Back to Journals » International Journal of General Medicine » Volume 17
HE4 Serum Levels are Associated with Poor Prognosis in Patients with Acute Heart Failure Combined with Chronic Kidney Disease
Authors Tang Y, Hu Z, Liu Z, Peng S, Liu T, Xiao Y, Peng J, Pan H, Zheng Z, He J
Received 14 October 2023
Accepted for publication 13 March 2024
Published 2 April 2024 Volume 2024:17 Pages 1273—1280
DOI https://doi.org/10.2147/IJGM.S444680
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Redoy Ranjan
Yi Tang,1,* Zhengqi Hu,2,* Zhibin Liu,2 Siling Peng,2 Tiancheng Liu,2 Yaoyuan Xiao,2 Jianqiang Peng,1 Hongwei Pan,1 Zhaofen Zheng,1 Jin He1
1Department of Cardiology, Hunan Provincial People’s Hospital, the First Affiliated Hospital of Hunan Normal University, Clinical Medicine Research Center of Heart Failure of Hunan Province, Hunan Normal University, Changsha, People’s Republic of China; 2Department of Cardiology, Hunan Provincial People’s Hospital, the First Affiliated Hospital of Hunan Normal University, Changsha, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jin He, Email [email protected]
Purpose: The levels of human epididymis protein 4 (HE4) is associated not only with the prognosis of patients with acute heart failure (AHF), but also with chronic kidney disease (CKD). Our study aims to understand the prediction value of HE4 on prognosis in patients with AHF combined with CKD.
Patients and Methods: This study prospectively enrolled patients diagnosed with AHF combined with CKD at the Department of Cardiology of Hunan Provincial People’s Hospital from March 2019 to December 2022. Serum levels of HE4 were measured using a chemiluminescence microparticle immunoassay. The endpoint events included heart failure readmission and cardiovascular death.
Results: A total of 130 patients with AHF combined with CKD were included in the stud. The median age is 73 years (interquartile range: 65– 79 years). Among the patients, 94 experienced the endpoint events. The multivariable Cox analysis reveals that LnHE4 (HR=2.280, 95% CI 1.300– 3.998, P = 0.004) and age (HR=1.024, 95% CI 1.003– 1.045, P = 0.025) are independent predictors of the endpoint events. The Kaplan-Meier survival curve demonstrates that patients with HE4 levels> 276.15 pmol/L has a significantly higher incidence of endpoint events compared to those with HE4 levels≤ 276.15 pmol/L (Log rank test: χ 2=19.689, P < 0.001). After adjusting for age and gender, the HR is 2.520 (95% CI: 1.626– 3.906, P < 0.001).
Conclusion: HE4 is an independent predictor of heart failure readmission and cardiovascular death in patients with AHF combined with CKD.
Keywords: Human epididymis protein 4, Acute heart failure, Chronic kidney disease, Prognosis
Introduction
Acute heart failure (AHF) is a severe cardiovascular condition that requires urgent treatment and intervention due to its rapid onset or progression of symptoms and signs. It may occur as a first episode (de novo) or more commonly as a result of acute decompensation of chronic heart failure.1 The primary causes of AHF include acute myocardial damage, increased cardiac load, and arrhythmia.2,3 AHF is significantly associated with poor prognosis in patients, with readmission rates ranging from 32% to 44% and a mortality rate of up to 30% within one year.2,4 Approximately half of the patients with heart failure also have concurrent chronic kidney disease (CKD). The presence of CKD similarly demonstrated a significant risk of cardiovascular events.5 As a result, it is crucial to conduct prognostic assessments of patients with AHF combined with CKD, especially, a new biomarker to evaluate the prognosis of patients with AHF combined with CKD.
Currently, several biomarkers are useful for predicting the prognosis of AHF, including N-Terminal B-type natriuretic peptide (NT-proBNP), Soluble ST2 (sST2), Galectin-3 (Gal-3), and Growth differentiation factor-15 (GDF-15).6 In clinical practice, NT-proBNP is widely used for the prognostic assessment of AHF. However, NT-proBNP levels are affected by various factors and diseases such as renal function, age, weight, pulmonary embolism, sepsis, and stroke, which can reduce its prognostic value. Particularly in patients with renal insufficiency, as NT-proBNP needs to be cleared through the kidneys, a decrease in estimated glomerular filtration rate (eGFR) can lead to an elevation in plasma NT-proBNP levels.7
Human epididymis protein 4 (HE4), also known as WAP four-disulfide core domain 2 (WFDC2), was initially discovered in human epididymal epithelial cells and was found to be involved in sperm maturation.8 In 2003, HE4 was identified as a biomarker for ovarian cancer.9 Our previous research findings demonstrated that HE4 could predict the prognosis in patients with ischemic cardiomyopathy and idiopathic pulmonary hypertension with right heart failure.10,11 In 2013, de Boer et al12 found that HE4 levels could predict the poor prognosis in patients with AHF. Arnold Piek et al13 also found that HE4 are associated with heart failure severity in patient with chronic heart failure. Furthermore, LeBleu VS et al14 revealed that HE4 was involved in the process of renal fibrosis, and several studies confirmed that HE4 levels were closely related to the severity of chronic kidney disease (CKD),15,16 and Huang Y et al17 demonstrated that HE4 was a predictor of AHF in patients with CKD during hospitalization.
Although HE4 could predict prognosis in patients with AHF and was closely associated with the severity of CKD, there was no research reporting the predictive value of serum HE4 levels in patients with AHF combined with CKD. Therefore, the aim of this study is to investigate the predictive value of HE4 in the prognosis of patients with AHF combined with CKD.
Materials and Methods
Study Population
This study prospectively enrolled 150 adult patients with AHF combined with CKD who were admitted to the Department of Cardiovascular Medicine at Hunan Provincial People’s Hospital from March 2019 to December 2022. The diagnosis of AHF was based on the rapid onset or worsening of at least one symptom (dyspnea, orthopnea, or edema) and one sign of heart failure (rales, edema, ascites, or pulmonary vascular congestion on chest radiography).1 The diagnosis of AHF was confirmed by two cardiologists. CKD was defined as abnormalities in renal structures and functions for>3 months, with an estimated glomerular filtration rate (eGFR) < 60 mL /min/1.73 m2.18 The Modification of Diet in Renal Disease (MDRD) formula was used to calculate the estimated glomerular filtration rate (eGFR).19 eGFR = 175 ´[Scr (mg/dL)] − 1.154 ´age − 0.203 (´0.742 if patient is female). Patients with the following conditions were excluded: 1. Malignant tumor; 2. Hepatic sclerosis or a medical history of hepatic fibrosis; 3. Females who are pregnant or planning to get pregnant; 4. Cystic pulmonary fibrosis and pulmonary tuberculosis; 5. Autoimmune diseases.
Clinical Assessment and Follow-Up
Each patient underwent initial clinical evaluation, including medical history collection, physical examination, biochemical tests, and echocardiography. NT-proBNP was determined at baseline using the chemiluminescence immunoassay method (Wantaikairui, XiaMen, China) in the Department of Laboratory Medicine of Hunan Provincial People’s Hospital. Follow-up visits were conducted at 1, 3, 6, and 12 months after discharge, and subsequently every 6 months, recording the occurrence of endpoint events during the follow-up period. Endpoint events were defined as cardiovascular death and heart failure readmission. The follow-up ended on March 21, 2023.
HE4 Measurement
Peripheral venous blood samples were collected the following morning after admission. Blood samples were centrifuged at 1000 rpm for 15 min, and the upper serum was frozen and stored at −80°C. Serum levels of HE4 were measured using a chemiluminescence microparticle immunoassay (Abbott, Germany) in the Department of Nuclear Medicine of Hunan Provincial People’s Hospital. The detectable dose of human HE4 ranged from 15 to 1500 pmol/L.
Statistical Method
Continuous variables with a normal distribution are expressed as the mean ± standard deviation (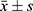 ), and continuous variables with a nonnormal distribution are represented by the median and quartile (IQR). Comparison between the two groups was performed using Student’s t-test or Mann–Whitney U-test, depending on the distribution of the data. Comparison among the three groups was conducted using Kruskal–Wallis test. The categorical variables are expressed as n (%), and the Chi-square (χ²) test was used for categorical variables. Pearson or Spearman correlation coefficients were used for bivariate correlation analysis. Receiver Operating Characteristic (ROC) Curve was used to judge the performance of variables in prognostic prediction. Univariate and multivariate Cox proportional hazards model and Kaplan-Meier curve were used for survival analysis. The statistical analyses were performed using SPSS version 25.0 (SPSS, Inc.). Two-tailed P value < 0.05 was statistically significant.
), and continuous variables with a nonnormal distribution are represented by the median and quartile (IQR). Comparison between the two groups was performed using Student’s t-test or Mann–Whitney U-test, depending on the distribution of the data. Comparison among the three groups was conducted using Kruskal–Wallis test. The categorical variables are expressed as n (%), and the Chi-square (χ²) test was used for categorical variables. Pearson or Spearman correlation coefficients were used for bivariate correlation analysis. Receiver Operating Characteristic (ROC) Curve was used to judge the performance of variables in prognostic prediction. Univariate and multivariate Cox proportional hazards model and Kaplan-Meier curve were used for survival analysis. The statistical analyses were performed using SPSS version 25.0 (SPSS, Inc.). Two-tailed P value < 0.05 was statistically significant.
Results
Baseline Characteristics
A total of 150 patients with AHF combined with CKD were initially enrolled, 20 patients were excluded due to loss of follow-up. Ultimately, 130 patients were included in the study analysis. The median age of the patients is 73 years (interquartile range: 65–79 years), and males accounted for 65.4% of the cohort. A total of 94 experienced endpoint events, among them, 69 patients experienced heart failure readmission and 25 patients experienced cardiovascular death. The median follow-up duration is 581 days(interquartile range: 461-1060 days).
The median HE4 level is 276.15 pmol/L. The patients were divided into high-level and low-level groups according the median HE4 level. There are no statistically significant differences between the two groups in age and baseline medical history (hypertension, diabetes, coronary heart disease, atrial fibrillation) (all P>0.05). However, compared to the low-level group, the high-level group has significantly higher levels of NT-proBNP and higher NYHA functional classification (all P < 0.05). Furthermore, the Serum HE4 levels increased as the cardiac function deteriorated, and there is a statistically significant difference among the three groups (P < 0.001) (Figure 1). Compared to the low-level group, the high-level group has lower eGFR, BMI, hemoglobin, albumin, and total bilirubin levels. The usage rate of ACEI/ARB/ARNI and aldosterone antagonists are also lower in the high-level group (P < 0.05) (Table 1).
 |
Table 1 Clinical Characteristics of the Cohort |
 |
Figure 1 Comparison of serum HE4 in different NYHA functional classification (II–IV). |
Correlation Analysis
Based on Spearman correlation analysis (Figures 2 and 3), the serum HE4 levels are positively correlated with the NT-proBNP levels (r = 0.567, P < 0.001), and negatively correlated with the eGFR levels (r = −0.755, P < 0.001). The NT-proBNP levels are significantly negatively correlated with the eGFR levels (r = −0.521, P < 0.001), and the serum HE4 levels are not correlated with the age (r = 0.010, P = 0.907), and the left ventricular ejection fraction (r = 0.153, P = 0.082).
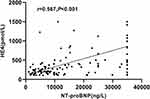 |
Figure 2 The association between HE4 and NT-proBNP levels. |
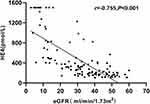 |
Figure 3 The association between HE4 and eGFR levels. |
HE4 and Clinical Outcomes
The results of the univariate analysis indicate that NT-proBNP, eGFR, age, hemoglobin, LDL-C, and LnHE4 (logarithmically transformed HE4 value) could serve as prognostic indicators for AHF patients with CKD. The variables with P < 0.05 (LnHE4, NT-proBNP, eGFR, age, hemoglobin, LDL-C, and NYHAIII-IV) in the univariate Cox regression were included in the multivariate analysis using the method of input variables. The results indicates that LnHE4 levels (HR = 2.280, 95% CI: 1.300–3.998, P = 0.004) and age (HR = 1.024, 95% CI: 1.003–1.045, P = 0.025) are independent predictive factors for the occurrence of endpoint events in patients with AHF combined with CKD (Table 2).
 |
Table 2 Univariate and Multivariate Cox Analysis of Proportional Risks for Events in AHF Patients with CKD |
The study conducts a ROC analysis (Figure 4) to evaluate the predictive value of serum HE4 levels. The analysis shows that the area under the curve (AUC) is 0.670 (P = 0.003, 95% CI: 0.565–0.775). The sensitivity and specificity are 69.1% and 63.9%, respectively.
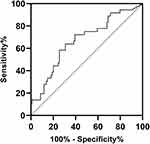 |
Figure 4 Receiver operating characteristic curve showing the sensitivity and specificity of HE4. HE4 human epididymis protein 4. |
The Kaplan-Meier survival curve (Figure 5) indicates that patients with HE4 levels > 276.15 pmol/L have a significantly higher risk of experiencing endpoint events compared to patients with HE4 levels ≤ 276.15 pmol/L (Log rank test: χ2=19.689, P < 0.001). The unadjusted hazard ratio (HR) is 2.597 (95% CI: 1.676–4.024, P < 0.001), and the HR adjusted for age and gender is 2.520 (95% CI: 1.626–3.906, P < 0.001).
 |
Figure 5 Kaplan-Meier analysis of human epididymis protein (HE4) for cardiovascular death and heart failure rehospitalization. Adjusted HR indicates hazard ratio (HR) adjusted for age and gender. |
Discussion
To the best of our knowledge, this is the first study investigating the prognostic value of HE4 in patients with AHF combined with CKD. Our results show that serum HE4 levels increase with renal function and cardiac function deterioration, HE4 levels are positively correlated with NT-proBNP levels. HE4 is an independent predictor of cardiovascular death and heart failure readmission in patients with AHF combined with CKD. Furthermore, patients with HE4>276.15 pmol/L has a 2.52-fold higher risk of experiencing endpoint events compared to patients with HE4 ≤ 276.15 pmol/L.
HE4, also known as WAP four-disulfide core domain 2 (WFDC2), is a protease inhibitor, which can inhibit serine, aspartic, and cysteine proteases.20 HE4 is expressed in a variety of tissues, including the reproductive tract, respiratory tract, and kidneys.21 Studies reported that HE4 was associated with AHF severity and poor prognosis in patients with AHF, and also had positively correlated with Galectin-3, a biomarker of cardiac fibrosis.12 Yamamoto et al22 reported that HE4 could act on cardiac fibroblasts and was involved in cardiac fibrosis. These findings indicated that HE4 may play a role in the occurrence and progression of heart failure as it involved in the fibrotic process. Consistent with previous studies, our study also shows an increase in serum HE4 levels as cardiac function worsen and serum HE4 levels have a positive correlation with NT-proBNP levels.
Although NT-proBNP is widely used to assess the severity and prognosis of AHF, because NT-proBNP undergoes renal metabolism, its evaluation in patients with AHF combined with CKD remain controversial.7 Multiple studies reported that NT-proBNP lose its value in predicting prognosis in patients with AHF combined with CKD.23–25 Our study also reveals that NT-proBNP could not serve as an independent predictor of prognosis in patients with AHF combined with CKD, which was consisted with the results of previous studies.
LeBleu et al14 found that serum HE4 levels were significantly elevated in patients with renal fibrosis, furthermore, HE4 was involved in renal fibrosis via inhibiting the activity of serine proteases and matrix metalloproteinases. The severity of renal fibrosis is associated with higher serum HE4 levels,15 Wang L et al16 found that there was a strong negative correlation between serum HE4 levels and eGFR. our study results are consistent with previous studies. More importantly, our study also demonstrates that HE4, rather than NT-proBNP, is an independent predictor of prognosis in patients with AHF combined with CKD.
Our study has several limitations. First, it is a single-center study with a relatively small sample size. Secondly, we only measured the baseline levels of HE4 whether the levels of HE4 after treatment or at discharge could better predict prognosis is unknown. A multicenter, large-scale study with serial measurements of HE4 will be needed to validate these findings.
Conclusion
HE4 is an independent predictor of heart failure readmission and cardiovascular death in patients with acute heart failure combined with chronic kidney disease.
Data Sharing Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Statement of Ethics
The study complied with the Declaration of Helsinki. This study protocol was reviewed and approved by the Ethics Committee of Hunan Provincial People’s Hospital, approval number (2023-232). All patients gave their written informed consent to participate.
Acknowledgment
We thank all subjects and colleagues for participating in the study.
Author Contributions
Yi Tang and Zhengqi Hu contributed equally to this work and share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Science and Technology Innovation Program of Hunan Province (2020SK50923), the Research Project of Hunan Provincial Health Commission (202103012371), Renshu Fund Project of Hunan Provincial People’s Hospital (RS2022A12).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi:10.1093/eurheartj/ehw128
2. Mauro C, Chianese S, Cocchia R, et al. Acute Heart Failure: diagnostic-Therapeutic Pathways and Preventive Strategies-A Real-World Clinician’s Guide. J Clin Med. 2023;12(3). doi:10.3390/jcm12030846
3. Arrigo M, Jessup M, Mullens W, et al. Acute heart failure. Nat Rev Dis Primers. 2020;6(1):16. doi:10.1038/s41572-020-0151-7
4. Bazmpani MA, Papanastasiou CA, Kamperidis V, et al. Contemporary Data on the Status and Medical Management of Acute Heart Failure. Curr Cardiol Rep. 2022;24(12):2009–2022. doi:10.1007/s11886-022-01822-1
5. Gallo G, Lanza O, Savoia C. New Insight in Cardiorenal Syndrome: from Biomarkers to Therapy. Int J Mol Sci. 2023;24(6):5089. doi:10.3390/ijms24065089
6. Al-Sadawi M, Saad M, Ayyadurai P, Shah NN, Bhandari M, Vittorio TJ. Biomarkers in Acute Heart Failure Syndromes: an Update. Curr Cardiol Rev. 2022;18(3):e090921196330. doi:10.2174/1573403X17666210909170415
7. Oremek GM, Passek K, Holzgreve F, von der Eltz V, Droge J. The biomarkers BNP and NT-proBNP. Zentralbl Arbeitsmed Arbeitsschutz Ergon. 2023;73(2):89–95. doi:10.1007/s40664-022-00491-9
8. Kirchhoff C, Habben I, Ivell R, Krull N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod. 1991;45(2):350–357. doi:10.1095/biolreprod45.2.350
9. Hellstrom I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63(13):3695–3700.
10. Tang Y, Wang Y, Xu X, et al. Human epididymis protein 4: a novel predictor of ischemic cardiomyopathy. BMC Cardiovascular Disorders. 2021;21(1):511. doi:10.1186/s12872-021-02319-5
11. Jin Q, Tang Y, Liu Z, et al. Serum human epididymis protein 4 level as a predictor of clinical worsening in idiopathic pulmonary arterial hypertension: a pilot study. BMC Cardiovascular Disorders. 2020;20(1):175. doi:10.1186/s12872-020-01461-w
12. de Boer RA, Cao Q, Postmus D, et al. The WAP four-disulfide core domain protein HE4: a novel biomarker for heart failure. JACC Heart Fail. 2013;1(2):164–169. doi:10.1016/j.jchf.2012.11.005
13. Piek A, Meijers WC, Schroten NF, Gansevoort RT, de Boer RA, Silljé HH. HE4 Serum Levels Are Associated with Heart Failure Severity in Patients With Chronic Heart Failure. J Card Fail. 2017;23(1):12–19. doi:10.1016/j.cardfail.2016.05.002
14. LeBleu VS, Teng Y, O’Connell JT, et al. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nature Med. 2013;19(2):227–231. doi:10.1038/nm.2989
15. Wan J, Wang Y, Cai G, et al. Elevated serum concentrations of HE4 as a novel biomarker of disease severity and renal fibrosis in kidney disease. Oncotarget. 2016;7(42):67748–67759. doi:10.18632/oncotarget.11682
16. Wang L, Sun Y, Cai X, Fu G. The diagnostic value of human epididymis protein 4 as a novel biomarker in patients with renal dysfunction. Int Urol Nephrol. 2018;50(11):2043–2048. doi:10.1007/s11255-018-1930-x
17. Huang Y, Jiang H, Zhu L. Human Epididymis Protein 4 as an Indicator of Acute Heart Failure in Patients with Chronic Kidney Disease. Lab Med. 2020;51(2):169–175. doi:10.1093/labmed/lmz041
18. Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M; Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
19. Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 2007;53(4):766–772. doi:10.1373/clinchem.2006.077180
20. Chhikara N, Saraswat M, Tomar AK, Dey S, Singh S, Yadav S. Human epididymis protein-4 (HE-4): a novel cross-class protease inhibitor. PLoS One. 2012;7(11):e47672. doi:10.1371/journal.pone.0047672
21. Karlsen NS, Karlsen MA, Hogdall CK, Hogdall EV. HE4 tissue expression and serum HE4 levels in healthy individuals and patients with benign or malignant tumors: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2285–2295. doi:10.1158/1055-9965.EPI-14-0447
22. Yamamoto M, Hanatani S, Araki S, et al. HE4 Predicts Progressive Fibrosis and Cardiovascular Events in Patients With Dilated Cardiomyopathy. J Am Heart Assoc. 2021;10(15):e021069. doi:10.1161/JAHA.120.021069
23. Bayes-Genis A, Zamora E, de Antonio M, et al. Soluble ST2 serum concentration and renal function in heart failure. J Card Fail. 2013;19(11):768–775. doi:10.1016/j.cardfail.2013.09.005
24. Liu C, Liang W, He X, et al. Prognostic Value of Cysteine-Rich Protein 61 Combined with N-Terminal Pro-B-Type Natriuretic Peptide for Mortality in Acute Heart Failure Patients with and without Chronic Kidney Disease. Cardiorenal Med. 2020;10(1):11–21. doi:10.1159/000501929
25. de la Espriella R, Bayes-Genis A, Llacer P, et al. Prognostic value of NT-proBNP and CA125 across glomerular filtration rate categories in acute heart failure. Eur J Internal Med. 2022;95:67–73. doi:10.1016/j.ejim.2021.08.024
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Association of the Systemic Immune-Inflammation Index with Outcomes in Acute Coronary Syndrome Patients with Chronic Kidney Disease
Shi S, Kong S, Ni W, Lu Y, Li J, Huang Y, Chen J, Lin K, Li Y, Ke J, Zhou H
Journal of Inflammation Research 2023, 16:1343-1356
Published Date: 27 March 2023
