Back to Journals » Journal of Inflammation Research » Volume 16
High-Dose Vitamin C Alleviates Pancreatic Necrosis by Inhibiting Platelet Activation Through the CXCL12/CXCR4 Pathway in Severe Acute Pancreatitis
Authors Gui M , Huang J, Sheng H , Chen Y, Yang Z , Ma L, Wang D, Xu L, Sun W, Liu J, Xu Y, Chen E, Zhao B, Mao E
Received 5 April 2023
Accepted for publication 6 July 2023
Published 10 July 2023 Volume 2023:16 Pages 2865—2877
DOI https://doi.org/10.2147/JIR.S415974
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Menglu Gui,1,* Jun Huang,2,* Huiqiu Sheng,1,* Ying Chen,1 Zhitao Yang,1 Li Ma,1 Daosheng Wang,3 Lili Xu,1 Wenwu Sun,1 Junling Liu,4 Yanyan Xu,4 Erzhen Chen,1 Bing Zhao,1 Enqiang Mao1
1Department of Emergency in Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China; 2Department of Cardiovascular Medicine, State Key Laboratory of Medical Genomics, Shanghai Key Laboratory of Hypertension, Shanghai Institute of Hypertension, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China; 3Department of Laboratory Medicine in Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China; 4Department of Biochemistry and Molecular Cell Biology, Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Enqiang Mao; Bing Zhao, Department of Emergency in Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, People’s Republic of China, Tel +8613501747906 ; +8618017117871, Email [email protected]; [email protected]
Background: Platelet activation in the early stage of pancreatitis is the key step developing into pancreatic necrosis. Studies suggested that vitamin C (Vit C) can inhibit platelet activity by targeting CXCL12/CXCR4 pathway. High-dose Vit C were showed to reduce pancreatic necrosis in severe acute pancreatitis (SAP) but the mechanism remains unclear. Here we speculate high-dose Vit C reduce pancreatic necrosis by inhibiting platelet activation through downregulating CXCL12/CXCR4 pathway.
Methods: The pancreatic microcirculation of rats was observed by intravital microscopy. The platelet activity of SAP rats treated with or without high-dose Vit C was analyzed by platelet function test. Besides, the activity of platelets preincubated with high-dose Vit C or vehicle from SAP patients was also evaluated. Then, the TFA (CXCR4 agonist) and rCXCL12 were used to neutralize the effect of high-dose Vit C in SAP rats treated with high-dose Vit C. Meanwhile, the levels of enzymes and inflammatory cytokines in rat plasma, and rats’ pancreatic histopathology and mortality were assessed.
Results: Platelets from animals and patients with SAP are more sensitive to agonists and are more easily activated. Administration of high-dose Vit C significantly ameliorated excessive activation of platelets in SAP rats, ultimately increasing the microvessel density and inducing microthrombus and blood stasis; these results were consistent with clinical sample analysis. Moreover, high-dose Vit C significantly inhibited the release of amylase, lipase, TNF-α, and IL-6 in SAP rat plasma, reducing pancreatic damage and the mortality of SAP rats. However, using TFA and rCXCL12 significantly reversed the effect of high-dose Vit C on excessive activation of platelets, aggravating microcirculation impairment and pancreatic damage.
Conclusion: The present study suggests that high-dose Vit C can ameliorate pancreatic necrosis by improving microcirculation disorders of SAP. For the first time, the underlying mechanism is related with inhibiting platelet activation through the CXCL12/CXCR4 pathway.
Keywords: severe acute pancreatitis, microcirculation impairment, platelets activity, vitamin C, CXCL12/CXCR4 pathway
Introduction
According to a recent study, the incidence of acute pancreatitis has increased over time with an average annual percent change (AAPC) of 3.07%.1 Unfortunately, 5–10% of patients with acute pancreatitis (AP) develop acute necrotizing pancreatitis (ANP), which leads to multiple organ failure (MOF) and infected pancreatic necrosis (IPN) with high mortality.2,3 The pathogenesis of ANP is quite complex. It is widely accepted that the development of ANP is strongly associated with early impairment of pancreas microcirculation, characterized by microthrombus due to platelet activation.4–6 Thus, inhibiting platelet activity at an early stage is essential for preventing pancreatic necrosis.
Vitamin C (Vit C) is an important anti-oxidant to detoxify exogenous oxidants radical species and an enzyme cofactor for many important biological reactions.7 Besides, previous studies found that intravenous Vit C administration may inhibit platelet function in mice with sepsis.8 And our previous study has suggested that Vit C may significantly improve pancreatic damage and decrease mortality in rats of SAP.9 Therefore, we speculate High-dose Vit C alleviates pancreatic necrosis by inhibiting platelet activation in SAP.
In the preliminary experiment, we have proved the inhibition on platelet activity and the reverse on pancreatic microcirculation injury after administration of high-dose Vit C in SAP rats. Yet, the exact mechanism of action between the high-dose Vit C and platelet activity is still not fully understood. Hence, through the bioinformatic analysis, we confirmed CXCL12 as the target of Vit C with therapeutic potential for coagulopathy in SAP.
CXCL12, also known as stromal-derived factor 1 (SDF-1), belongs to the chemokine family, and functions by binding to its receptors CXC chemokine receptor 4 (CXCR4) and CXCR7.10 Reportedly, CXCL12 induced platelet aggregation and thrombus formation by ligating to CXCR4 on platelet surface, driving receptor internalization, mobilization of intracellular calcium reserves, and thromboxane A2 release and dense granule secretion.11,12 And CXCL12 expressed highly in thrombotic diseases such as acute lower-extremity deep venous thrombosis and stroke13,14 or inflammatory disease such as SAP.15 Thus, we speculated that the pro-thrombotic response of CXCL12 may play an important role the development of ANP. Noticeably, previous studies have confirmed that Vit C could suppress the production of an inflammatory chemokine by inhibiting the nuclear factor kappa-B(NF-κB) pathway.7,16 Yet, the relationship between Vit C and CXCL12 or CXCL12/CXCR4-CXCR7 is unclear. Therefore, in this study, we investigated the effect of high-dose Vit C on microcirculation disorders and the potential pathway CXCL12/CXCR4-CXCR7 in a rat model of SAP.
Materials and Methods
Antibodies and Reagents
Thrombin, collagen, ADP, apyrase, prostaglandin E1 (PGE1), tetraethyl rhodamine isothiocyanate (TRITC) -conjugated phalloidin, recombination CXCL12 (rCXCL12), and sodium taurocholate were purchased from Sigma-Aldrich (St Louis, MO, United States). Anti-CXCL12 antibody was purchased from Arigo (Taiwan, China), Anti-CXCR4 antibody, anti-CXCR7 antibody were purchased from Abcam (Cambridge, United Kingdom). Anti-GAPDH antibody and horseradish peroxidase (HRP)-linked second antibody were purchased from Proteintech (Chicago, IL, United States). CXCR4 agonist ATI-2341 TFA (TFA) was purchased from MedChemExpress (Shanghai, China). APC anti-rat CD62P was purchased from Biolegend (San Diego, CA, United States).
Animals
Sprague-Dawley rats, 7–8 weeks old, were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Science. All the animals were housed in an environment with a temperature of 22 ± 1 °C, a relative humidity of 50 ± 1%, and a light/dark cycle of 12/12 hr and fed with standard rat chow and tap water ad libitum.
SAP Model
Before surgery, rats were fasted overnight and had free access to water. Surgical procedures were conducted under anesthesia with 5% pentobarbital. SAP was induced as previously described.17 Retrograde infusion of a 5% sodium taurocholate solution (1 mL/kg) was performed into the biliopancreatic duct at a constant rate of 3 mL/h. After completing the infusion, the part of the duct that enters the duodenum was clipped for 5 minutes. Next, the clip was removed, and the wounds were sutured. In the control group, only an abdominal incision and sutures were made.
Animal Experimental Design
In the first stage, rats were randomly divided into three groups: the sham surgery (Control), SAP, SAP + 500 mg/kg Vit C. Vit C was injected into the femoral vein of the rats after the establishment of the SAP model. The SAP group rats received the same volume of saline. All rats were sacrificed after 6h, or 24 h and blood and pancreatic tissue were collected for further analysis.
In the second stage, rats were randomly divided into five groups: the sham surgery (Control), SAP, SAP + 500 mg/kg Vit C, SAP + 500 mg/kg Vit C+ 0.2umol/kg TFA, SAP + 500 mg/kg Vit C+ 30ng/kg rCXCL12. Blood samples were centrifuged at 3000 rpm for 10 minutes to separate the plasma and stored at −80 °C until analysis. Each pancreatic tissue sample section was immediately fixed with 4% paraformaldehyde for HE staining analysis.
Intravital Microscopy (IVM)
Fluorescein isothiocyanate (FITC)-dextran (FD2000S, sigma) was injected into the femoral vein in a dose of 5mg/kg. Then, imaging was performed on the Multiphoto Laser Scanning Microscopy (Olympus FVMPE-RS, Japan).18 The vascular images were processed using Image J software.
Platelet Isolation and Aggregation
Washed rat platelets were prepared as previously described19 with a minor modification. Briefly, rat blood obtained from the abdominal aorta was anticoagulated with 3.2% citrate (9:1) and mixed with an equal volume of normal saline. Samples were then mixed with PGE1 (100 ng/mL) and apyrase (1 U/mL) and centrifuged at 230 g for 10 min at room temperature to obtain platelet-rich plasma (PRP). Then, samples were mixed with 1 U/mL Apyrase and 5 mM EDTA and centrifuged at 800 g for 10 min to obtain the platelets, which were then resuspended in Tyrode’s buffer (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 10 mM HEPES, pH 7.4). Next, the concentration of platelets was counted with a globulimeter (HEMAVET, USA) and adjusted to a final concentration of 4.0 × 10^8/mL.
Washed platelets were left for 1 h at 37°C, and then further stimulated by ADP (80 μM), thrombin (0.25 U/mL), or collagen (8 μg/mL). The maximum platelet aggregation rate was detected with an aggregometer (CHRONO-LOG, Havertown, USA) within 5 min for continuous stirring.
Human Platelet Preparation and Aggregation
Venous human blood from healthy, drug-free volunteers and SAP-confirmed patients was drawn in 3.2% trisodium citrate (9:1). In brief, platelets were extracted as mentioned above and resuspended to a final concentration of 3.0 × 10^8/mL. After still standing, the platelets were stimulated by ADP (80 μM), thrombin (0.05 U/mL), or collagen (5 μg/mL). The maximum platelet aggregation rate was detected with an aggregometer within 5 min for continuous stirring.
Platelet Adhesion on Collagen-Coated Surface
Glass coverslips were coated with 100 µg/mL collagen in 5% glucose at 4°C overnight. Washed platelets were added to collagen-coated coverslips and incubated at 37°C for 1 h in the presence of 2 mM CaCl2.20 After being washed with phosphate-buffered saline, adherent platelets were fixed with 4% paraformaldehyde, permeabilized with labeling buffer (0.5% Triton X-100, 0.5% bovine serum albumin), and stained with TRITC-conjugated phalloidin (66 µM) in the dark at room temperature for 60 min. Adherent platelets were observed under an inverted fluorescence microscope (AXIO ScopeA1, Zeiss, Germany) and processed using Image J software.
Platelet Flow Cytometry
The platelets 4.0 x 10^8 /mL in 100μL volume were stimulated with or without thrombin (0.1 U/mL) at 37°C within 20 min, then, adding 400μL Tyrode’s Buffer to stop the reaction. Next, the APC-anti-CD62P was added for 1μL for per million platelets in 100μL volume and incubated for 20 min at room temperature in the dark. Last, platelets were analyzed with a FACSCalibur flow cytometer (BD, USA). For each sample, 30000 platelets were analyzed.21
Analysis of Biochemical Indicators
Arterial blood samples were processed immediately after collection by centrifugation at 3000 rpm for 10 minutes to obtain plasma. The plasma levels of amylase and lipase were measured using commercial assay kits (Jiancheng Bioengineering Institute, Nanjing, China).
Enzyme-Linked Immunosorbent Assay (ELISA)
Quantification of CXCL12 (Novus biological, NBP3-11823), IL-6 (Multiscienc, EK306/3), and TNF-α (Thermofisher, 88–7340-88) in the rat plasma was performed using an ELISA kit according to the manufacturer’s instructions.
Western Blot
The total protein of lysates platelets from rats was separated by SDS-polyacrylamide gel and transferred to polyvinylidene fluoride membranes (Millipore, Temecula, CA, USA). After blocking with 5% nonfat milk, membranes were immunoblotted with the indicated antibodies at 4°C overnight and then with an HRP-linked second antibody at room temperature for 1 h. The detection was performed by Western HRP Substrate (Yamei, PA, USA), and the results were visualized using the Tanon 5500 Imaging System (Shanghai, China). The intensity of bands in Western blot assay were processed using Image J software.
Histological Examination
After fixation in 4% paraformaldehyde for 24 h, pancreatic tissues were processed for routine histology. Tissue sections (5 µm) were stained with hematoxylin and eosin (H&E). Pancreatic histopathology scoring was performed according to Van Laethem et al.22 Briefly, edema was graded from 0 to 3 (0: absent; 1: focally increased between lobules; 2: diffusely increased between lobules; 3: acinar disrupted and separated). Inflammatory cell infiltration was graded from 0 to 3 [0: absent; 1: in ducts (around ductal margins); 2: in the parenchyma, <50% of the lobules; 3: in the parenchyma, >50% of the lobules]. Acinar necrosis was graded using a 0–3 score: 0: absent; 1: periductal necrosis, <5%; 2: focal necrosis, 5–20%; 3: diffuse parenchymal necrosis, 20–50%.
Prediction of Putative Targets of Vitamin C with Therapeutic Potential in Coagulopathy for Severe Acute Pancreatitis
One dataset “GSE109227_series_matrix” of AP in mice induced by caerulein was collected from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) by systematical retrieval, which contained 11 pancreas samples in total, and analyzed in R language.
A total of 339 known target genes of vitamin C were obtained from a previous report (Supplementary Table 1),23 and 204 therapeutic targets for coagulation were collected using the Online Mendelian Inheritance (OMIM) (http://www.omim.org/) database (Supplementary Table 2–1), DrugBank (http://www.drugbank.ca/) database (Supplementary Table 2–2), and GeneCards (www.genecards.org/) database (Supplementary Table 2–3). Next, the targets of vitamin C with therapeutic potential for coagulopathy in AP were identified.
Statistical Analysis
All data were analyzed using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA). Quantified data are presented as mean ± SEM with the indicated number of independent observations. P value of < 0.05 was considered statistically significant and was determined using non-parametric test, unpaired Student’s t-test or ANOVA, as appropriate.
Results
High-Dose Vit C Treatment Ameliorates the Severity of SAP Rats
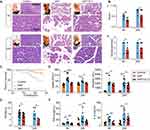
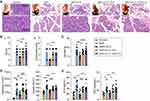
To define how high-dose Vit C impacts SAP, we compared the histological injury of pancreas. Hyperaemia and exudation appear immediately in pancreatic tissue after the retrograde infusion of taurocholate into the biliopancreatic duct, accompanied by slight edema. And 6 hours and 24 hours later, the rats were killed, whose pancreatic tissues were taken. There was massive destruction of the pancreatic tissue structure accompanied by severe edema in the SAP group, while high-dose Vit C reduced the destruction of the pancreatic tissue structure and edema (Figure 1A). The results of histological examination showed that the edema and inflammation with necrosis in pancreatic tissues from SAP rats were more severe, whereas the pathological changes of pancreatic tissues from SAP rats treated with high-dose Vit C were clearly mild (Figure 1A); also, the results showed that histological score in SAP rats increased significantly compared to the control group, while SAP rats treated with high-dose Vit C had a reduced histological score compared to the SAP group (Figure 1Bi). Consistently, high-dose Vit C treatment significantly reduced necrosis scores of pancreatic tissues in SAP rats (Figure 1Bii). The mortality was higher in the SAP group than in the SAP + high-dose Vit C group (50% vs 18.75%, Figure 1C). In addition, there were massive ascites in SAP rats compared to the control group, while the volume of ascites in SAP group treated with high-dose Vit C was significantly lower than that in the SAP group (Figure 1D).
We further found that high-dose Vit C significantly lowered amylase and lipase activity and the level of IL-6 and TNF-α of SAP rats’ plasma (Figure 1E and F). SAP usually accompanied by multiple organ dysfunction syndrome (MODS) and systemic inflammatory response syndrome (SIRS), poses a great threat to the lives of patients.24 As show in Figure S1, high-dose Vit C could improve the liver injury and cardiac injury in SAP rats. Taken together, these data confirmed that high-dose Vit C treatment might reduce sodium taurocholate-induced pancreatic pathological damage, attenuate the systemic inflammatory response and organ dysfunction, ultimately reducing mortality in SAP rats.
High-Dose Vit C Treatment Improves the Microcirculatory Disturbance of SAP Rats
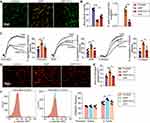
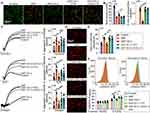
To confirm the microcirculatory changes in SAP rats, intravital microscopy was used to observe the capillaries of the pancreas. Compared to the control group, mass microthrombus in the capillaries were seen in the model group, leading to blood stasis and decreased capillaries quantity (Figure 2A and B), which is consistent with previous studies.25,26
Next, we tested the function of platelets, which have a critical role in the formation of microthrombus from three aspects. Platelet aggregation rate and the individual surface coverage of platelet spreading on immobilized collagen of SAP rats increased significantly compared to the control group (Figure 2C–E). Moreover, the positive rate of P-selectin on the platelet surface was calculated to detect the degranulation of platelets.20 After being induced by 0.1 U/ mL thrombin, P-selectin, encompassed in α-granules, quickly mobilizes towards the cell surface.27 We found that the positive rate of P-selectin in SAP rats was clearly higher than in the control group (Figure 2F). These data showed that platelets from SAP rats were more sensitive to agonists and were more easily activated which resulted in microthrombus and blood stasis; this was consistent with previous studies.28,29 On the other hand, high-dose Vit C treatment significantly inhibited excessive activation of platelets (Figure 2C–2F). Together, these data demonstrated that high-dose Vit C improved microcirculatory disturbance, manifested with fewer microthrombus and blood stasis and more capillary quantity by inhibiting platelet activity (Figure 2A and B), ultimately improving the dysfunction of pancreatic microcirculation in SAP rats.
High-Dose Vit C Reduces the Excessive Activation of Platelet from SAP Patients
To determine the clinical effects of high-dose Vit C, we detected the activity of platelets from SAP patients and healthy volunteers. Washed platelets from SAP patients were preincubated with 1000uM Vit C (from clinical data) or vehicle (0.9% NaCl) respectively, for 1h. In addition, washed platelets from healthy volunteers (control group) were preincubated with a vehicle. Consistent with the results of our animal experiments, the platelet aggregation rate and individual surface coverage of platelet from SAP patients significantly increased compared to the healthy volunteers (Figure 3A–C). High-dose Vit C was able to cause significant inhibition in activator-stimulated platelet aggregation and less platelet spreading on the collagen-coated surface than that of the SAP group (Figure 3A–C). These data revealed that high-dose Vit C could correct the coagulopathy caused by excessive platelet activation during SAP.
High-Dose Vit C Attenuates the Excessive Activation of Platelet via the CXCL12/CXCR4 Pathway in SAP Rats
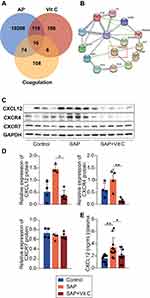
We searched for previously publicly available data and used the bioinformatic analysis to identify the key molecules whose abundance changed and were associated with the platelet activation after Vit C treatment. A total of 10,497 differentially expressed genes (DEGs) of AP were identified to meet the selection criteria of| log 2FoldChange| > 0 and adjusted p-value < 0.05 (Supplementary Table 3). We focused on the intersecting part among therapeutic targets of Vit C, regulation targets of coagulopathy, and DEGs for AP, and 16 proteins were identified (Figure 4A). Of these, 5 were clotting factors, 1 regulated the fibrinolytic system, 2 adjusted the activity of the platelets, 1 was associated with the production of clotting factors, 5 participated in cancer and cell development, and 1 controlled monocyte migration. After importing the 16 target genes into the STRING database, which were visualized with the interaction score set to the highest confidence (0.900), a network of the targets of Vit C with therapeutic potential in coagulopathy regulation for SAP was generated (Figure 4B). Combining the PPI results and literature review, CXCL12, one of the chemokines, was screened as the target that correlates most strongly with the microcirculation and microthrombosis in SAP by adjusting the activity of platelets.
To verify the result of bioinformatic analysis, we performed RT-qPCR of pancreas tissue from SAP rats to evaluate the expression of CXCL12 (Supplementary Materials). Consistent with the previous research,15 the transcription of CXCL12 significantly increased at 6h after the establishment of SAP (Figure S2). Next, we detected the protein level of CXCL12, CXCR4, and CXCR7 in platelets. The CXCR7 expression was unaltered, while CXCL12 and CXCR4 were upregulated in SAP platelets. High-dose Vit C significantly inhibited the synthesis of CXCL12 and CXCR4 (Figure 4C and D). We further evaluated the expression of these proteins in clinical samples. As show in Figure S3, CXCL12, CXCR4 and CXCR7 were upregulated in SAP platelets. High-dose Vit C (8g/d for 3 days) treatment reduced the expression of CXCL12, CXCR4 and CXCR7 on platelets in SAP patients. Next, we found that high-dose Vit C significantly lowered the level of CXCL12 in the plasma of SAP rats (Figure 4E). Together, these results point to the CXCL12/CXCR4 pathway influenced by Vit C in regulating platelet activity in SAP rats.
High-Dose Vit C Treatment Ameliorates the Severity of SAP Rats by Improving Microcirculation Disorders Through CXCL12/CXCR4 Pathway
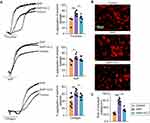
To unravel the role of the CXCL12/CXCR4 pathway involved in the microcirculatory disturbance and platelet activation, we used TFA and rCXCL12 to neutralize the effect of high-dose Vit C. The results showed that TFA and rCXCL12 aggravated microcirculation disturbance in the pancreas, owing to more microthrombus, larger area of blood stasis, and much fewer capillaries in SAP rats treated with high-dose Vit C (Figure 5A and B). Meanwhile, TFA and rCXCL12 also reversed the alleviation of excessive platelet activity in SAP rats treated with high-dose Vit C (Figure 5C–F).
Next, we evaluated the overall impact of the CXCL12/CXCR4 pathway in SAP rats. The results showed that TFA and rCXCL12 exacerbated the pathological damages of pancreatic tissues with much higher histological scores and necrosis scores, significantly increased the volume of ascites, and the activity of amylase and lipase as well as the level of IL-6 and TNF-α in plasma in SAP rats treated with high-dose Vit C (Figure 6A–E). Thus, these data indicated that high-dose Vit C treatment could inhibit excessive activation of platelets, improve microcirculation disorders, and ultimately ameliorate the severity of SAP rats through CXCL12/CXCR4 pathway.
Discussion
In the present study, we illustrated that platelets from animals and patients with SAP are more sensitive to agonists and are more easily activated. High-dose Vit C at the early stage of SAP could improve coagulopathy and microcirculation failure and alleviate pancreatic damage by significantly inhibiting activation of platelets through CXCL12/CXCR4 pathway.
Our results are consistent with previous studies, as our data confirmed mass microthrombus and microvascular failure and the enhanced platelet function at the early stage of SAP. Moreover, Tsaroucha et al and Fan et al found increased P-selectin expression on platelets in AP patients,5,28 leading to thrombosis and aggravating histopathology of acinar necrosis.30,31 However, they indirectly illustrated the changes in platelet activity at the molecular level. To the best of our knowledge, this study first reported the more sensitive to agonists and more easily activated of the platelets at the cellular level, manifested as enhanced platelets aggregation and spreading in both SAP patients and rats. Besides, our results illustrated that an antiplatelet drug might be another alternative to antiplatelet therapy as an intervention for regulating the balance between bleeding and thrombosis can reduce thrombosis without increasing the bleeding risk.32
Previous studies have confirmed that Vit C is closely associated with coagulation by inhibiting platelet activity,33,34 which is consistent with our results. Although reactive oxygen species mediate platelet activation, high-dose Vit C promotes oxidative stress.35 Therefore, the inhibition of high-dose Vit C on platelet activation cannot be explained by anti-oxidative stress alone. Our bioinformatic analysis suggested that CXCL12 is the target linking inflammation and coagulation by regulating platelet activity. This study verified the increased expression of CXCL12 and CXCR4 in platelets and demonstrated that CXCL12 promoted platelet activation by binding to CXCR4, which was proved by reversing the inhibited effect of high-dose Vit C on platelet activity in SAP rat. And this study also illustrated the downregulation of CXCL12/CXCR4 pathway by high-dose Vit C in SAP rats, which was consistent with previous studies7,16 that Vit C could suppress the production of an inflammatory chemokine by inhibiting the NF-κB pathway. Besides, in in vitro experiments, Vit C illustrated a dose-response similar to chemokines36 (Figure S4A). TFA or rCXCL12 significantly reverses the effect of Vit C on platelet activity (Figure S4B). This phenomenon cannot be interpreted by the production inhibition of CXCL12/CXCR4. That means, in a specific range of concentration, Vit C inhibits the binds of CXCL12 and CXCR4 or interrupts the signal transmission of the CXCL12/CXCR4 pathway. Taken together, these results confirmed that high-dose Vit C alleviates pancreatic microcirculation disturbance, improving pancreatic necrosis by inhibiting platelet activation via downregulating the CXCL12/CXCR4 pathway or intercepting the signal transmission of the CXCL12/CXCR4 pathway in SAP.
However, the present study also has some limitations. We did not illustrate the specific pathway or mechanism effected by high-dose Vit C that regulated the production of CXCL12 since it is derived from stroma cells in discrete anatomical locations.37 At the same time, the details of intercepting the signal transmission of the CXCL12/CXCR4 pathway is yet clarified. These mechanisms should be further expounded in subsequent studies.
Conclusions
In this study, we illustrated that platelets from animals and patients with SAP are more sensitive to agonists and are more easily activated, inducing pancreatic microcirculation disturbance and necrosis. We also confirmed that suppressing by Vit C on the CXCL12/CXCR4 pathway inhibits the excessive platelet activation and ameliorates the microthrombus and microcirculation blood stasis, alleviating the severity of SAP. These data not only expand the research scope of the mechanism of high-dose Vit C but also provide evidence for further clinical investigations and applications.
Abbreviations
ANP, Acute necrotizing pancreatitis; AP, Acute pancreatitis; SAP, Severe acute pancreatitis; Vit C, Vitamin C; MOF, Multiple organ failure; IPN, Infected pancreatic necrosis; TFA, ATI-2341 TFA; rCXCL12, recombination CXCL12; SDF-1, Stromal-derived factor 1; CXCR4, CXC chemokine receptor 4; PGE1, Prostaglandin E1; TRITC, Tetraethyl rhodamine isothiocyanate; HRP, horseradish peroxidase; FITC, Fluorescein isothiocyanate; PRP, Platelet-rich plasma; GEO, Gene Expression Omnibus; OMIM, Online Mendelian Inheritance; DEGs, Differentially expressed genes; RT-qPCR, Real-time Quantitative Polymerase Chain Reaction; NF-κB, nuclear factor kappa-B.
Data Sharing Statement
The data sets used and analyzed in our study are available from the corresponding author upon reasonable request.
Animal Ethical Approval Statement
All animal studies (including the rats euthanasia procedure) were done in compliance with the regulations and guidelines of Shanghai Jiaotong University institutional animal care and conducted according to the AAALAC and the IACUC guidelines. All animal experiments were approved by the Ethics Committee of Ruijin Hospital of Shanghai Jiao Tong University School of Medicine.
Ethics Approval and Informed Consent
The study followed guidelines enshrined in the Declaration of Helsinki and was duly granted ethical clearance by Ethics Committee of Ruijin Hospital of Shanghai Jiao Tong University School of Medicine vide their approval number (2018) 临伦审第 (145) 号, dated 29th August 2018. All participants read and understood the information leaflet and signed the consent form prior to recruitment.
Acknowledgments
We thank all contributors of the Core Facility of Basic Medical Sciences, Shanghai Jiao Tong University School of Medicine.
Author Contributions
Menglu Gui, Jun Huang, and Huiqiu Sheng share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by Clinical Research Project of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (2018CR004), and shanghai natural science fund (21ZR1440400).
Disclosure
The authors declare that they have no competing interests.
References
1. Iannuzzi JP, King JA, Leong JH, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162(1):122–134. doi:10.1053/j.gastro.2021.09.043
2. Schepers NJ, Bakker OJ, Besselink MG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68(6):1044–1051. doi:10.1136/gutjnl-2017-314657
3. L. K, Zhou J, Mao W, et al. Immune enhancement in patients with predicted severe acute necrotising pancreatitis: a multicentre double-blind randomised controlled trial. Intensive Care Med. 2022;48(7):899–909. doi:10.1007/s00134-022-06745-7
4. Dobosz M, Hac S, Mionskowska L, Dobrowolski S, Wajda Z. Microcirculatory disturbances of the pancreas in cerulein-induced acute pancreatitis in rats with reference to L-arginine, heparin, and procaine treatment. Pharmacol Res. 1997;36(2):123–128. doi:10.1006/phrs.1997.0200
5. Fan C, Song Y, Wang X, Mao C, Xiong Y. Identification of early derangements of coagulation, hematological and biochemical profiles in patients with acute pancreatitis. Clin Biochem. 2022;109–110:37–43. doi:10.1016/j.clinbiochem.2022.08.005
6. Osada J, Wereszczynska-Siemiatkowska U, Dabrowski A, Dabrowska MI. Platelet activation in acute pancreatitis. Pancreas. 2012;41(8):1319–1324. doi:10.1097/MPA.0b013e31824bd89f
7. Marik PE. Vitamin C for the treatment of sepsis: the scientific rationale. Pharmacol Ther. 2018;189:63–70. doi:10.1016/j.pharmthera.2018.04.007
8. Secor D, Li F, Ellis CG, et al. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med. 2010;36(11):1928–1934. doi:10.1007/s00134-010-1969-3
9. Xu LL, Zhao B, Sun SL, et al. High-dose vitamin C alleviates pancreatic injury via the NRF2/NQO1/HO-1 pathway in a rat model of severe acute pancreatitis. Ann Transl Med. 2020;8(14):852. doi:10.21037/atm-19-4552
10. Janssens R, Struyf S, Proost P. The unique structural and functional features of CXCL12. Cell Mol Immunol. 2018;15(4):299–311. doi:10.1038/cmi.2017.107
11. Chatterjee M, Rath D, Gawaz M. Role of chemokine receptors CXCR4 and CXCR7 for platelet function. Biochem Soc Trans. 2015;43(4):720–726. doi:10.1042/BST20150113
12. Walsh TG, Harper MT, Poole AW. SDF-1alpha is a novel autocrine activator of platelets operating through its receptor CXCR4. Cell Signal. 2015;27(1):37–46. doi:10.1016/j.cellsig.2014.09.021
13. Sun S, Chai S, Zhang F, Lu L. Overexpressed microRNA-103a-3p inhibits acute lower-extremity deep venous thrombosis via inhibition of CXCL12. IUBMB Life. 2020;72(3):492–504. doi:10.1002/iub.2168
14. Schutt RC, Burdick MD, Strieter RM, Mehrad B, Keeley EC. Plasma CXCL12 levels as a predictor of future stroke. Stroke. 2012;43(12):3382–3386. doi:10.1161/STROKEAHA.112.660878
15. Li ZF, Xia XM, Huang C, et al. Emodin and baicalein inhibit pancreatic stromal derived factor-1 expression in rats with acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8(2):201–208.
16. Horio F, Kiyama K, Kobayashi M, Kawai K, Tsuda T. Ascorbic acid deficiency stimulates hepatic expression of inflammatory chemokine, cytokine-induced neutrophil chemoattractant-1, in scurvy-prone ODS rats. J Nutr Sci Vitaminol. 2006;52(1):28–32. doi:10.3177/jnsv.52.28
17. Silva-Vaz P, Abrantes AM, Castelo-Branco M, et al. Murine models of acute pancreatitis: a critical appraisal of clinical relevance. Int J Mol Sci. 2019;20(11):2794. doi:10.3390/ijms20112794
18. Masedunskas A, Weigert R. Intravital two-photon microscopy for studying the uptake and trafficking of fluorescently conjugated molecules in live rodents. Traffic. 2008;9(10):1801–1810. doi:10.1111/j.1600-0854.2008.00798.x
19. Y. X, Ouyang X, Yan L, et al. Sin1 (stress-activated protein kinase-interacting protein) regulates ischemia-induced microthrombosis through integrin alphaiibbeta3-mediated outside-in signaling and hypoxia responses in platelets. Arterioscler Thromb Vasc Biol. 2018;38(12):2793–2805. doi:10.1161/ATVBAHA.118.311822
20. Huang M, Deng M, Nie W, et al. Naringenin inhibits platelet activation and arterial thrombosis through inhibition of phosphoinositide 3-kinase and cyclic nucleotide signaling. Front Pharmacol. 2021;12:722257. doi:10.3389/fphar.2021.722257
21. Ding X, Liu TD, Xie ZL, et al. A naphthalenic derivative ND-1 inhibits thrombus formation by interfering the binding of fibrinogen to integrin alphaIIbbeta3. Biomed Res Int. 2016;2016:8587164. doi:10.1155/2016/8587164
22. Van Laethem JL, Marchant A, Delvaux A, et al. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108(6):1917–1922. doi:10.1016/0016-5085(95)90158-2
23. Zhu N, Huang B, Jiang W. Targets of vitamin C with therapeutic potential for cardiovascular disease and underlying mechanisms: a study of network pharmacology. Front Pharmacol. 2020;11(11):591337. doi:10.3389/fphar.2020.591337
24. Yang R, Tenhunen J, Tonnessen TI. HMGB1 and histones play a significant role in inducing systemic inflammation and multiple organ dysfunctions in severe acute pancreatitis. Int J Inflam. 2017;2017:1817564. doi:10.1155/2017/1817564
25. Klar E, Messmer K, Warshaw AL, Herfarth C. Pancreatic ischaemia in experimental acute pancreatitis: mechanism, significance and therapy. Br J Surg. 1990;77(11):1205–1210. doi:10.1002/bjs.1800771104
26. Du BQ, Yang YM, Chen YH, Liu XB, Mai G. N-acetylcysteine improves pancreatic microcirculation and alleviates the severity of acute necrotizing pancreatitis. Gut Liver. 2013;7(3):357–362. doi:10.5009/gnl.2013.7.3.357
27. Hackert T, Büchler MW, Werner J. Targeting P-selectin in acute pancreatitis. Expert Opin Ther Targets. 2010;14(9):899–910. doi:10.1517/14728222.2010.504717
28. Tsaroucha AK, Schizas D, Vailas MG, et al. E and P selectins as potential markers in the assessment of the severity of acute pancreatitis. Pancreas. 2018;47(4):406–411. doi:10.1097/MPA.0000000000001009
29. Hartman H, Abdulla A, Awla D, et al. P-selectin mediates neutrophil rolling and recruitment in acute pancreatitis. Br J Surg. 2012;99(2):246–255. doi:10.1002/bjs.7775
30. Jin H, Gebska MA, Blokhin IO, et al. Endothelial PPAR-gamma protects against vascular thrombosis by downregulating P-selectin expression. Arterioscler Thromb Vasc Biol. 2015;35(4):838–844. doi:10.1161/ATVBAHA.115.305378
31. Hackert T, Sperber R, Hartwig W, et al. P-selectin inhibition reduces severity of acute experimental pancreatitis. Pancreatology. 2009;9(4):369–374. doi:10.1159/000212098
32. Mayer K, Hein-Rothweiler R, Schupke S, et al. Efficacy and safety of revacept, a novel lesion-directed competitive antagonist to platelet glycoprotein VI, in patients undergoing elective percutaneous coronary intervention for stable ischemic heart disease: the randomized, double-blind, placebo-controlled ISAR-PLASTER Phase 2 Trial. JAMA Cardiol. 2021;6(7):753–761. doi:10.1001/jamacardio.2021.0475
33. Cordova C, Musca A, Violi F, Perrone A, Alessandri C. Influence of ascorbic acid on platelet aggregation in vitro and in vivo. Atherosclerosis. 1982;41(1):15–19. doi:10.1016/0021-9150(82)90064-8
34. Tousoulis D, Antoniades C, Tountas C, et al. Vitamin C affects thrombosis/ fibrinolysis system and reactive hyperemia in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2003;26(10):2749–2753. doi:10.2337/diacare.26.10.2749
35. Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016;22(6):463–493. doi:10.1111/odi.12446
36. Tchernychev B, Ren Y, Sachdev P, et al. Discovery of a CXCR4 agonist pepducin that mobilizes bone marrow hematopoietic cells. Proc Natl Acad Sci U S A. 2010;107(51):22255–22259. doi:10.1073/pnas.1009633108
37. Scala S. Molecular pathways: targeting the CXCR4-CXCL12 axis--untapped potential in the tumor microenvironment. Clin Cancer Res. 2015;21(19):4278–4285. doi:10.1158/1078-0432.CCR-14-0914
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.