythms can reduce CD4 counts. Finally, aging has also been implicated by a study from Ireland where 9 (4.3%) of 209 very elderly individuals satisfied criteria for ICL.103
 |
Table 3 Differential Diagnosis of CD4 Lymphocytopenia |
Therapy
The treatment of ICL revolves around the treatment of presenting illness, appropriate prophylaxis and screening, and the treatment of ICL itself.
Therapy of Presenting Illness
ICL presents most often with OI followed by autoimmune conditions and neoplasias. Most such infections have been successfully managed using standard therapeutic regimens. When the infection is refractory to chemotherapy addition of agents such as IL2 have been tried with success.57 The use of maintenance therapy is more controversial. Zonios et al in their series of patients with cryptococcosis and ICL reported a relapse rate of only 12%.70 Also, considering ICL is non-progressive and may in some cases improve spontaneously, maintenance therapy may not be required.6 However, as long as the CD4 counts are depressed, there is a risk of relapse and hence it may be prudent to give maintenance therapy till CD4+ counts normalizes.104 In malignancy, again standard therapy has usually been given.67 In cutaneous malignancy, especially HPV-associated, recurrence and dissemination have been commonly reported.8,81 Autoimmune conditions are also treated as per standard protocols using steroids and other immunomodulators. This added immunosuppression may precipitate opportunistic infections in patients with ICL and so close follow up is necessary.97
Prophylaxis and Screening
Subjects with ICL are at risk for OI, and it appears prudent to provide prophylaxis. The suggested regimes for prophylaxis of OI are given in Table 4. The recommended prophylaxis and vaccinations according to the CD4 counts as per standard guidelines for prophylaxis in HIV/AIDS may be applied in subjects with ICL also.105 In addition, there is some evidence to suggest that lower CD8+ counts may be associated with increased risk of infections.5 In such individuals, prophylaxis may be started at higher CD4 counts. Regular screening for HPV-associated malignancy (both mucosal and cutaneous) must be considered. Kirtava et al reported that six of 115 (5.2%) patients with primary Sjogren’s syndrome had ICL and one developed lymphoma on follow up.106 Other authors have also reported lymphomas on follow up. Hence, prolonged follow up with regular blood counts and clinical evaluation should be offered to all these patients.
 |
Table 4 Prophylaxis for Opportunistic Infections |
Therapy of ICL
As the underlying pathophysiology is poorly understood and the condition is rare, therapy of ICL is mostly experimental.
Interleukin 7 (IL-7)
IL-7 levels are known to be inversely correlated with CD4 counts in patients with HIV/AIDS or chemotherapy, and is likely a compensatory response.38 Some authors like Harel et al have reported successful therapy of ICL with IL-7.107 Subsequently, Sheikh et al reported a Phase I/2A trial of recombinant human IL-7 in subjects with ICL at doses of 3µg/kg (3 subjects), 10µg/kg (5 subjects) and 20µg/kg (1 subject) subcutaneous weekly for 3 weeks. They concluded that therapy with IL-7 results in increase in the number of circulating CD4 and CD8 T cells and tissue-resident CD3 T cells in the gut mucosa and bone marrow.25
Interleukin 2 (IL-2)
IL-2 is a potent mitogen and growth factor in antigen-stimulated CD4 cells.108 IL-2 has been studied in HIV and has been shown to increase CD4 counts.109 Trojan et al reported a patient and reviewed four others with ICL who received IL-2 therapy. Four of them had failed chemotherapy for underlying infections, which they successfully cleared with IL-2 therapy. These subjects also showed increase in CD4 counts.57 In a Korean lady with ICL and monoclonal gammopathy, IL-2 therapy helped achieve remission and improved renal functions.110 Regent et al reported IL-2 therapy in six of the 40 subjects with ICL. Four showed increase in CD4 counts.7 More recently, Yarmohammadi et al reported on four patients of 24 subjects with ICL who received IL-2 (3 for recalcitrant warts and 1 for tuberculous lung infection). All four had a meaningful clinical response to IL-2 therapy.8 IL-2 appears to be well tolerated in this group.8
Interferon Gamma (INFγ)
INF-γ is an immunomodulator whose action is mediated by hundreds of cell-specific INF-γ-controlled genes.111 Overall it promotes and maintains TH1 type of cell response.111 Netea et al reported two patients with cryptococcal meningitis and ICL who were successfully treated with INF-γ.112 Sternfeld et al similarly reported two subjects with MAC infection treated with a combination of IL-2 and INF-γ.58 It has been suggested that this therapy may be useful in ICL presenting with an OI whose clearance is mediated by TH1 response.78
Stem Cell Transplant
Bone marrow transplantation for indications secondary to the associated conditions of ICL may result in amelioration of ICL post-transplant. Petersen et al first described allogenic bone marrow transplant in a 20-year-old man with ICL, recurrent OI and aplastic anaemia. Post-transplant, they reported a normalization of CD4 counts with ability to mount a normal antibody response to diphtheria, tetanus and polio vaccines.113 Lum et al performed stem cell transplant following rituximab therapy in a patient with ICL and EBV related MALT lymphoma. Following the transplant, the subject achieved remission of lymphoma and cure of ICL.34 Subsequently, Cervera et al reported a 40-year-old man with ICL presenting with recurrent OI. They performed a non-myeloablative hematopoietic stem cell transplant from HLA identical sibling. At 35 months follow up, he had increased CD4 counts and no infections. A non-myeloablative regimen with fludarabine and single dose of whole-body irradiation was used to obtain a stable mixed or full chimerism and low toxicity.114 Hamidieh et al reported successful transplant using a reduced intensity conditioning regimen in a paediatric patient with ICL.115 Thus, hematopoietic stem cell transplant with low-intensity conditioning regimens may be considered for therapy of ICL per se also.
Other Agents
Though immunoglobulin levels are typically normal in ICL, there is an overlap between combined variable immunodeficiency and ICL.116 Anecdotal use of Intravenous immunoglobulin8 suggests that such patients in the overlap zone may benefit from it.
Overall, therapy is directed at the presenting condition. Prophylaxis is considered based on the CD4 counts and possibly CD8 counts. Screening for malignancy should be offered. Live vaccines should be avoided. Specific therapy for ICL is yet to be established, though IL-7 looks promising.
Knowledge Gaps
ICL continues to remain an elusive condition. Immune dysregulation with increased apoptosis, senescence, sequestration and cytokine imbalance have been proposed as immediate causes for the reduction in CD4 counts.54 The contribution of each of these factors and their interaction with each other is not known. This immune dysregulation may be brought about by genetic factors. Though isolated genetic defects have been reported,31 to date no genome-wide association studies have been carried out. While novel manifestations of ICL continue to be reported, some patients with ICL remain asymptomatic. We do not understand what determines how a patient presents. The natural history of ICL is also poorly understood with some subjects recovering over time. Research into ICL has been hampered by the relative rarity of the condition and difficulty in diagnosis. Though some research interest groups in France7 and US5 have cohorts of these subjects, a worldwide registry of such patients may boost research into this condition.
Conclusion
Idiopathic CD4 lymphocytopenia is characterised by low CD4+ T cell counts and is likely multifactorial in aetiology. It commonly presents as opportunistic infections, autoimmune diseases and/or neoplasias. A high index of suspicion is necessary for diagnosis. Currently, management revolves around treatment of the presenting symptoms and close follow up. More research into this obscure disease will provide us with better insights into its pathology and open new avenues for therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Centers for Disease Control. Unexplained CD41 T-lymphocyte depletion in persons without evident HIV infection. Morb Mortal Wkly Rep. 1992;41.
2. Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ lymphocytopenia without HIV infection–an investigation of cases in the United States. N Engl J Med. 1993;328(6):373–379. doi:10.1056/NEJM199302113280601
3. Busch MP, Valinsky JE, Pagileroni T, et al. Screening of blood donors for idiopathic CD4 lymphocytopenia. Transfusion. 1994;34:192–197. doi:10.1046/j.1537-2995.1994.34394196614.x
4. Bofill M, Janossy G, Lee CA, et al. Laboratory control values for CD4 and CD* lymphocytes- Implications of HIV-1 diagnosis. Clin Exp Immunol. 1992;88. doi:10.1111/j.1365-2249.1992.tb03081.x
5. Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287–294. doi:10.1182/blood-2007-12-127878
6. Ahmad DS, Esmadi M, Steinmann WC. Idiopathic CD4 Lymphocytopenia: spectrum of opportunistic infections, malignancies, and autoimmune diseases. Avicenna J Med. 2013;3:37–47. doi:10.4103/2231-0770.114121
7. Régent A, Autran B, Carcelain G, et al. Idiopathic CD4 lymphocytopenia: clinical and immunologic characteristics and follow-up of 40 patients. Medicine (Baltimore). 2014;93:61–72. doi:10.1097/MD.0000000000000017
8. Yarmohammadi H, Cunningham-Rundles C. Idiopathic CD4 lymphocytopenia: pathogenesis, etiologies, clinical presentations and treatment strategies. Ann Allergy Asthma Immunol. 2017;119:374–378. doi:10.1016/j.anai.2017.07.021
9. Zhang Y, Davila ML. T Lymphocytes. In: Greer JP, Arber DA, Glader B, editors. Wintrobe’s Clinical Hematology. Philadelphia: Lipincott Williams & Wilkins; 2018:453–481.
10. Park C. Lymphocytes and lymphatic organs. In: Greer JP, Arber DA, Glader B, editors. Wintrobe’s Clinical Hematology. Philadelphia: Lipincott Williams & Wilkins; 2018:226–259.
11. Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM. T cell responses: naive to memory and everything in between. Adv Physiol Educ. 2013;37:273–283. doi:10.1152/advan.00066.2013
12. Gorska MM, Alam R. A mutation in the human uncoordinated 119 gene impairs TCR signaling and is associated with CD4 lymphopenia. Blood. 2012;119:1399–1406. doi:10.1182/blood-2011-04-350686
13. Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi:10.1182/blood-2008-05-078154
14. Zhu J. T helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol. 2018;10(10):a030338. doi:10.1101/cshperspect.a030338
15. Marrack P, Scott-Browne J, MacLeod MK. Terminating the immune response. Immunol Rev. 2010;236:5–10. doi:10.1111/j.1600-065X.2010.00928.x
16. Zhan Y, Carrington EM, Zhang Y, Heinzel S, Lew AM. Life and death of activated T Cells: how are they different from naïve T cells? Front Immunol. 2017;8. doi:10.3389/fimmu.2017.01809
17. Ducloux D, Courivaud C, Bamoulid J, et al. Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J Am Soc Nephrol. 2010;21:868–875. doi:10.1681/ASN.2009090976
18. Bugault F, Benati D, Mouthon L, et al. Altered responses to homeostatic cytokines in patients with idiopathic CD4 lymphocytopenia. PLoS One. 2013;8:e55570. doi:10.1371/journal.pone.0055570
19. Bignon A, Regent A, Klipfel L, et al. DUSP4-mediated accelerated T-cell senescence in idiopathic CD4 lymphopenia. Blood. 2015;125:2507–2518. doi:10.1182/blood-2014-08-598565
20. Kovacs SB, Sheikh V, Thompson WL, et al. T-Cell depletion in the colonic mucosa of patients with idiopathic CD4 + lymphopenia. J Infect Dis. 2015;212(10):1579–1587. doi:10.1093/infdis/jiv282
21. Fruhwirth M, Clodi K, Heitger A, Neu N. Lymphocyte diversity in a 9-year-old boy with idiopathic CD4+ T cell lymphocytopenia. Int Arch Allergy Immunol. 2001;125:80–85. doi:10.1159/000053800
22. Lee PI, Ciccone EJ, Read SW, et al. Evidence for translocation of microbial products in patients with idiopathic CD4 lymphocytopenia. J Infect Dis. 2009;199:1664–1670. doi:10.1086/598953
23. Weitkamp JH, Lewis DB, Levy O. Immunology of the fetus and newborn. In: Gleason CA, Juul SE, editors. Avery’s Diseases of the Newborn.
24. Signorini S, Pirovano S, Fiorentini S, et al. Restriction of T-cell receptor repertoires in idiopathic CD4+ lymphocytopenia. Br J Haematol. 2000;110:434–437. doi:10.1046/j.1365-2141.2000.02166.x
25. Sheikh V, Porter BO, DerSimonian R, et al. Administration of interleukin-7 increases CD4 T cells in idiopathic CD4 lymphocytopenia. Blood. 2016;127:977–988. doi:10.1182/blood-2015-05-645077
26. Volpe E, Sambucci M, Battistini I, Borsellino G. Fas-fas ligand: checkpoint of t cell functions in multiple sclerosis. Front Immunol. 2016;7. doi:10.3389/fimmu.2016.00382
27. Luo L, Li T. Idiopathic CD4 lymphocytopenia and opportunistic infection–an update. FEMS Immunol Med Microbiol. 2008;54:283–289. doi:10.1111/j.1574-695X.2008.00490.x
28. Laurence J, Mitra D, Steiner M, Lynch DH, Siegal FP, Staiano-Coico L. Apoptotic depletion of CD4+ T cells in idiopathic CD4+ T lymphocytopenia. J Clin Invest. 1996;97:672–680. doi:10.1172/JCI118464
29. Roger PM, Bernard-Pomier G, Counillon E, Breittmayer JP, Bernard A, Dellamonica P. Overexpression of fas/CD95 and fas-induced apoptosis in a patient with idiopathic CD4+ T lymphocytopenia. Clin Infect Dis. 1999;28:1012–1016. doi:10.1086/514739
30. Freeman AF, Sereti I. Idiopathic CD4 lymphopenia. In: Sullivan KE, Stiehm ER, editors. Stiehm's Immune Deficiencies. Vol. 2. Amsterdam: Academic Press; 2014:243–250.
31. Serwas NK, Cagdas D, Ban SA, et al. Identification of ITK deficiency as a novel genetic cause of idiopathic CD4+ T-cell lymphopenia. Blood. 2014;124:655–657. doi:10.1182/blood-2014-03-564930
32. Kuijpers TW, Ijspeert H, van Leeuwen EM, et al. Idiopathic CD4+ T lymphopenia without autoimmunity or granulomatous disease in the slipstream of RAG mutations. Blood. 2011;117:5892–5896. doi:10.1182/blood-2011-01-329052
33. Abdollahpour H, Appaswamy G, Kotlarz D, et al. The phenotype of human STK4 deficiency. Blood. 2012;119(15):3450–3457. doi:10.1182/blood-2011-09-378158
34. Lum SH, Bonney D, Cheesman E, Wright NB, Hughes S, Wynn R. Successful curative therapy with rituximab and allogeneic haematopoietic stem cell transplantation for MALT lymphoma associated with STK4 -mutated CD4+ lymphocytopenia. Pediatr Blood Cancer. 2016;63:1657–1659. doi:10.1002/pbc.26048
35. Li F-Y, Chaigne-Delalande B, Kanellopoulou C, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475(7357):471–476. doi:10.1038/nature10246
36. Lundström W, Fewkes NM, Mackall CL. IL-7 in human health and disease. Semin Immunol. 2012;24:218–224. doi:10.1016/j.smim.2012.02.005
37. Ponchel F, Cuthbert RJ, Goeb V. IL-7 and lymphopenia. Clin Chim Acta. 2011;412:7–16. doi:10.1016/j.cca.2010.09.002
38. Puronen CE, Thompson WL, Imamichi H, et al. Decreased interleukin 7 responsiveness of T lymphocytes in patients with idiopathic CD4 lymphopenia. J Infect Dis. 2012;205:1382–1390. doi:10.1093/infdis/jis219
39. Contento RL, Molon B, Boularan C, et al. CXCR4-CCR5: a couple modulating T cell functions. Proc Natl Acad Sci. 2008;105(29):10101–10106. doi:10.1073/pnas.0804286105
40. O’Brien TR, Diamondstone L, Fried MW, et al. Idiopathic CD4+ T lymphocytopenia in HIV seronegative men with hemophilia and sex partners of HIV seropositive men. Am J Hematol. 1995;49.
41. Garry RF, Fermin CD, Kohler PF, Markert ML, Luo H. Antibodies against retroviral proteins and nuclear antigens in a subset of idiopathic CD4+ T lymphocytopenia patients. AIDS Res Hum Retroviruses. 1996;12:931–940. doi:10.1089/aid.1996.12.931
42. Griffiths TW, Stevens SR, Cooper KD. Acute erythroderma as an exclusion criterion for idiopathic CD4+ T lymphocytopenia. Arch Dermatol. 1994;130. doi:10.1001/archderm.1994.01690120066009
43. Esenten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44.
44. Malaspina A, Moir S, Chaitt DG, et al. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood. 2007;109:2086–2088. doi:10.1182/blood-2006-06-031385
45. Isgro A, Sirianni MC, Gramiccioni C, Mezzaroma I, Fantauzzi A, Aiuti F. Idiopathic CD4+ lymphocytopenia may be due to decreased bone marrow clonogenic capability. Int Arch Allergy Immunol. 2005;136:379–384. doi:10.1159/000084258
46. Salit RB, Hankey KG, Yi R, Rapoport AP, Mann DL. Detection of CD4(+) T-cell antibodies in a patient with idiopathic CD4 T lymphocytopenia and cryptococcal meningitis. Br J Haematol. 2007;139:133–137. doi:10.1111/j.1365-2141.2007.06781.x
47. Le Deist F, Moshous D, Howe SJ, et al. Combine B and T cell immunodeficiencies. In: Rezaei N, Ahamohammadi A, Notarangelo LD, editors. Primary Immune Deficiencies.
48. Piccin A, Eisendle K, Rovigatti U, et al. Transition of idiopathic CD4 + lymphocytopenia into mycosis fungoides? Leuk Lymphoma. 2014;55:1649–1651. doi:10.3109/10428194.2013.840779
49. Gholamin M, Bazi A, Abbaszadegan MR. Idiopathic lymphocytopenia. Curr Opin Hematol. 2015;22:46–52. doi:10.1097/MOH.0000000000000102
50. Gupta N, Banerjee S, Sharma R, et al. Osteomyelitis due to multiple rare infections in a patient with idiopathic CD4 lymphocytopenia. Intractable Rare Dis Res. 2017;6(3):206–210. doi:10.5582/irdr.2017.01029
51. Schimizu S, Takashima Y, Maya Y, et al. Disseminated Mycobacterium intercellulare infection that led to recognition of idiopathic CD4 lymphocytopenia. J Dtsch Dermatol Ges. 2019;17:1066–1068. doi:10.1111/ddg.13946
52. Zollner TM, Stracke S, Neumeister B, et al. Idiopathic CD4+ T lymphocytopenia presenting as mycetoma in a patient with a mutation in the cystic fibrosis transmembrane regulator gene. Arch Dermatol. 1996;132:1247–1249. doi:10.1001/archderm.1996.03890340113023
53. Tanaka S, Teraguchi M, Hasui M, Taniuchi S, Ikemoto Y, Kobayashi Y. Idiopathic CD4+ T-lymphocytopenia in a boy with down syndrome. Report of a patient and a review of the literature. Eur J Pediatr. 2004;163:122–123. doi:10.1007/s00431-003-1375-8
54. Zonios D, Sheikh V, Sereti I. Idiopathic CD4 lymphocytopenia: a case of missing, wandering or ineffective T cells. Arthritis Res Ther. 2012;14:222. doi:10.1186/ar4027
55. Walker U, Warnatz K. Idiopathic CD4 lymphocytopenia. Curr Opin Rheumatol. 2006;18:389–395.
56. Mahmood M, Ajmal S, Abu Saleh OM, Bryson A, Marcelin JR, Wilson JW. Mycobacterium genavense infections in non-HIV immunocompromised hosts: a systematic review. Infect Dis. 2018;50:329–339. doi:10.1080/23744235.2017.1404630
57. Trojan T, Collins R, Khan DA. Safety and efficacy of treatment using interleukin 2 in a patient with idiopathic CD4+ lymphocytopenia and Mycobacterium Avium-Intercellulare. Clin Exp Immunol. 2009;156:440–445.
58. Sternfeld T, Niggs A, Belohradsky BH, Bogner JR. Treatment of relapsing Mycobacterium avium infection with interferon-gamma and interleukin 2 in an HIV negative patient with low CD4 syndrome. Int J Infect Dis. 2010;14:e198–e201. doi:10.1016/j.ijid.2009.08.004
59. Hayashi T, Hinoda Y, Takahashi T, et al. Idiopathic CD4+ T-lymphocytopenia with bowen’s disease. Intern Med. 1997;36:822–824. doi:10.2169/internalmedicine.36.822
60. Ohashi DK, Crane JS, Spira TJ, Courrege ML. Idiopathic CD4 lymphocytopenia with verrucae, basal cell carcinomas and chronic tinea corporis infections. J Am Acad Dermatol. 1994;31.
61. Mazzucchelli I, Vezzoli M, Ottini E, Paulli M, Boveri E, Mazzone A. A complex immunodeficiency. Idiopathic CD4+ T-lymphocytopenia and hypogammaglobulinemia associated with HHV8 infection, Kaposi’s sarcoma and gastric cancer. Haematologica. 1999;84:378–380.
62. Aghoram R, Narayan SK. Progressive multifocal leukoencephalopathy in idiopathic CD4+ lymphocytopenia. J Neurovirol. 2018;24:526–528. doi:10.1007/s13365-018-0638-0
63. Purnell D, Ilchyshyn A, Jenkins D, Salim A, Seth R, Snead D. Isolated human papillomavirus 18-positive extragenital bowenoid papulosis and idiopathic CD4+ lymphocytopenia. Br J Dermatol. 2001;144:619–621. doi:10.1046/j.1365-2133.2001.04097.x
64. Mc Lane NJ, Weems JJ, Antsworth MV. Cytomegalovirus retinitis in a patient with idiopathic CD4+ T lymphocytopenia. Clin Infect Dis. 1994;18.
65. Gupta M, Jardeleza MSR, Kim I, Durand ML, Kim L, Lobo A-M. Varicella zoster virus necrotizing retinitis in two patients with idiopathic CD4 lymphocytopenia. Ocul Immunol Inflamm. 2016;24:544–548. doi:10.3109/09273948.2015.1034376
66. Dziadzio M, Chee R, McNamara C, Deheragoda M, Wagner T, Seneviratne SL. EBV-driven diffuse large B-cell lymphoma confined to the liver in a patient with a history of idiopathic CD4 lymphocytopenia. BMJ Case Rep. 2013;2013:bcr2013009721–bcr2013009721. doi:10.1136/bcr-2013-009721
67. Niino D, Tsukasaki K, Torii K, et al. Human Herpes virus 8-negative primary effusion lymphoma with BCL6 rearrangement in a patient with idiopathic CD4 positive T-lymphocytopenia. Haematologica. 2008;93(1):e22–e23. doi:10.3324/haematol.12085
68. Wakeel A, Urbaniak S, Armstrong SS, et al. Idiopathic CD4 lymphocytopenia associated with chronic pruritic papules. Br J Dermatol. 1994;131.
69. Xia XJ, Shen H, Xu AE. Cutaneous Penicillium marneffei infection in a patient with idiopathic CD4(+) lymphocytopenia. J Dermatol. 2015;42:812–814. doi:10.1111/1346-8138.12899
70. Zonios DI, Falloon J, Huang CY, Chaitt D, Bennett JE. Cryptococcosis and idiopathic CD4 lymphocytopenia. Medicine (Baltimore). 2007;86:78–92. doi:10.1097/md.0b013e31803b52f5
71. Jayachandran V, Gjorgova-Gjeorgjievski S, Siddique H. Pulmonary nocardiosis in a patient with idiopathic CD4 lymphocytopenia. Respirol Case Rep. 2018;6:e00283.
72. Relia N, Kavimandan A, Sinha S, Sharma SK. Disseminated histoplasmosis as the first presentation of idiopathic CD4+ T-lymphocytopenia. J Postgrad Med. 2010;56:39–40. doi:10.4103/0022-3859.62426
73. Sethi J, Ramachandran R, Kohli HS, Gupta KL. Isolated renal mucormycosis in a patient with idiopathic CD4 lymphocytopenia. BMJ Case Rep. 2018;2018:bcr–2018.
74. Legarth RA, Christensen M, Calum H, Kazenstein TL, Helweg-Larsen J. Cryptococcal rib osteomyelitis as primary and only symptom of idiopathic CD4penia. Med Mycol Case Rep. 2014;4:16–18. doi:10.1016/j.mmcr.2014.02.002
75. Jundt MC, Ayalew AM, Hartman TE, Roden AC, Koo CW. Idiopathic CD4 lymphocytopenia with fulminant pneumocystis jirovecii pneumonia. Am J Respir Crit Care Med. 2019;199:e35–e36. doi:10.1164/rccm.201802-0267IM
76. Kodjikian L, Garweg JG, Nguyen M, Schaffner T, Deplazese P, Zimmerli S. Intraocular microsporidiosis due to Encephalitozoon cuniculi in a patient with idiopathic CD4+ T-lymphocytopenia. Int J Med Microbiol. 2005;294:529–533. doi:10.1016/j.ijmm.2004.09.013
77. Plonquet A, Bassez G, Authier FJ, Dray JM, Farcet JP, Gherardi RK. Toxoplasmic myositis as a presenting manifestation of idiopathic CD4 lymphocytopenia. Muscle Nerve. 2003;27:761–765. doi:10.1002/mus.10376
78. Fox-Lewis A, Lockwood DNJ. Visceral leishmaniasis complicating idiopathic CD4+ T-cell lymphocytopenia: 2 case reports. PLoS Negl Trop Dis. 2017;11:e0005412. doi:10.1371/journal.pntd.0005412
79. Stetson CL, Rapini RP, Tyring SK, Kimbrough RC. CD4+ T lymphocytopenia with disseminated HPV. J Cutan Pathol. 2002;29:502–505. doi:10.1034/j.1600-0560.2002.290809.x
80. Lopez PM, De Morales JM, González IR, Prieto MA. Cutaneous infections by papillomavirus, herpes zoster and Candida albicans as the only manifestation of idiopathic CD4+ T lymphocytopenia. Int J Dermatol. 1999;38:119–121. doi:10.1046/j.1365-4362.1999.00364.x
81. Ladoyanni E, North J, Tan CY. Idiopathic CD4+ T-cell lymphocytopaenia associated with recalcitrant viral warts and squamous malignancy. Acta Derm Venereol. 2007;87:76–77. doi:10.2340/00015555-0150
82. Mijares MC, Aldahan AS, Gonzalez HH, Benhayoun N, Alboukrek D. Hypomyopathic dermatomyositis presenting with idiopathic CD4 lymphocytopenia and delayed anti-MDA5 positivity. Cureus. 2019;11.
83. Yamauchi PS, Nguyen NQ, Grimes PE. Idiopathic CD4+T-cell lymphocytopenia associated with vitiligo. J Am Acad Dermatol. 2002;46:779–782. doi:10.1067/mjd.2002.119672
84. Baroudjian B, Viguier M, Battistella M, et al. Psoriasis associated with idiopathic CD4+ T-cell lymphopenia: a regulatory T-cell defect? Br J Dermatol. 2014;171:186–189. doi:10.1111/bjd.12922
85. Sasidharanpillai S, Khader A, Puravoor J, Riyaz N. Cutaneous T-cell non Hodgkin lymphoma in a patient with idiopathic CD4+ lymphocytopenia. Indian J Dermatol Venereol Leprol. 2013;79(6):831–833. doi:10.4103/0378-6323.120748
86. Sancesario G, Palmieri G, Viola G, et al. Difficulty diagnosing chronic cryptococcal meningitis in idiopathic CD4+ lymphocytopenia. Neurol Sci. 2011;32:519–524. doi:10.1007/s10072-011-0496-5
87. Puri V, Duggal AK, Chaudhry N. Idiopathic CD4 lymphocytopenia with sensorimotor polyneuropathy. Ann Indian Acad Neurol. 2016;19:381–384. doi:10.4103/0972-2327.165470
88. Kojima M, Sakurai S, Morita Y, Nakamura N, Sugihara S, Shimano S. EBV(+) B-cell lymphoproliferative disorder associated with subsequent development of burkitt lymphoma in a patient with idiopathic CD4(+) T-lymphocytopenia. J Clin Exp Hematop. 2008;48:55–59. doi:10.3960/jslrt.48.55
89. Sinicco A, Maiello A, Raiteri R, et al. Pneumocystis carinii in a patient with pulmonary sarcoidosis and idiopathic CD4+ T lymphocytopenia. Thorax. 1996;51:
90. Nair JP, Athavale AU, Gawande S, et al. Disseminated cryptococcosis with caverno-oesophageal fistula in a case of idiopathic CD4+ T-lymphocytopenia. J Assoc Physicians India. 2014;62:66–69.
91. Kortsik C, Elmer A, Tamm I. Pleural effusion due to Histoplasma capsulatum and idiopathic CD4 lymphocytopenia. Respiration. 2003;70:118–122. doi:10.1159/000068426
92. Hamanishi T, Nakao T, Nishino M, et al. Idiopathic CD4+ T lymphocytopenia disclosed by the onset of empyema thoracis. Intern Med. 1999;38:40–44. doi:10.2169/internalmedicine.38.40
93. Lin JC, Tripathi HM. Pure red cell aplasia and idiopathic CD4 t-lymphocytopenia. Clin Infect Dis. 1994;18:651–652.
94. Hequet O, Salles G, Espinousse D, et al. Multifocal progressive leukoencephalopathy occurring after refractory anemia and multiple infectious disorders consecutive to severe lymphopenia. Ann Hematol. 2002;81:340–342. doi:10.1007/s00277-002-0458-3
95. Mandl T, Bredberg A, Jacobsson LT, Manthorpe R, Henriksson G. CD4+ T-lymphocytopenia–a frequent finding in anti-SSA antibody seropositive patients with primary Sjogren’s syndrome. J Rheumatol. 2004;31:726–728.
96. Bordin G, Ballare M, Paglino S, et al. Idiopathic CD4+ lymphocytopenia and systemic vasculitis. J Intern Med. 1996;240:37–41. doi:10.1046/j.1365-2796.1996.447785000.x
97. Denu RA, Rush PS, Ahrens SE, Westergaard RP. Idiopathic CD4 lymphocytopenia with giant cell arteritis and pulmonary mucormycosis. Med Mycol Case Rep. 2014;6:73–75. doi:10.1016/j.mmcr.2014.10.002
98. Rijnders RJ, van den Ende IE, Huikeshoven FJ. Suspected idiopathic CD4+ T-lymphocytopenia in a young patient with vulvar carcinoma stage IV. Gynecol Oncol. 1996;61:423–426. doi:10.1006/gyno.1996.0167
99. Yamada Y, Okada M, Kamitamari A, et al. Multiple immune abnormalities in a patient with idiopathic CD4+ T-lymphocytopenia. Intern Med. 2009;48:1967–1971. doi:10.2169/internalmedicine.48.2623
100. Díaz Betancourt ML, Klínger Hernández JC, Niño Castaño VE. Profound CD4+ T lymphocytopenia in human immunodeficiency virus negative individuals, improved with anti-human herpes virus treatment. Colomb Med (Cali). 2008;43:729–734. doi:10.25100/cm.v43i4.1159
101. Calvo KR, Vinh DC, Maric I, et al. Myelodysplasia in autosomal dominant and sporadic monocytopenia immunodeficiency syndrome: diagnostic features and clinical implications. Haematologica. 2011;96:1221–1225. doi:10.3324/haematol.2011.041152
102. Hsu AP, MacReynolds LJ, Holland SM. GATA2 deficiency. Curr Opin Allergy Clin Immunol. 2015;15(1):104–109. doi:10.1097/ACI.0000000000000126
103. Rea IM, Alexander HD, Crockard AD, Morris TC. CD4 lymphopenia in very elderly people. Lancet. 1996;347(8997):328–329. doi:10.1016/S0140-6736(96)90504-8
104. Yuanjie Z, Julin G, Fubing C, Jianghan C. Recurrent pulmonary cryptococcosis in a patient with idiopathic CD4 lymphocytopenia. Med Mycol. 2008;46:729–734. doi:10.1080/13693780802256083
105. Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Altanta, USA: Centers for Disease Control NIoHaHMAotIDSoA; 2019.
106. Kirtava Z, Blomberg J, Bredberg A, Henriksson G, Jacobsson L, Manthorpe R. CD4+ T-lymphocytopenia without HIV infection: increased prevalence among patients with primary Sjogren’s syndrome. Clin Exp Rheumatol. 1995;13:609–616.
107. Harel A, Horng S, Gustafson T, Ramineni A, Farber RS, Fabian M. Successful treatment of progressive multifocal leukoencephalopathy with recombinant interleukin-7 and maraviroc in a patient with idiopathic CD4 lymphocytopenia. J Neurovirol. 2018;24:652–655. doi:10.1007/s13365-018-0657-x
108. Ross SH, Cantrell DA. Signalling and function of Interleukin 2 in T lymphocytes. Annu Rev Immunol. 2018;26:411–433.
109. The INSIGHT-ESPIRIT study group and SILCAAT scientific committee. Interleukin 2 therapy in patients with HIV infection. N Engl J Med. 2009;361.
110. Wilhelm M, Weissinger F, Kunzmann V, Muller JG, Fahey JL. Idiopathic CD4+ T cell lymphocytopenia evolving to monoclonal immunoglobulins and progressive renal damage responsive to IL-2 therapy. Clin Immunol. 2001;99:298–304. doi:10.1006/clim.2001.5016
111. Castro F, Cardoso AP, Goncalves RM, Serre K, Oliviera MJ. Interferon gamma at the cross roads of tumor immune surveillance or evasion. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.00847
112. Netea MG, Brouwer AE, Hoogendoorn EH, et al. Two patients with cryptococcal meningitis and idiopathic CD4 lymphopenia: defective cytokine production and reversal by recombinant interferon- gamma therapy. Clin Infect Dis. 2004;39:e83–e87. doi:10.1086/425121
113. Petersen EJ, Rozenberg-Arska M, Dekker AW, Clevers HC, Verdonck LF. Allogeneic bone marrow transplantation can restore CD4+ T-lymphocyte count and immune function in idiopathic CD4+ T-lymphocytopenia. Bone Marrow Transplant. 1996;18:813–815.
114. Cervera C, Fernandez-Aviles F, de la Calle-martin O, et al. Non-myeloablative hematopoietic stem cell transplantation in the treatment of severe idiopathic CD4+ lymphocytopenia. Eur J Haematol. 2011;87:87–91. doi:10.1111/j.1600-0609.2011.01619.x
115. Hamidieh AA, Pourpak Z, Hamdi A, Nabavi M, Ghavamzadeh A. Successful fludarabine based hematopoietic stem cell transplantation in pediatric patient with idiopathic CD4+ lymphocytopenia. Pediatr Transplant. 2013;17:e109–e111. doi:10.1111/petr.12086
116. Kaczmarski RS, Webster AD, Moxham J, Davision F, Sutherland S, Mufti GJ. CD4+ lymphocytopenia due to common variable immunodeficiency mimicking AIDS. J Clin Pathol. 1994;47:364–366. doi:10.1136/jcp.47.4.364
117. Galie M, Cassone M, Ausiello C, Serra P. Idiopathic CD4+ T-lymphocyte deficiency: the clinical evolution of a case. Ann Ital Med Int. 1997;12:233–237.
118. von Bernuth H, Knochel B, Winkler U, Roesler J, Schlesier M, Gahr M. Immunodeficiency with recurrent panlymphocytopenia, impaired maturation of B lymphocytes, impaired interaction of T and B lymphocytes, and impaired integrity of epithelial tissue: a variant of idiopathic CD4+ T lymphocytopenia? Pediatr Allergy Immunol. 2002;13:381–384. doi:10.1034/j.1399-3038.2002.01100.x
119. Heredia A, Hewlett IK, Soriano V, Epstein JS. Idiopathic CD4+ T lymphocytopenia: a review and current perspective. Transfus Med Rev. 1994;8:223–231. doi:10.1016/S0887-7963(94)70114-0
120. Brooks JP, Gaffari G. Idiopathic CD4 lymphocytopenia. Allergy Asthma Proc. 2016;37(6):501–504. doi:10.2500/aap.2016.37.3992
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

