Back to Journals » Journal of Inflammation Research » Volume 16
Association of the Systemic Immune-Inflammation Index with Outcomes in Acute Coronary Syndrome Patients with Chronic Kidney Disease
Authors Shi S, Kong S, Ni W, Lu Y, Li J, Huang Y, Chen J, Lin K, Li Y, Ke J, Zhou H
Received 15 November 2022
Accepted for publication 10 March 2023
Published 27 March 2023 Volume 2023:16 Pages 1343—1356
DOI https://doi.org/10.2147/JIR.S397615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Sanling Shi, Shuting Kong, Weicheng Ni, Yucheng Lu, Junfeng Li, Yuheng Huang, Jinxin Chen, Ken Lin, Yuanmiao Li, Jiayu Ke, Hao Zhou
Department of Cardiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China
Correspondence: Hao Zhou, Email [email protected]
Background: The systemic immune-inflammation index (SII; neutrophil × platelet/lymphocyte) is a novel marker for immune and inflammatory status and is associated with adverse prognosis in cardiovascular disease.
Methods: In total, 744 patients diagnosed with acute coronary syndrome (ACS) and chronic kidney disease (CKD) were included in our study, received standard therapies, and were followed up. Patients were divided into high and low SII groups according to the baseline SII. The primary endpoint was major cardiovascular events (MACEs), defined as cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.
Results: During a median follow-up of 2.5 years, a total of 185 (24.9%) MACEs were recorded. Analysis of the ROC curve revealed that the best cutoff value of SII was 1159.84× 109/L for predicting MACEs. The Kaplan–Meier analysis showed that those patients in the low SII group had higher survival rates than those in the high SII group (p < 0.001). Compared to those in the low SII group, patients in the high SII group were at significantly higher risk of MACEs (134 (38.8%) vs 51 (12.8%), p < 0.001). Univariate and multivariable Cox regression analyses revealed that a high SII level was independently associated with MACEs in ACS patients with CKD (adjusted hazard ratio [HR]: 1.865, 95% confidence interval [CI]: 1.197– 2.907, p = 0.006).
Conclusion: The present study showed that an elevated SII is associated with adverse cardiovascular outcomes in ACS with CKD patients, suggesting that SII may be a valuable predictor of poor prognosis in ACS with CKD patients. Further studies are needed to confirm our findings.
Keywords: acute coronary syndrome, chronic kidney disease, inflammation, prognosis
Introduction
Chronic kidney disease (CKD) is common in patients with cardiovascular disease; an estimated 30% to 40% of patients with myocardial infarction (MI) have CKD.1 Indeed, CKD is an independent predictor for adverse cardiovascular events, further leading to poor clinical outcomes for patients.2 Acute coronary syndrome (ACS) and CKD are related, making the condition more complicated. Creatinine showed a close association with cardiovascular events and several risk scores containing creatinine, such as PRECISION DAPT, ACEF, and SYNTAX II, have been developed to predict the occurrence of clinical outcomes.3–5 However, patients with advanced CKD have been widely excluded from clinical trials in this area. Therefore, no standard regimen has been established for such patients, and there are limited data on outcomes in this patient population. Thus, it is necessary to explore more effective biomarkers and adjunctive therapeutic strategies to improve the prognosis of ACS patients with CKD.
Vascular inflammation drives atherosclerotic plaque formation and destabilization and contributes to the progression of ACS.6 Additionally, CKD is a chronic inflammatory disease involving many causes and inflammation may represent a marker of poor outcomes in CKD patients.7 According to the findings of the CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study) trial, anti-inflammatory medication effectively lowers the risk of major adverse cardiovascular events in CKD patients.8,9 Studies have revealed a significant association between immunological and inflammatory responses and atherosclerosis development, and numerous biomarkers have been used as a predictors in coronary artery disease (CAD) patients.10–12 The neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) are significant inflammatory biomarkers that may be associated with atherosclerosis and predict various cardiovascular events.13,14
Recently, Hu et al created a new inflammatory index, the systemic immune-inflammation index (SII; neutrophil × platelet/lymphocyte) to assess the inflammatory and immune status.15 SII is associated with poor prognosis in individuals with several types of cancers, suggesting that SII may be a prognostic indicator in various malignant diseases.15–17 Moreover, recent studies have shown that SII is strongly linked to adverse outcomes in patients with cardiovascular disease.18,19 In addition, CKD has been considered a major risk factor for congestive heart failure (CHF), and the risk increases with the deterioration of renal function.20 A study has shown that SII has predictive value for both long-term mortality and appropriate ICD therapy in patients having HF with reduced ejection fraction (HFrEF).21 In patients with chronic coronary syndrome (CCS), SII served as an independent predictor for functionally substantial coronary stenosis.22 However, the relationship between SII in patients with ACS and CKD in clinical practice has not been fully elucidated and remains unclear. To explore the association of SII with cardiovascular disease, we investigated the predictive significance of SII in ACS patients with CKD who received a percutaneous coronary intervention (PCI) in this study.
Methods
Study Design and Baseline Characteristics
This was a single-center retrospective observational study based in the cardiovascular department of the First Affiliated Hospital of Wenzhou Medical University. This study complies with the Declaration of Helsinki and was approved by the Ethics Committee. All study subjects signed informed written consent forms.
As shown in the flowchart (Figure 1), from January 2013 to August 2021, 1222 patients with ACS and CKD diagnoses participated in the current study. Among them, 320 patients who did not undergo PCI were excluded. PCI was performed by experienced interventional physicians following standard techniques. Additionally, patients with missing crucial baseline data, loss of follow-up, severe infection, autoimmune disorders, severe liver insufficiency, thyroid dysfunction, hematology disorders, and malignant tumors were also excluded from this study. Finally, 744 patients in total received standard treatments and were followed up. According to the ideal cutoff value for SII, patients were divided into low SII (<1159.84×109/L, n = 399) and high SII (>1159.84×109/L, n = 345) groups.
 |
Figure 1 Flowchart of the study. ACS, acute coronary syndrome; CKD chronic kidney disease; PCI, percutaneous coronary intervention. |
Diagnosis of Clinical Diseases
The diagnosis of ACS, including STEMI and non-ST-segment elevation acute coronary syndrome (NSTE-ACS), was made following the American Heart Association (AHA) and the American College of Cardiology (ACC). STEMI was defined as persistent chest pain for more than 30 minutes, arriving at the hospital within 12 hours of the onset of symptoms, having a 12-lead electrocardiogram (ECG) with new left bundle branch block or ST-segment elevation >0.1 mV in 2 consecutive leads, and increased cardiac markers (creatine kinase-MB or troponin I).23 NSTEMI and UA were part of NSTE-ACS. NSTEMI was described as ischemic symptoms without ST-segment elevation on the ECG with raised cardiac markers. Newly developed/accelerated chest symptoms on exertion or resting angina within 2 weeks without the release of biomarkers were considered to be UA.24 Utilizing the estimated glomerular filtration rate (eGFR), renal function was evaluated. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration algorithm from the creatinine level (CKD-EPI). Based on the definition of CKD, an eGFR <60 mL/min/1.73 m2 was considered to indicate CKD. This formula was used to determine SII: total peripheral platelet count (P) × neutrophil-to-lymphocyte ratio (N/L) (SII = P × N/L ratio).15 Systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg during the baseline hospitalization or known hypertension with antihypertensive medication were considered to be signs of hypertension. Diabetic mellitus (DM) was identified by fasting plasma glucose (FPG) ≥7.0 mmol/L (126 mg/dL), hemoglobin A1c (HbA1c) levels ≥6.5%, 2-hour blood glucose of oral glucose tolerance test ≥11.1 mmol/L (200 mg/dL), or prior DM diagnosis treated with oral hypoglycemic drugs or insulin treatment. Patients with fasting serum triglyceride (TG) ≥1.7 mmol/L, total cholesterol (TC) ≥5.2 mmol/L, low-density lipoprotein cholesterol (LDL-C) ≥3.4 mmol/L, high-density lipoprotein cholesterol (HDL-C) <1.0 mmol/L, and/or receiving lipid-lowering drugs were considered to have dyslipidemia.
Data Collection
Based on outpatient follow-up outcomes, all clinical data were gathered from the electronic medical record system. The demographic data of the patients included age, sex, hypertension, diabetes mellitus, dyslipidemia, smoking status, previous MI, and revascularization (PCI or coronary artery bypass grafting [CABG]). The clinical information comprised the primary diagnosis at admission, physical, laboratory examination, imaging data, and the medication regimen at discharge.
Follow-Up and Clinical Outcome Definitions
These patients received follow-up care by telephone and/or in the outpatient clinic, which was implemented by competent medical professionals or nurses. The primary endpoint was MACEs, which are a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The secondary endpoints included all-cause death, cardiovascular death, nonfatal myocardial infarction, congestive heart failure, nonfatal stroke, and revascularization (PCI).
Statistical Analysis
The statistics of continuous variables were expressed as the mean ± standard deviation (SD) or as the median (25th-75th percentile). Each categorical variable is shown as frequencies and percentages. Student’s t-test or the Mann–Whitney U-test was used to compare continuous variables between groups. Fisher’s exact test or the chi-square test was performed to identify significant differences in categorical variables between the two groups. The primary and secondary clinical outcomes were expressed as total percentages and presented as proportions with 95% confidence intervals (CIs). In the Kaplan‒Meier (KM) survival analysis, binary recursive partitioning analysis (BRPA) was used to identify the most discriminatory cutoff value of high vs low SII for the composite endpoint (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke). The prognostic differences and event-free survival rates of patients in different SII groups were analyzed using Kaplan‒Meier survival analysis, and significance was determined using the Log rank test. Univariate and multivariate Cox regression analyses were used to estimate the hazard ratios (HRs) of the combined and individual endpoints with 95% confidence intervals (CIs) after adjusting for individual risk factors. Baseline clinical factors with significant differences (p < 0.05) between the two groups in the univariate analysis were included in the multivariate analysis. The multivariable Cox analysis included SII, age, diabetes mellitus, dyslipidemia, multivessel lesions, LVEF, CRP, albumin, glucose, creatinine, eGFR, ACEI/ARBs, β blocker, diuretics, statins, NLR and PLR, due to their statistical significance in univariate analysis. To evaluate whether the accuracy of the prediction of adverse cardiovascular events would improve with the addition of the SII to a basic model with known risk factors (ie, age, sex, hypertension, diabetes, dyslipidemia, and current smoker), the C-index, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated. A 2-tailed P value < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS software version 26.0.
Results
Baseline Characteristics
Among the 1222 patients screened, 744 patients (mean age 71.9±10.7 years, 74.6% male) who were diagnosed with ACS and CKD were retrospectively enrolled in the current study. The optimal cutoff value for SII was determined by analyzing MACEs, including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. As shown in Figure 2, SII had the best predictive power for MACEs compared with NLR and PLR. The ROC analysis evaluated the SII cutoff point (1159.84×109/L) that was predictive of MACEs (Figure 2). We split the study population into two groups based on the SII cutoff value. Table 1 shows the baseline characteristics of patients grouped by SII. Compared with the low SII group, patients with a higher SII were older with a higher prevalence of cardiogenic shock, STEMI, and no-reflow phenomenon after PCI. The patients in the higher SII group had a significantly higher neutrophil count, platelet count, and CRP but a lower lymphocyte count, albumin, and left ventricular ejection fraction. In addition, the proportion of patients treated with statins, ACEIs/ARBs, and β-blockers was also lower in the high SII group.
 |
Table 1 Baseline Characteristics of Patients Classified by Systemic Immune-Inflammation Index Level |
Clinical Outcomes in Different Groups Stratified by SII
The clinical outcomes of the patients in the low SII group and high SII group are shown in Table 2. During a median follow-up of 2.5 years, there were 107 cardiovascular deaths, 55 nonfatal myocardial infarctions, 84 congestive heart failures, 30 nonfatal strokes, and 101 patients who underwent coronary revascularization (Table 2). Patients in the high SII group had a higher prevalence of MACEs than those in the low SII group [134 (38.8%) vs 51 (12.8%), p < 0.001]. Patients in the high SII group had a greater prevalence of all-cause death, cardiovascular death, and congestive heart failure than those in the low SII group. Although it seemed that the low-SII group had a higher incidence of future revascularization, there did not appear to be a significant difference between the two groups. The K-M survival curve with the Log rank test also showed that the higher-SII group was substantially related to a greater risk of MACEs (Figure 3A), all-cause death (Figure 3B), cardiovascular death (Figure 3C), nonfatal myocardial infarction (Figure 3D), and congestive heart failure (Figure 3E). These findings suggest that a greater SII was related to an increased risk for cardiovascular events.
 |
Table 2 Clinical Outcome in Patients According to SII Score |
 |
Figure 3 Kaplan-Meier survival curve analysis showing (A) MACEs, (B) all-caused death, (C) cardiovascular death, (D) nonfatal MI, and (E) congestive heart failure. |
Subgroup and Multivariate Analysis of Clinical Outcomes in ACS with CKD Patients
We conducted univariate and multivariate Cox regression analyses to identify the independent indicators of the presence of MACEs and all-cause death in ACS with CKD patients (Table 3 and Table 4). According to the Cox regression analysis, SII ≥1159.84×109/L (HR:1.865; 95% CI: 1.197–2.907; p = 0.006), was an independent predictor related to the occurrence of MACEs. The analysis also revealed that the associations of SII (HR: 7.709; 95% CI: 5.083–11.693; p < 0.001) with all-cause death remained significant in study subjects. The Cox proportional hazard regression model analysis performed in three separate models to detect independent predictors of clinical outcomes is presented in Table 5. The findings demonstrated that a high SII was independently associated with an increased risk of MACEs (HR: 2.742; 95% CI: 1.943–3.871; p < 0.001), all-cause death (HR: 4.889; 95% CI: 3.165–7.550; p < 0.001), cardiovascular death (HR: 4.690; 95% CI: 2.548–8.633; p < 0.001), nonfatal myocardial infarction (HR: 2.715; 95% CI: 1.526–4.829; p = 0.001), and congestive heart failure (HR: 2.175; 95% CI: 1.368–3.459; p = 0.001) after adjusting for age, sex, smoking, history of hypertension, diabetes, dyslipidemia, myocardial infarction, left ventricular ejection fraction, CRP, β-blockers use and statin use. In the subgroup analysis, we determined whether the incidence of MACEs was affected by other covariates in various subgroups (Figure 4). There was a significant interaction between SII and hypertension (p = 0.011). However, there was no discernible difference in hypertension between the two groups. The MACEs in the high SII group were consistent among subgroups. The subgroup analysis further identified the robustness of the association between a high SII and major adverse cardiovascular events.
 |
Table 3 The Univariate and Multivariate Cox Regression Analysis Estimating the Influence of the SII on MACEs in Patients |
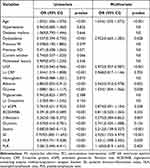 |
Table 4 The Univariate and Multivariate Cox Regression Analysis Estimating the Influence of the SII on All-Cause Death in Patients |
 |
Table 5 The Association of High SII (≥1159.84×109/L) and Future Adverse Events in Patients |
 |
Figure 4 Subgroup analysis of the predictive value of high SII vs low SII for MACEs in ACS and CKD patients. |
The Additional Prediction of Adverse Cardiovascular Events After Adding SII to the Baseline Model
Adding the SII to a fundament model with conventional risk factors enhanced the predictive value of MACEs (p < 0.001), cardiovascular death (p < 0.001), congestive heart failure (p = 0.006), and revascularization (p = 0.0261), as displayed by the significant increase in the C-index (Table 6). Additionally, reclassification with the addition of SII also improved integrated discrimination improvement (IDI) of 0.269 (p < 0.001), with a 3.5% improvement in net reclassification analysis (NRI) (p < 0.001) in MACEs, and cardiovascular death (NRI: 0.039, P < 0.001; IDI: 0.310, P < 0.001), suggesting that the addition of SII could provide significantly better prediction than conventional risk factors in ACS with CKD patients (Table 6).
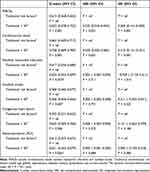 |
Table 6 Evaluation of Predictive Models for Cardiovascular Events |
Discussion
To the best of our knowledge, this is the first study to investigate whether the inflammatory biomarker SII functions as an independent indicator of poor clinical prognosis in ACS with CKD patients. Importantly, a high SII remained independently associated with MACEs, all-cause death, cardiovascular death, nonfatal myocardial infarction, and congestive heart failure in patients with ACS and CKD even after controlling for age and cardiovascular risk factors. In addition, we have demonstrated that adding SII to the model with existing conventional risk factors can significantly improve the risk stratification of MACEs, and cardiac death in patients with ACS and CKD. Accordingly, SII may be used as a reliable and convenient indicator for better identification of high-risk patients with ACS and CKD.
Patients with CKD have a higher cardiovascular burden than those without renal dysfunction.25,26 Chronic kidney disease is frequently encountered in patients suffering from acute coronary syndrome.27 In addition, patients with ACS and CKD are more likely to experience adverse cardiovascular events.28 However, research has demonstrated that 75% of the published trials for coronary artery disease excluded patients with CKD from participation.29 According to the research findings, CKD is recognized as an independent predictor of outcomes in patients with ACS.2,30 Patients suffering from chronic kidney disease exhibit elevated levels of inflammatory biomarkers.31 In patients with CKD, elevated levels of inflammatory biomarkers are linked to atherosclerotic vascular disease incidents and mortality.7 Various inflammatory cells are essential for the initiation and development of the atherosclerotic process.32 The rupture of coronary atherosclerotic plaques and the formation of thrombi result in a blood flow blockade in an infarct-related artery area and are closely associated with acute coronary syndrome.33 Inflammation is crucial to the initiation and progression of this process as well as its poor consequences.34 Additionally, innate immunity and adaptive immunity are essential for atherosclerotic plaque rupture.35
Leukocyte are an independent predictor of inflammation and immune responses.36 Neutrophils are the most abundant leukocyte subtype in circulating blood and have been considered essential in the inflammatory response.37,38 Neutrophils secrete inflammatory cytokines, which may cause endothelial dysfunction and degeneration of the vascular walls.39 The neutrophil-to-lymphocyte ratio (NLR) has been gradually considered a reliable marker for systemic inflammation and immune responses. A meta-analysis showed that NLR is associated with poor survival in various solid tumors.40 In recent years, NLR has attracted attention because of its close association with adverse cardiovascular disease outcomes. Many previous studies suggested that a higher NLR level was associated with cardiovascular disease and a predictor for adverse cardiovascular events.13,41–48 Platelets play an essential role in the pathophysiology of atherosclerosis and the development of acute thrombotic events.49,50 A greater baseline platelet count is a significant and independent predictor for poor prognostic in AMI patients.51 Moreover, the platelet-to-lymphocyte ratio (PLR) is a powerful prognosis indicator of poor outcomes in cardiovascular diseases.14,52
Recently, the systemic immune-inflammation index was developed as a new indicator of systemic inflammation based on lymphocyte, neutrophil, and platelet counts. Based on the previous theory, SII could provide a more balanced and comprehensive reflection of humans’ immunological and inflammatory responses.53 Increased SII values have been associated with tumor progression and poor survival outcomes in different types of malignancies.15,16,54 These findings further promote the exploration of the association of SII in populations with cardiovascular disease. Importantly, increasing research has been conducted to demonstrate the predictive significance of SII in CAD. A prior study found that SII can detect the occurrence of coronary artery disease with unique sensitivity, specificity, and predictive values when compared to NLR, PLR, and C-reactive protein (CRP).55 Additionally, SII was considered a better predictor for the severity of coronary artery lesions than ratios such as NLR and PLR.56 A similar pattern of results was obtained in our study. Lütfi et al19 reported the efficacy of SII in predicting in-hospital and long-term mortality in STEMI patients. Kerim et al57 found that the SII level was related to the no-reflow phenomenon (NRP) in STEMI patients undergoing primary PCI. Saban et al58 claimed that the increase in SII score was independently correlated with CIN formation in NSTEMI patients undergoing PCI.
In our current study, we evaluated the adverse outcome impact of SII in ACS with CKD patients. Our study found that SII was an independent risk indicator for adverse events in patients with ACS and CKD, and the increased risk of MACEs, all-cause death, cardiovascular death, nonfatal myocardial infarction, and congestive heart failure in patients with high SII was significantly higher than in patients with low SII after considering cardiovascular risk factors. In this study, we added SII to the clinical model, which enhanced the predictability for MACEs and cardiovascular death in patients with ACS and CKD (as evaluated by OCR curves) and reclassified subjects into different risk categories through the IDI and the NRI. This finding demonstrated that elevated SII is an independent predictor of adverse events and enhances the predictive ability of adverse events in patients with ACS and CKD.
Limitations
Several limitations need to be acknowledged and addressed in this study. First, this is a retrospective and observational study in a single center and therefore may be subject to selection bias. Moreover, the sample size was relatively modest, and the follow-up period was brief, limiting the drawing of broad conclusions. Second, we only evaluated the baseline SII level; we did not examine how dynamic variations in SII levels affected cardiovascular events over the course of the follow-up period. Last, because this was an observational and retrospective study, no causal link could be established beyond association. Therefore, further studies with large sample sizes and long-term follow-up will be required to confirm these conclusions.
Conclusions
In conclusion, our study suggests that among ACS with CKD patients, compared to low SII patients, those with high SII have higher odds of MACEs, all-cause death, and cardiovascular death. An elevated SII level may be an independent predictor of adverse cardiovascular outcomes in ACS with CKD patients. Importantly, exploring anti-inflammatory pathways as potential therapeutic targets is warranted, and SII as an available and low-cost indicator may offer new therapeutic perspectives.
Acknowledgments
We gratefully acknowledge the assistance of the investigators of the First Affiliated Hospital of Wenzhou Medical University and the participant’s support.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Fox CS, Muntner P, Chen AY, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the national cardiovascular data acute coronary treatment and intervention outcomes network registry. Circulation. 2010;121(3):357–365. doi:10.1161/CIRCULATIONAHA.109.865352
2. Jankowski J, Floege J, Fliser D, Bohm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi:10.1161/CIRCULATIONAHA.120.050686
3. Tanik VO, Cinar T, Arugaslan E, et al. The predictive value of PRECISE-DAPT score for in-hospital mortality in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2019;70(5):440–447. doi:10.1177/0003319718807057
4. Hayiroglu MI, Keskin M, Uzun AO, et al. Predictive value of SYNTAX score II for clinical outcomes in cardiogenic shock underwent primary percutaneous coronary intervention; a pilot study. Int J Cardiovasc Imaging. 2018;34(3):329–336. doi:10.1007/s10554-017-1241-9
5. Cinar T, Hayiroglu MI, Seker M, et al. The predictive value of age, creatinine, ejection fraction score for in-hospital mortality in patients with cardiogenic shock. Coron Artery Dis. 2019;30(8):569–574. doi:10.1097/MCA.0000000000000776
6. Weissberg PL, Bennett MR. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(24):1928–1929.
7. Amdur RL, Feldman HI, Dominic EA, et al. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings from the CRIC study. Am J Kidney Dis. 2019;73(3):344–353. doi:10.1053/j.ajkd.2018.09.012
8. Ebert T, Pawelzik SC, Witasp A, et al. Inflammation and premature ageing in chronic kidney disease. Toxins. 2020;12:4. doi:10.3390/toxins12040227
9. Ridker PM, MacFadyen JG, Glynn RJ, et al. Inhibition of interleukin-1beta by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71(21):2405–2414. doi:10.1016/j.jacc.2018.03.490
10. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
11. Oh TJ, Ahn CH, Kim BR, et al. Circulating sortilin level as a potential biomarker for coronary atherosclerosis and diabetes mellitus. Cardiovasc Diabetol. 2017;16(1):92. doi:10.1186/s12933-017-0568-9
12. Yu P, Zhao J, Jiang H, et al. Neural cell adhesion molecule-1 may be a new biomarker of coronary artery disease. Int J Cardiol. 2018;257:238–242. doi:10.1016/j.ijcard.2017.12.040
13. Adamstein NH, MacFadyen JG, Rose LM, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42(9):896–903. doi:10.1093/eurheartj/ehaa1034
14. Kurtul A, Murat SN, Yarlioglues M, et al. Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol. 2014;114(7):972–978. doi:10.1016/j.amjcard.2014.07.005
15. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
16. Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–3302. doi:10.7150/jca.25691
17. Zhang Y, Lin S, Yang X, Wang R, Luo L. Prognostic value of pretreatment systemic immune-inflammation index in patients with gastrointestinal cancers. J Cell Physiol. 2019;234(5):5555–5563. doi:10.1002/jcp.27373
18. Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230. doi:10.1111/eci.13230
19. Öcal L, Keskin M, Cerşit S, et al. Systemic immune-inflammation index predicts in-hospital and long-term outcomes in patients with ST-segment elevation myocardial infarction. Coron Artery Dis. 2022;33(4):251–260. doi:10.1097/MCA.0000000000001117
20. Silverberg D, Wexler D, Blum M, Schwartz D, Iaina A. The association between congestive heart failure and chronic renal disease. Curr Opin Nephrol Hypertens. 2004;13(2):163–170. doi:10.1097/00041552-200403000-00004
21. Hayiroglu MI, Cinar T, Cinier G, et al. Evaluating systemic immune-inflammation index in patients with implantable cardioverter defibrillator for heart failure with reduced ejection fraction. Pacing Clin Electrophysiol. 2022;45(2):188–195. doi:10.1111/pace.14436
22. Erdogan M, Erdol MA, Ozturk S, Durmaz T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark Med. 2020;14(16):1553–1561. doi:10.2217/bmm-2020-0274
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;61(4):e78–e140. doi:10.1016/j.jacc.2012.11.019
24. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;64(24). e139-e228. doi:10.1016/j.jacc.2014.09.017
25. Hausenloy DJ, Chilian W, Crea F, et al. The coronary circulation in acute myocardial ischaemia/reperfusion injury: a target for cardioprotection. Cardiovasc Res. 2019;115(7):1143–1155. doi:10.1093/cvr/cvy286
26. Li Q, Chen Y, Yu L, et al. The relationship between lipoprotein(a) and cardiovascular events in acute coronary syndrome patients with and without chronic kidney disease. Atherosclerosis. 2022;349:204–210. doi:10.1016/j.atherosclerosis.2022.04.007
27. Washam JB, Herzog CA, Beitelshees AL, et al. Pharmacotherapy in chronic kidney disease patients presenting with acute coronary syndrome: a scientific statement from the American heart association. Circulation. 2015;131(12):1123–1149. doi:10.1161/CIR.0000000000000183
28. Santopinto JJ, Fox KA, Goldberg RJ, et al. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the global registry of acute coronary events (GRACE). Heart. 2003;89(9):1003–1008. doi:10.1136/heart.89.9.1003
29. Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int. 2006;70(11):2021–2030. doi:10.1038/sj.ki.5001934
30. Best PJ, Lennon R, Ting HH, et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39(7):1113–1119. doi:10.1016/S0735-1097(02)01745-X
31. Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7(12):1938–1946. doi:10.2215/CJN.03500412
32. Medina-Leyte DJ, Zepeda-Garcia O, Dominguez-Perez M, Gonzalez-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci. 2021;22(8):3850. doi:10.3390/ijms22083850
33. Kaufmanova J, Stikarova J, Hlavackova A, et al. Fibrin clot formation under oxidative stress conditions. Antioxidants. 2021;10:6. doi:10.3390/antiox10060923
34. Fracassi F, Crea F, Sugiyama T, et al. Healed culprit plaques in patients with acute coronary syndromes. J Am Coll Cardiol. 2019;73(18):2253–2263. doi:10.1016/j.jacc.2018.10.093
35. Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol. 2014;9:73–102. doi:10.1146/annurev-pathol-020712-163936
36. Meyer-Lindemann U, Mauersberger C, Schmidt AC, et al. Colchicine impacts leukocyte trafficking in atherosclerosis and reduces vascular inflammation. Front Immunol. 2022;13:898690.
37. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi:10.1038/nri3024
38. Van Avondt K, Maegdefessel L, Soehnlein O. Therapeutic targeting of neutrophil extracellular traps in atherogenic inflammation. Thromb Haemost. 2019;119(4):542–552. doi:10.1055/s-0039-1678664
39. Ikeda U, Ikeda M, Oohara T, Kano S, Yaginuma T. Mitogenic action of interleukin-1 alpha on vascular smooth muscle cells mediated by PDGF. Atherosclerosis. 1990;84(2–3):183–188. doi:10.1016/0021-9150(90)90089-2
40. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:10.1093/jnci/dju124
41. Kim S, Eliot M, Koestler DC, Wu WC, Kelsey KT. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson heart study and modification by the Duffy antigen variant. JAMA Cardiology. 2018;3(6):455–462. doi:10.1001/jamacardio.2018.1042
42. Guo TM, Cheng B, Ke L, et al. Prognostic value of neutrophil to lymphocyte ratio for in-hospital mortality in elderly patients with acute myocardial infarction. Curr Med Sci. 2018;38(2):354–359. doi:10.1007/s11596-018-1887-0
43. Li X, Ji Y, Kang J, Fang N. Association between blood neutrophil-to-lymphocyte ratio and severity of coronary artery disease: evidence from 17 observational studies involving 7017 cases. Medicine. 2018;97(39):e12432. doi:10.1097/MD.0000000000012432
44. Park JS, Seo KW, Choi BJ, et al. Importance of prognostic value of neutrophil to lymphocyte ratio in patients with ST-elevation myocardial infarction. Medicine. 2018;97(48):e13471. doi:10.1097/MD.0000000000013471
45. Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102(6):653–657. doi:10.1016/j.amjcard.2008.05.006
46. Zhang S, Diao J, Qi C, et al. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. BMC Cardiovasc Disord. 2018;18(1):75. doi:10.1186/s12872-018-0812-6
47. Arbel Y, Shacham Y, Ziv-Baran T, et al. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long-term all-cause mortality in ST-elevation myocardial infarction patients. Can J Cardiol. 2014;30(10):1177–1182. doi:10.1016/j.cjca.2014.05.010
48. Zhao Y, Zhang S, Yi Y, et al. Neutrophil-to-lymphocyte ratio as a predictor for cardiovascular diseases: a cohort study in Tianjin, China. J Hum Hypertens. 2022. doi:10.1038/s41371-022-00724-7
49. Schrottmaier WC, Mussbacher M, Salzmann M, Assinger A. Platelet-leukocyte interplay during vascular disease. Atherosclerosis. 2020;307:109–120. doi:10.1016/j.atherosclerosis.2020.04.018
50. Siegel-Axel D, Daub K, Seizer P, Lindemann S, Gawaz M. Platelet lipoprotein interplay: trigger of foam cell formation and driver of atherosclerosis. Cardiovasc Res. 2008;78(1):8–17. doi:10.1093/cvr/cvn015
51. Nikolsky E, Grines CL, Cox DA, et al. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the Cadillac trial). Am J Cardiol. 2007;99(8):1055–1061. doi:10.1016/j.amjcard.2006.11.066
52. Kurtul A, Yarlioglues M, Murat SN, et al. Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Am J Cardiol. 2014;114(3):342–347. doi:10.1016/j.amjcard.2014.04.045
53. Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8(1):10566. doi:10.1038/s41598-018-28646-w
54. Ji Y, Wang H. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: a meta-analysis. World J Surg Oncol. 2020;18(1):197. doi:10.1186/s12957-020-01974-w
55. Liu Y, Ye T, Chen L, et al. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron Artery Dis. 2021;32(8):715–720. doi:10.1097/MCA.0000000000001037
56. Candemir M, Kiziltunc E, Nurkoc S, Sahinarslan A. Relationship Between Systemic Immune-Inflammation Index (SII) and the Severity of Stable Coronary Artery Disease. Angiology. 2021;72(6):575–581. doi:10.1177/0003319720987743
57. Esenboga K, Kurtul A, Yamanturk YY, Tan TS, Tutar DE. Systemic immune-inflammation index predicts no-reflow phenomenon after primary percutaneous coronary intervention. Acta Cardiol. 2022;77(1):59–65. doi:10.1080/00015385.2021.1884786
58. Kelesoglu S, Yilmaz Y, Elcik D, et al. Systemic immune inflammation index: a novel predictor of contrast-induced nephropathy in patients with non-ST segment elevation myocardial infarction. Angiology. 2021;72(9):889–895. doi:10.1177/00033197211007738
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

