Back to Journals » Journal of Pain Research » Volume 16
Hypoconnectivity of the Amygdala in Patients with Low-Back-Related Leg Pain Linked to Individual Mechanical Pain Sensitivity: A Resting-State Functional MRI Study
Authors Wang Z , Wang Y , Ji Y, Yang Z , Pei Y, Dai J, Zhang Y, Zhou F
Received 14 June 2023
Accepted for publication 16 October 2023
Published 8 November 2023 Volume 2023:16 Pages 3775—3784
DOI https://doi.org/10.2147/JPR.S425874
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Ziyun Wang,1,2,* Yao Wang,1,2,* Yuqi Ji,1,2 Ziwei Yang,1,2 Yixiu Pei,3 Jiankun Dai,4 Yong Zhang,5 Fuqing Zhou1,2
1Department of Radiology, The First Affiliated Hospital, Nanchang University, Nanchang, 330006, People’s Republic of China; 2Neuroradiology Laboratory, Jiangxi Province Medical Imaging Research Institute, Nanchang, 330006, People’s Republic of China; 3Department of Radiology, The Affiliated Ganzhou Hospital of Nanchang University, Ganzhou, Jiangxi, 341000, People’s Republic of China; 4MR Advanced Application, GE Healthcare, Beijing, 100176, People’s Republic of China; 5Department of Pain Clinic, The First Affiliated Hospital, Nanchang University, Nanchang, Jiangxi Province, 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong Zhang, Department of Pain Clinic, The First Affiliated Hospital, Nanchang University, 17 Yongwaizheng Street, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel +86 791 8869 5036, Email [email protected] Fuqing Zhou, Department of Radiology, The First Affiliated Hospital, Nanchang University, 17 Yongwaizheng, Street, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel +86 791 8869 5132, Email [email protected]
Purpose: To explore resting-state functional connectivity (rsFC) of the amygdala in patients with low-back-related leg pain (LBLP).
Patients and Methods: For this prospective study, a total of 35 LBLP patients and 30 healthy controls (HCs) were included and underwent functional MRI and clinical assessments. Then, patients with LBLP were divided into acute LBLP (aLBLP) and chronic LBLP (cLBLP) subgroups. We further evaluated the between-group rsFC differences using left and right amygdala seeds in a whole-brain voxel analysis strategy. Finally, we performed correlation analysis between the rsFC values of altered regions and clinical indices.
Results: Compared to HCs, hypoconnectivity of the amygdala was observed in LBLP patients (P < 0.01, with correction). The amygdala’s rsFC pattern was different between aLBLP and cLBLP patients: decreased the amygdala’s FC to the right putamen, to the right paracentral lobule (PCL), or to the right posterior temporal lobe in aLBLP patients, while right amygdala to the bilateral anterior cingulate cortex (ACC) and the left postcentral gyrus (PoCG) in cLBLP patients. Correlation analysis showed that lower rsFC of the left amygdala to the right PCL was correlated with the von Frey filament (vF) test values of the left lumbar (p = 0.025) and right lumbar (p = 0.019) regions, and rsFC of the right amygdala to the left PoCG was correlated with lower vF test values of the left lumbar (p = 0.017), right lumbar spine (p = 0.003); to right PoCG was correlated with calf (p = 0.015); the rsFC of the right amygdala to bilateral ACC was negatively correlated with the pain rating index (p = 0.003).
Conclusion: LBLP patients showed amygdala hypoconnectivity, and the altered pattern of amygdala rsFC was different in the acute and chronic phases. Moreover, the amygdala hypoconnectivity was related to individual mechanical sensitivity (vF test) in LBLP patients.
Keywords: amygdala, resting-state functional MRI, low-back-related leg pain, acute pain, chronic pain
Introduction
Low-back-related leg pain (LBLP) is one of the most common subgroups of low-back pain caused by intervertebral disc degeneration, disc herniation and nerve root compression.1 Patients with LBLP can exhibit a variety of clinical symptoms, including dull or achy, stinging, burning pain or numbness from the low back to the backs of the thighs and even some pain comorbidities/symptoms, such as anxiety, depression, and cognitive impairment.2,3 Additionally, LBLP patients are usually associated with more serious disability, poorer outcomes and negative emotion than those with low-back pain alone.4 Negative emotion will not only increase physiological pain sensitivity but also increase the incidence of complications, such as sleep disorders and abnormal decision-making, through the transition from acute low back pain to chronic low back pain.5 These factors seriously affect patients’ quality of social life, pain prognosis, and even outcomes.6,7
Multiple studies have demonstrated that the amygdala plays an important role in pain processing and regulation. For example, hyperactivity of basolateral amygdala (BLA) neurons and central amygdala (CeA) neurons leads to the impairment of amygdala-cortical control mechanisms, which allows the development of amygdala pain plasticity.8 A neuroimaging meta-analysis showed that the location of peak activation within and outside the amygdala subregion in acute and chronic pain had great agreement with neuroanatomical landmarks derived from laboratory and neuroimaging modalities under the experimental induction, such as mechanical compression, thermal stimulation and capsaicin stimulation.9 In patients with chronic pain exposed to threat and scare stimuli, it was observed that negative emotional states correlated with increased resting-state functional connectivity (rsFC) between the amygdala and the supramarginal gyrus.10 Hyperconnectivity of the amygdala has also been observed in patients with irritable bowel syndrome-related abdominal pain.11
Furthermore, compared to acute low back pain, more emotion-related structural and functional alterations in the brain were observed in patients with chronic pain. This may be due to large-scale changes in the brain during the transition from acute pain to chronic pain, manifested by a shift from acute pain-related brain regions to chronic emotion-related circuits involving the amygdala.12 However, functional connectivity in the amygdala has rarely been studied in patients with different pain durations of LBLP. In other words, the role of the amygdala in acute and chronic LBLP still remains unclear.
Therefore, we hypothesized that LBLP patients exhibit abnormal amygdala rsFCs, and the altered rsFC pattern is different in the acute phase and the chronic phase. We aimed to elucidate the amygdala functional connectivity differences between brain regions related to emotion arousal and pain processing matrix regions. For all participants, we selected the left and right amygdala as seeds, calculated whole-brain rsFC, compared the rsFC differences between LBLP patients or their subgroups (acute LBLP and chronic LBLP) and controls at the voxel-based level, and evaluated the relationship between rsFC and pain intensity, pain rating, mechanical sensitivity and mood (anxiety, depression).
Materials and Methods
Participants
Thirty-five clinically defined LBLP patients were recruited from the First Affiliated Hospital of Nanchang University from October 2021 to March 2023. The inclusion criteria included the following: 1) aged 22–65 years and voluntarily joined the study; 2) a diagnosis and full evidence of discogenic compression on back-computed tomography (CT) and/or magnetic resonance imaging (MRI) (≥1 ruptured annulus fibrosus with compressed soft tissue); 3) visual analog scale (VAS) scores > 3/10; and 4) ineffective prior conservative treatment with medications, eg, anti-inflammatory drugs (Motrin, Advil and Naproxen) and acetaminophen (eg, Tylenol) without opioids, exercise and physical therapy. The exclusion criteria for the LBLP group were a history of spinal stenosis due to calcification on the spinal protrusion, lateral recess stenosis, spinal stenosis, piriformis syndrome, or sciatica; a history of head and spinal cord injury or a major systemic disease; and a history of significant cardiac events. Pain for more than 3 months was defined as chronic pain. In this study, 21 patients were defined as having chronic LBLP (cLBLP), while 17 patients were defined as having acute LBLP (aLBLP). Healthy controls (HCs) were recruited by advertisements. All of the HCs were screened using the Clinical Diagnostic Interview Nonpatient Version and did not have significant cognitive disorders, head trauma, or magnetic resonance imaging (MRI) contraindications. All subjects provided informed written consent to the procedures approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Nanchang University in accordance with the Declaration of Helsinki.
Clinical Assessment
Before MRI scanning, each LBLP patient completed a series of clinical evaluations, including (1) the Japanese Orthopaedic Association (JOA) Back Pain Evaluation questionnaire (score range, −6 to 29) to examine the impact of neuropathic or nociceptive pain on quality of life; (2) the visual analogue scale (VAS) for pain intensity, ranging from 0 (no pain) to 10 (worst pain); (3) the Short-Form McGill Pain Questionnaire (SF-MPQ) to evaluate the pain intensity of subjects, including four major groups, sensory, affective, evaluative, and miscellaneous, with the sum of the rank values for each descriptor termed the pain rating index (PRI); (4) a manual von Frey filaments (vF) test (Item No. 514000-20C, Danmic Global, LLC, Inc., San Jose, CA, USA) for assessing the mechanical pain sensitivity of 8 regions (bilateral hand, back, calf, and foot), where a higher value indicates lower mechanical pain sensitivity;13–15 and (5) the Hamilton Depression Rating Scale (HAMD) and the Hamilton Anxiety Scale (HAMA) for evaluating the symptoms of anxiety and depression.
Imaging Data Acquisition
All MRI data were acquired on a 3.0T MRI (Signa Pioneer, GE Healthcare, Milwaukee, CA, USA) at the First Affiliated Hospital of Nanchang University. A high-resolution 3D T1-weighted imaging scanner was used with the following parameters: repetition time (TR)/echo time (TE) =8.0 ms/3.0 ms, field of view (FOV) = 256 mm × 256 mm, matrix = 256 × 256, thickness/gap = 1.0/0 mm and 176 sagittal slices. To acquire resting-state fMRI data, we used an echo planar image (EPI) sequence with the following parameters: TR/TE = 2000 ms / 25 ms, FOV = 190 × 190 mm, matrix = 64 × 64, flip angle = 90°, slice thickness = 3.5 mm, 40 axial slices, and 240 volumes over 8 min. While participants were lying in the scanner, they were asked to follow the instruction: “Keep your eyes closed, avoid thinking about anything and maintain a clear head”.
Data Preprocessing
All fMRI data were processed and analyzed using the toolbox for Data Processing & Analysis of Brain Imaging (DPABI v6.0; http://rfmri.org/dpabi). The processing steps were as follows: the first 10 image (volumes) were discarded to keep the signal steady, slice timing and head motion correction were performed; and images were segmented into white matter, gray matter, and cerebrospinal fluid (CSF) with the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) algorithm and normalized to Montreal Neurological Institute (MNI) space; Furthermore, we excluded images in which head motion exceeded 3 mm translation or 3° rotation according to the criteria of Van Dijk et al.16 After normalization, we continued resampling the image to 3 mm isotropic voxels and spatial smoothing (6 mm using a full width at half-maximum Gaussian kernel). Additionally, detrended linear drift and nuisance linear regression, including the white matter, CSF and head motion parameters, were enforced according to the Friston-24 model.17 Finally, to diminish the influence of low-frequency drift and high-frequency physiological noise, we applied temporal bandpass filtering (0.01–0.1 Hz).
Seed Definition and Functional Connectivity Analysis
We selected the left and right amygdala as seeds based on the Harvard–Oxford atlas, which was implemented in DPABI v6.0 (http://rfmri.org/dpabi) and presented by the BrainNet Viewer. Subject-level rsFC was performed by calculating the Pearson correlation coefficient of average time courses of blood oxygenation level-dependent signals between the seed and all other brain voxels. For further statistical analysis, all correlation values were transformed by Fisher r-to-z transformation to fit the Gaussian distribution. A two-sample t-test was assessed for the LBLP and HC groups with age, sex and mean frame displacement (FD) as covariates (two-tailed, voxel-level P < 0.01, Gaussian random field (GRF) theory correction at cluster-level P < 0.05). In addition, two sub-groups comparisons for the aLBLP vs HC and cLBLP vs HC groups were conducted.
Clinical Statistical Analysis
SPSS 23.0 package (SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis. We used Shapiro–Wilk tests and histograms to test the normality of the data, and we used means and standard deviations to characterize normally distributed data. Age, sex, mean FD and observed clinical scores among the groups were assessed through different statistical methods. Normally distributed data were analyzed by two-samples t tests, whereas the Man-Whitney U-test was used for the non-normally distributed data. The chi-square test was used for categorical data, and all significance levels were set at P<0.05. The log transformation was to reduce skewness of vF test data to approximately conform to normality. Partial or Spearman correlations were performed between the altered rsFC values and clinical assessments, with sex and age as covariates.
Results
Clinical and Demographic Analysis
Two healthy controls were excluded from the final analysis due to excessive 3 mm translation or 3° rotation during the scanning procedure. Table 1 summarizes the demographic and clinical data of the LBLP patients and HCs included in this study. There was no significant difference in age or sex between the LBLP and HC groups, between the aLBLP and HC groups (Table S1), or between the cLBLP and HC groups (Table S1). The average vF test values were 39.2–588 millinewton (mN), indicating significantly decreased mechanical sensitivity in LBLP patients, which the normal range is 0.08–0.70 mN (Table 1). There was no significant difference in the vF test values between aLBLP and cLBLP patients (Table S1, P: 0.376–0.973). There was no significant difference in the clinical questionnaires between the aLBLP and cLBLP groups (P > 0.05) (Table S1).
 |
Table 1 Demographic Data and Clinical Characteristics of the LBLP and HC Groups |
Hypoconnectivity of the Amygdala in LBLP
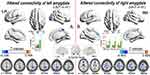
Compared to HCs, LBLP patients showed decreased rsFC between the left amygdala and right paracentral lobule (PCL), between the right amygdala and bilateral anterior cingulate cortex (ACC), and between the right amygdala and bilateral postcentral gyrus (PoCG) (voxel-level P < 0.01, Gaussian random field [GRF] correction at cluster-level P < 0.05) (Figure 1 and Table 2).
 |
Table 2 Brain Regions with Altered Amygdala Functional Connectivity of LBLP and LBLP Subgroups (Voxel-Level P < 0.01, GRF Correction at Cluster-Level P < 0.05) |
Different Connectivity Patterns of the Amygdala in aLBLP and cLBLP
Figure 2A–C presents the altered connectivity of the amygdala in aLBLP and cLBLP patients (Table 2). Although the alterations in functional connectivity were all decrease, the amygdala rsFC patterns were different in acute and chronic LBLP patients (Figure 2D). Specifically, aLBLP patients showed lower rsFC between the amygdala and the bilateral putamen, right PCL, and right posterior temporal lobe, while cLBLP patients mainly exhibited lower rsFC between the right amygdala and the bilateral ACC, between the right amygdala and left PoCG.
Correlation Analysis
When exploring the regions with altered amygdala connectivity in the LBLP group, we observed that lower rsFC of the left amygdala to the right PCL was related to the lg(vF) of the left lumbar (r = 0.390, p = 0.025) and right lumbar spine (r = 0.406, p = 0.019) (Figure 3A). Decreased rsFC of the right amygdala to the bilateral ACC was related to higher PRI scores (r = 0.497, p = 0.003), while lower rsFC of the right amygdala to the left PoCG was related to lower lg(vF) of left lumbar (r = 0.411, p = 0.017) and right lumbar spine (r = 0.496, p = 0.003), the right amygdala to right PoCG showed lower lg(vF) of left calf regions (r = 0.421, p = 0.015) (Figure 3B).
In acute or chronic LBLP subgroups, similar positive associations were found between the rsFC and the lg(vF) value (see Figure S1).
Discussion
In this study, we examined rsFC of the amygdala in patients with LBLP, as well as associations between rsFC and clinical assessments within the patient group. The main findings were as follows: 1) LBLP patients showed a hypoconnectivity of the amygdala, mainly in the right PCL, bilateral ACC and bilateral PoCG, compared with HCs. More specifically, decreased amygdala rsFC in aLBLP patients was mainly located in the pain matrix, while decreased amygdala rsFC in cLBLP patients was mainly located in the affective/emotional processing area (ACC). 2) Hypoconnectivity of the amygdala was associated with the lower mechanical pain sensitivity (vF test values) and higher pain intensity (PRI scores).
Hypoconnectivity of the Amygdala in LBLP Patients
In this study, we found decreased amygdala rsFC in LBLP patients, specifically to the right PCL, bilateral ACC and PoCG. The PCL controls motor and sensory innervation of the contralateral calf and plays a key role in somatosensory perception.18 Reduced region homogeneity and degree centrality were found in the PCL of persistent somatoform pain disorder patients,19,20 and such abnormal alterations may be related to diminished inhibition of pain perception and mood disorders, which has been similarly reported in neuroimaging studies of trigeminal neuralgia.21 In the negative stimuli of pain and fear, the activated PCL was associated with enhanced self-perceived pain responses.22 The ACC is a part of the limbic system that is activated by noxious stimuli, most importantly, it is involved in affective pain processing. The amygdala participates in the limbic-cortical circuit, which is involved in pain processing and receives pain information input from the ACC.23 Studies have found that injecting trace excitatory amino acids into the ACC-induced avoidance learning behavior, and blocking excitatory amino acid receptors in the ACC prevented avoidance learning from peripheral noxious stimuli, suggesting that the ACC modulates affective and motivational pain responses through neural projections to the amygdala.24 The PoCG is well known as part of the brain network of the pain matrix information processing, there is extensive activation in the PoCG in patients with pain, which indicates that it plays an important role in the transmission and processing of sensory information of pain. Our previous study found that the amplitude of low-frequency fluctuation (ALFF) in LBLP patients was reduced mainly in the default mode network and processing system of the pain matrix (thalamus, midbrain, PoCG). In addition, decreased ALFF of the PoCG was inversely correlated with two-point tactile discrimination in the legs, which represents a fine modality measure, indicating a heightened sensitivity to pain and sensory disturbances in patients with LBLP.25 We speculated that this may be related to the occurrence of abnormal functional damage in brain regions of pain-related afferent pathways in LBLP patients, and weaker connectivity predicted decreased inhibitory control of pain perception and pain behavior.
By further comparing the between-group differences in the decreased amygdala functional connectivity between aLBLP and cLBLP patients, we found that the pattern of connectivity changes in the amygdala was different. For aLBLP patients, reduced rsFC occurred between the amygdala and the right PCL, putamen and posterior temporal lobe, mainly in the regions of the pain matrix processing pathway. The putamen is the main part of the basal ganglia, which processes pain information through the cortex-basal ganglia-thalamus-cortical loop, including receiving sensory and emotional input of pain.26 The volume of the putamen has been found to be reduced in patients with left trigeminal neuralgia.27 A recent study reported that the shape of the putamen is closely related to the volume and pain score in acute pain models.28 These results suggested that the putamen plays an important role in the perception of pain. The relationship between the temporal lobe and pain has not been well explored; a machine learning study of chronic low back pain found increased gray matter in the temporal lobe that correlated with unpleasant mood,29 and similar findings were shown in patients with fibromyalgia,30 suggesting that it may further represent the affective dimension of pain. However, cLBLP showed reduced rsFC only between the right amygdala and the bilateral ACC and PoCG, mainly located in the affective/emotional processing area. A study on the asymmetry of amygdala pain processing showed that during persistent pain in mice, the right amygdala played a dominant role in pain and emotion processing.31 Furthermore, the ACC mediates information on emotional behavior and cognitive function and is the center of coordinating multiple signals and emotional pain processing. A study on the activation of inhibitory neural circuits by optogenetic stimulation of the ACC showed that inhibition of ACC output significantly reduced pain behavior in transgenic mice.32
Associations Between rsFC Across All Altered Regions and Clinical Assessments in LBLP Patients
In patients with spine -related leg pain, according to the type of mechanism a sensory deficit of variable degrees can be present with different sensitivity to von Frey filaments, and the mechanical deficit can be associated with the neurological lesion that causes pain and not to altered central nervous system functionality.33 But in the present study, we found that patients with LBLP generally had decreased mechanical sensitivity, which was positively correlated with rsFC of left amygdala-right PCL and/or right amygdala-PoCG. It is consistent with a previous study of decreased tactile acuity (associated with gray matter volume of primary somatosensory cortex) in the low back pain.34 A previous study found that the amygdala, a core region of the threat-processing neural circuit, could reduce susceptibility to perceptual disturbance and thereby protect the body from potential threats.35 Therefore, the decreased rsFC between the amygdala and the right PCL and PoCG may be a compensatory or protective factor against the loss of protective sensation to cope with pain injuries in LBLP patients. At the same time, our study provides new evidence for amygdala connectivity link to individual mechanical pain sensitivity in patients with LBLP.
Moreover, PRI was used to evaluate the pain perception and pain emotion of LBLP patients. The lower rsFC coefficient of the right amygdala to the bilateral ACC was negatively correlated with PRI in LBLP patients, suggesting that decreased rsFC of the amygdala may be involved in more pain perception and emotional disorders. This finding may be an important implication for understanding the underlying pathophysiological mechanisms of pain-related affective disorders in LBLP patients.
Limitations
This study included a relatively small sample size of acute and chronic LBLP patients. Therefore, our results should be interpreted cautiously. We did not investigate the rsFC of subregions of the amygdala, although previous studies have suggested that different subregions may have different roles in pain, which should be further analyzed in future studies.
Conclusion
Taken together, our findings demonstrate that LBLP patients exhibited amygdala hypoconnectivity, and interestingly, the decreased rsFC was related to individual mechanical pain sensitivity. In acute LBLP patients, the decreased amygdala functional connections were mainly located in the pain perception cortex, while in the chronic LBLP patients, the decreased amygdala functional connections involved the emotional processing area.
Acknowledgments
To all of the participants in this study, we express our sincere thanks. This study was supported by the National Natural Science Foundation of China (82160331), Jiangxi Province Double Thousand Talent Plan (jxsq2023201039), the Traditional Chinese Medicine Project of Jiangxi Province (2018A088), the General Project of Jiangxi Provincial Department of Education (GJJ190065) and the Natural Science Foundation of Jiangxi Province (20202BABL206114). This project is implemented by the Jiangxi Clinical Research Center for Medical Imaging. The funders had no role in the study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kongsted A, Kent P, Albert H, Jensen TS, Manniche C. Patients with low back pain differ from those who also have leg pain or signs of nerve root involvement – a cross-sectional study. BMC Musculoskelet Disord. 2012;13(1):236. doi:10.1186/1471-2474-13-236
2. Michaelides A, Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med. 2019;131(7):438–444. doi:10.1080/00325481.2019.1663705
3. Pinel L, Perez-Nieto MA, Redondo M, Rodriguez-Rodriguez L, Mateos LL. The impact of cognitive anxiety and the rating of pain on care processes in a vigilance task: the important part played by age. Pain Res Manag. 2020;2020:3204720. doi:10.1155/2020/3204720
4. Konstantinou K, Hider SL, Jordan JL, Lewis M, Dunn KM, Hay EM. The impact of low back-related leg pain on outcomes as compared with low back pain alone: a systematic review of the literature. Clin J Pain. 2013;29(7):644–654. doi:10.1097/AJP.0b013e31826f9a52
5. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi:10.1016/j.jpain.2013.08.007
6. Gandhi W, Rosenek NR, Harrison R, Salomons TV. Functional connectivity of the amygdala is linked to individual differences in emotional pain facilitation. Pain. 2020;161(2):300–307. doi:10.1097/j.pain.0000000000001714
7. Garcia-Larrea L, Bastuji H. Pain and consciousness. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):193–199. doi:10.1016/j.pnpbp.2017.10.007
8. Thompson JM, Neugebauer V. Amygdala Plasticity and Pain. Pain Res Manag. 2017;2017:8296501. doi:10.1155/2017/8296501
9. Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp. 2014;35(2):527–538. doi:10.1002/hbm.22199
10. Timmers I, Lopez-Sola M, Heathcote LC, et al. Amygdala functional connectivity mediates the association between catastrophizing and threat-safety learning in youth with chronic pain. Pain. 2022;163(4):719–728. doi:10.1097/j.pain.0000000000002410
11. Qi R, Liu C, Ke J, et al. Abnormal amygdala resting-state functional connectivity in irritable bowel syndrome. AJNR Am J Neuroradiol. 2016;37(6):1139–1145. doi:10.3174/ajnr.A4655
12. Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136(Pt 9):2751–2768. doi:10.1093/brain/awt211
13. Kostek M, Polaski A, Kolber B, Ramsey A, Kranjec A, Szucs K. A protocol of manual tests to measure sensation and pain in humans. J Vis Exp. 2016;118:e54130.
14. Haloua MH, Sierevelt I, Theuvenet WJ. Semmes-Weinstein monofilaments: influence of temperature, humidity, and age. J Hand Surg Am. 2011;36(7):1191–1196. doi:10.1016/j.jhsa.2011.04.009
15. Geber C, Klein T, Azad S, et al. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre study. Pain. 2011;152(3):548–556. doi:10.1016/j.pain.2010.11.013
16. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–438. doi:10.1016/j.neuroimage.2011.07.044
17. Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-Related effects in fMRI time-series. Magn Reson Med. 1996;35(3):346–355. doi:10.1002/mrm.1910350312
18. Patra A, Kaur H, Chaudhary P, Asghar A, Singal A. Morphology and morphometry of human paracentral lobule: an anatomical study with its application in neurosurgery. Asian J Neurosurg. 2021;16(2):349–354. doi:10.4103/ajns.AJNS_505_20
19. Huang T, Zhao Z, Yan C, et al. Altered spontaneous activity in patients with persistent somatoform pain disorder revealed by regional homogeneity. PLoS One. 2016;11(3):e0151360. doi:10.1371/journal.pone.0151360
20. Liu Q, Zeng XC, Jiang XM, Zhou ZH, Hu XF. Altered brain functional hubs and connectivity underlie persistent somatoform pain disorder. Front Neurosci. 2019;13:415. doi:10.3389/fnins.2019.00415
21. Liu H, Zheng R, Zhang Y, et al. Alterations of degree centrality and functional connectivity in classic trigeminal neuralgia. Front Neurosci. 2022;16:1090462. doi:10.3389/fnins.2022.1090462
22. Khatibi A, Roy M, Chen JI, Gill LN, Piche M, Rainville P. Brain responses to the vicarious facilitation of pain by facial expressions of pain and fear. Soc Cogn Affect Neurosci. 2023;18(1). doi:10.1093/scan/nsac056
23. Thompson JM, Neugebauer V. Cortico-limbic pain mechanisms. Neurosci Lett. 2019;702:15–23. doi:10.1016/j.neulet.2018.11.037
24. Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7(4):398–403. doi:10.1038/nn1207
25. Zhou F, Gu L, Hong S, et al. Altered low-frequency oscillation amplitude of resting state-fMRI in patients with discogenic low-back and leg pain. J Pain Res. 2018;11:165–176. doi:10.2147/JPR.S151562
26. Starr CJ, Sawaki L, Wittenberg GF, et al. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134(Pt 7):1987–2004. doi:10.1093/brain/awr117
27. Tsai YH, Yuan R, Patel D, et al. Altered structure and functional connection in patients with classical trigeminal neuralgia. Hum Brain Mapp. 2018;39(2):609–621. doi:10.1002/hbm.23696
28. Torrecillas-Martinez L, Catena A, O’Valle F, Padial-Molina M, Galindo-Moreno P. Does experienced pain affects local brain volumes? Insights from a clinical acute pain model. Int J Clin Health Psychol. 2019;19(2):115–123. doi:10.1016/j.ijchp.2019.01.001
29. Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cerebral Cortex. 2014;24(4):1037–1044. doi:10.1093/cercor/bhs378
30. Schmidt-Wilcke T, Luerding R, Weigand T, et al. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–S116. doi:10.1016/j.pain.2007.05.010
31. Carrasquillo Y, Gereau R. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain. 2008;4:24. doi:10.1186/1744-8069-4-24
32. Gu L, Uhelski ML, Anand S, et al. Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons. PLoS One. 2015;10(2):e0117746. doi:10.1371/journal.pone.0117746
33. Schmid AB, Tampin B, Baron R, et al. Recommendations for terminology and the identification of neuropathic pain in people with spine-related leg pain. Outcomes from the NeuPSIG working group. Pain. 2023;164(8):1693–1704. doi:10.1097/j.pain.0000000000002919
34. Mai U, Mirarab S, Xia X. Log transformation improves dating of phylogenies. Mol Biol Evol. 2021;38(3):1151–1167. doi:10.1093/molbev/msaa222
35. Spengler FB, Scheele D, Kaiser S, Heinrichs M, Hurlemann R, Protective A. Mechanism against illusory perceptions is amygdala-dependent. J Neurosci. 2019;39(17):3301–3308. doi:10.1523/JNEUROSCI.2577-18.2019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.