Back to Journals » Journal of Pain Research » Volume 16
Global Trends and Hotspots on Microglia Associated with Pain from 2002 to 2022: A Bibliometric Analysis
Authors Dong G , Li H, Gao H , Chen Y, Yang H
Received 11 April 2023
Accepted for publication 28 July 2023
Published 15 August 2023 Volume 2023:16 Pages 2817—2834
DOI https://doi.org/10.2147/JPR.S413028
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Guoqi Dong, Hui Li, Hui Gao, Yingqi Chen, Huayuan Yang
School of Acupuncture-Moxibustion and Tuina, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China
Correspondence: Huayuan Yang, School of Acupuncture-Moxibustion and Tuina, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China, Email [email protected]
Background: Researchers have made significant progress in microglia associated with pain in recent years. However, more relevant bibliometric analyses are still needed on trends and directions in this field. The aim of this study is to provide a comprehensive perspective and to predict future directions of pain-related microglia research via bibliometric tools.
Methods: English articles and reviews related with pain and microglia were extracted from the Web of Science core collection (WosCC) database between 2002 to 2022. Bibliometric tools such as VOSviewer, CiteSpace, and Bibliometrix R package were used to analyze publication characteristics, countries, authors, institutions, journals, research hotspots, and trend topics.
Results: A total of 2761 articles were included in this analysis. Research on microglia associated with pain has increased significantly over the last two decades. China (n = 1020, 36.94%) and the United States (n = 751, 27.20%) contributed the most in terms of publications and citations, respectively. Kyushu University published the most articles in this field compared to other institutions, and Professor Inoue Kazuhide (n = 54) at this university made outstanding contributions in this field. Molecular Pain (n = 113) was the journal with the most publication, while Journal of Neuroscience had the highest number of citations. According to the authors keywords analysis, the research in this area can be summarized into 7 clusters such as “microglia activation pathways”, “pain treatment research”, “mental symptoms of chronic pain”, and so on.
Conclusion: This study provides a comprehensive analysis of pain-related microglia research in the past two decades. We identified the countries, institutions, scholars, and journals with the highest number of publications and the most influence in the field, and the research trends identified in this paper may provide new insights for future research.
Keywords: microglia, pain, bibliometrics, VOSviewer, CiteSpace
Introduction
Pain, defined as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” by the International Association for the Study of Pain (IASP),1 has imposed a significant burden on individual health and economy. Research has revealed that chronic pain is prevalent at a rate of 11% to 40% in the United States.2 In recent years, there has been an increasing number of basic and clinical research on specific diagnostic biomarkers and effective treatments for pain. However, chronic pain still has complex etiology and complicated mechanisms.
Microglia, one of the subtypes of glia, are distributed in the brain and spinal cord as resident macrophages and participate in various pathological pain, such as neuropathic pain, inflammatory pain, and cancer-induced pain. Microglia might be served as a diagnostic biomarker or a therapeutic target for pain. On the one hand, spinal cord microglia can increase calcitonin gene-related peptide (CGRP) terminals to modify synaptic plasticity.3 On the other hand, the extracellular matrix structure (perineural network) will be degraded by microglia during nerve injury, thus enhancing the neuronal projection activity and inducing pain behavior.4 In inflammatory pain, noxious stimulation leads to spinal microglia proliferation and migration. The cytokines, excitatory amino acids, and neuropeptides released by microglia enhance spinal hyperexcitability and promote pain induction.5 Although the research on microglia is increasing, the majority of previous studies on the role of microglia in pain have focused solely on a critical aspect, lacking a comprehensive analysis of the field. Although some reviews provided an overview of the research trends in the field,6,7 there is still a need for quantitative results. Therefore, it is crucial to conduct a bibliometric study on the function of microglia in relation to pain. In this study, we conducted a bibliometric analysis of microglia research related to pain to identify the current research hotspots and predict trends in this field to provide valuable insights for basic research and develop potential therapeutic strategies for pain management in the clinical study.
Materials and Methods
Data Collection and Cleaning
All information and data from the articles were retrieved from WosCC, including the Science Citation Index-expanded (SCI-E) and the Social Sciences Citation Index (SSCI). The searched items are shown in Supplementary Table 1. The following criteria were applied to screen the literature: (1) Topics for microglia associated with pain as illustrated in the search strategy; (2) Literature publication time was set from January 1, 2002, to December 31, 2022; (3) Only original studies and reviews were included, either clinical or experimental research; (4) The literature language was limited to English. Two reviewers (Hui Gao and Hui Li) conducted data retrieval and collection based on the search strategy and inclusion criteria, and a third senior reviewer (Huayuan Yang) was consulted to resolve any discrepancies in record selection. A total of 2795 pieces of articles were retrieved, and 34 irrelevant articles were excluded, including early access (13), proceeding paper (12), book chapters (5), retracted publication (3), and data paper (1). Ultimately, 2761 articles were saved as plain text files, along with the full record and cited references extracted from the record content. To make our result more convincing, we applied the thesaurus file to combine synonyms with duplicate information into one element and removed misspelled items. After the data was cleansed, we imported it into the bibliometric software, such as VOSviewer and CiteSpace, for subsequent analysis. The overall flow of this study is summarized in Figure 1.
 |
Figure 1 Flow chart of data retrieval and analysis in this study. |
Analytical Method and Tools
All detailed data were initially exported via the “Citation Report” function in WosCC. The information included the number of publications and citations, publication year, countries, H-index, institutions, journals, authors, references, and author keywords.
Microsoft Excel 365 was applied to generate trend diagrams of annual publications and citations and add the linear regression curve. Through the co-occurrence analysis function of VOSviewer (version 1.6.18.0), it is possible to visualize the cooperation between countries, authors, and institutions and identify the frontiers of a discipline. Pajek (version 5.16) was utilized as an auxiliary tool to adjust the map generated by VOSviewer. Scimago Graphica (version 1.0.26) is a chart-making software showing national publications, regional distribution, and cooperation strength. CiteSpace (version 6.1.R6) was used to analyze co-cited references, including cluster, timeline view, and the reference burst. In general, the cluster is considered highly credible if the silhouette value of a network exceeds 0.7.8 While the modularity value is greater than 0.3, cluster structure is considered significant. The parameters of CiteSpace were configured according to the following levels: 1) time span (from 2002 to 2022); 2) year per slice (1); 3) term source (title, abstract, author keywords); 4) selection criteria (g-index, k=25); 5) pruning: selecting Pathfinder and Pruning sliced networks. Finally, the trend topic map was generated via the Bibliometrix R package (version 4.0.1) in R tool based on high-frequency author keywords. R-Bibliometrix was configured according to the following parameters: 1) words minimum frequency = 15; 2) number of words per year = 5.
Results
Publication Characteristics
Annual publications can reveal the general trend of research. Over the past 20 years, the number of publications (NP) has grown from 7 (2002) to 256 (2022); meanwhile, the number of citations (NC) increased from 3 (2002) to 13,605 (2022), and the average citation per paper (ACPP) increased from 0.43 (2002) to 53.14 (2022). This indicates that scholars are increasingly focusing their attention on this area. As shown in Figure 2, the regression analysis of the number of publications shows that y = 14.22x – 28,488.57 and the correlation coefficient R2 = 0.97, and the predicted publications throughout the year 2023 will be 278, indicating more attention to pain-related microglia research is expected in the coming years. Meanwhile, the regression analysis of the number of citations shows that y = 739.62x – 1,482,701.70 and the correlation coefficient R2 = 0.94. In 2023, 13,550 citations can be forecasted.
 |
Figure 2 The trend of annual publications and citations over the past 20 years. |
Analysis of Countries
From 2002 to 2022, 69 countries contributed to the research on microglia associated with pain by publishing academic articles in WosCC. As shown in Table 1, the country with the most publications was China (1020, 36.94%), followed by the United States (751, 27.20%), Japan (337, 12.21%), Canada (165, 5.98%), and the United Kingdom (147, 5.32%). The United States ranked first in the number of citations (51,613), followed by China (22,427), Japan (16,021), Canada (13,901), and the United Kingdom (10,817).
 |
Table 1 The Top 10 Prolific Countries in Research on Microglia in Pain |
The annual trends of the top five countries in publications are shown in Figure 3. From the starting point of the trend, the United States participated in this field earlier than other countries. The United States maintained first in annual publications before 2013, and China overtook the United States for the first time in 2014. The trend of annual publication in Japan, Canada, and the United Kingdom remained relatively low and constant compared with the United States and China.
 |
Figure 3 The annual trends in 5 prolific countries. |
In order to explore the collaborative relationship, a global cooperation network was generated by SCImago Graphica (Figure 4). In the network, node size represents a total number of publications of a country, and the connection means the cooperation relationship. Figure 4A shows that 37 countries were divided into 7 clusters of different colors. The United States had the strongest connection strength and established cooperative relations with 33 countries (Figure 4B). Moreover, according to the total link strength (Table 1), East Asian countries (China, Japan, and South Korea), European countries (such as the United Kingdom, Germany, and Italy), and North American countries (the United States and Canada) were essential components of the cooperation network.
 |
Figure 4 Collaboration visualization between countries. (A) The international collaboration of countries. (B) The co-authorship map of countries. |
Analysis of Institutions
Over 2000 institutions were involved in the research on microglia associated with pain. Table 2 shows the top 10 institutions by the number of publications. Kyushu University contributed the most articles (79), followed by Shanghai Jiao Tong University (74), the University of California System (74), the University of London (72), and the University of Toronto (64). Therefore, these universities were influential institutions in this field. Research articles from Harvard University were the most cited (10,533), followed by the University of Toronto (8642), Kyushu University (8098), the University of London (6481), and the University of California System (4309). Figure 5 shows the collaborative network of different institutions. Harvard University, the University of Toronto, the University of California System, Sun Yat-Sen University, and Kyushu University were the top five institutions with the highest link strength, indicating that these universities are superior to other institutions in terms of international academic cooperation.
 |
Table 2 The Top 10 Prolific Institutions in Research on Microglia in Pain |
 |
Figure 5 Collaborations network of institutions on microglia associated with pain. |
Analysis of Authors
Over 10,000 authors were involved in this area, of which 415 published more than five articles. In terms of publications, Inoue Kazuhide (54) ranked first (Table 3), followed by Tsuda Makoto (50), Ji Ru-Rong (39), Watkins Linda R (31), and Wang Yong-Xiang (29). Figure 6A exhibits the network between authors, which shows that authors from the same country collaborated closely while those from different countries collaborated relatively less. Co-cited authors refer to those (at least two) who are cited at the same time. Of the 52,320 co-cited authors, 121 had more than 100 citations, and the network is shown in Figure 6B. It is worth noting that Tsuda Makoto, Ji Ru-Rong, Watkins Linda R, and Inoue Kazuhide ranked in the top ten in the number of citations. The same is true in the number of publications, indicating that these four authors had a great influence in this area.
 |
Table 3 The Top 10 Productive Authors and Co-Cited Authors |
 |
Figure 6 Authors contributing to the research on microglia associated with pain. (A) Author collaboration network. (B) Bibliometric coupling of co-cited authors based on VOSviewer. |
Journal Distribution
Research articles on pain-related microglia have been published in over 500 journals. The number of publications in the ten most productive journals accounts for 27.09% of the total publications (748 out of 2761), as presented in Table 4. Our results show that Molecular Pain (113 publications), Journal Of Neuroinflammation (103 publications) and Pain (103 publications) were the top three most published journals. Besides, Journal of Neuroscience (9868 citations), Pain (6820 citations), and Brain Behavior and Immunity (4460 citations) were the most cited journals. Moreover, six prolific journals had an impact factor greater than five, and most journals belonged to the category of Neuroscience in WOS.
 |
Table 4 The Top 10 Prolific Journals in Research on Microglia in Pain |
Co-Cited Reference Analysis
Table 5 shows the top ten most cited references.9–18 The most cited reference was conducted by Chaplan et al9 with 491 citations, validating a quantitative allodynia assessment technique (up-down method). The reference from Tsuda et al10 showed the second highest citation (426), demonstrating that P2X4R activation only affects mechanical pain production after nerve injury but not in normal animals. The third-most cited reference (349), provided by Coull Jam et al11 illustrated that brain-derived neurotrophic factor (BDNF) is a significant mediator of communication between microglia and neurons and is considered to be an important target in the treatment of pain.
 |
Table 5 The 10 Most Cited References in the Cocitation Analysis |
The network diagram of co-cited references generated by CiteSpace (Figure 7A) shows that there are 17 specific clusters based on references with significant weighted mean silhouette score and modularity Q score (S = 0.8609 and Q = 0.7207): including “#0 chronic pain”, “#1 neuroinflammation”, “#2 interleukin 1”, “#3 morphine tolerance”, “#4 ATP”, and so on. Timeline analysis contributes to a better understanding of hot spots and innovation in this field. As shown in Figure 7B, the timeline analysis based on references reveals that “interleukin-1” and “interleukin-4” were in the early stage of the study, and “NLRP3” appeared in the latest phase of the study.
In addition, CiteSpace was also utilized to identify the top 25 references with the strongest citation burst.10–16,19–36 As shown in Figure 8, since 2006, the strongest citation burst came from the review of Inoue et al34 aiming to reveal the latest advance in the study of microglial mechanisms related to neuropathic pain. Moreover, The reference from Milligan et al14 had the second strongest citation burst. This review mainly summarized the dual effects of glia on pain and proposed new targets for drug treatment of pain according to the anti-inflammatory characteristics of glia.
 |
Figure 8 Top 25 references with the strongest citation bursts based on CiteSpace. The bold column indicates the year when the references began to be cited frequently. |
Analysis of Author Keywords
From 1990, literature from the WosCC database began to contain author keywords.37 As shown in Table 6, The most frequent author keywords were “microglia” (1052 times), “neuropathic pain” (715 times), pain (365 times), astrocyte (328 times), and spinal cord (272 times). The author keywords with higher centrality can be regarded as the core keywords and turning points.38 The top five author keywords in centrality were “neuropathic pain”, “spinal cord”, “chronic pain”, “spinal dorsal horn”, and “spinal cord injury” with the centrality of 0.47, 0.29, 0.17, 0.12, and 0.11, respectively.
 |
Table 6 Top 10 Author Keywords with the Highest Frequency and Centrality |
To summarize the research content and hot spots in this field, the co-occurrence and clustering analysis of author keywords were performed via VOSviewer. Of the 4237 author keywords, 94 appeared more than 15 times. Figure 9 shows the author keywords clustering network diagram. It can be seen that the 94 keywords are divided into seven different clusters as follows: cluster 1: microglia activation pathways (red), cluster 2: pain treatment research (green), cluster 3: mental symptoms of chronic pain (dark blue), cluster 4: central nervous system research (yellow), cluster 5: immune cells and cytokines research (purple), cluster 6: mechanism research (light blue), and cluster 7: peripheral nervous system research (orange).
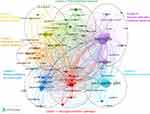 |
Figure 9 Network visualization map of author keywords co-occurrence analysis. |
The trend topics diagram was generated via Bibliometrix R packages based on the top 46 author keywords (Figure 10). The results showed that the duration of “fractalkine”, “ATP”, and “MAPK” were the most extended (10 years), followed by “interleukin-1”, “substance P”, “rat”, “purinergic signaling”, “spinal cord”, “GFAP”, “pain”, “astrocyte”, “glia”, “hyperalgesia”, and “central sensitization” (9 years). “NLRP3 inflammasome” began to appear in 2018; meanwhile, “autophagy”, “migraine”, and “HMGB1” began to appear in 2019. “NLRP3 inflammasome” and “HMGB1” had the highest frequency in 2021, while “migraine”, “autophagy”, “diabetic neuropathic pain”, “visceral pain”, and “anxiety” had the highest frequency in 2020.
 |
Figure 10 Trend topics of author keywords over time. |
Discussion
The role of microglia in pain is well-known due to the crucial efforts of relevant scholars. This study quantitatively analyzed the publications on microglia associated with pain from 2002 to 2022 via bibliometric analysis. Quantitative methods can provide the intellectual structure and help determine the future direction in this field.
The Trend Overview of the Development of Microglia in Pain
According to our analysis, annual publications and citations have increased significantly, indicating that microglia are receiving more and more attention in pain research.
Regarding country distribution, although China was the leading country in terms of the number of articles, its average citations per paper was lower than that of other countries. This result shows that Chinese scholars still need to make efforts on the quality of research. Of the ten institutions with the highest number of publications, five belong to China, two were from the United States and one each was from Japan, the United Kingdom and Canada. Of note, except for four institutions, the top fifty institutions with publications were all from the top ten countries, illustrating that the nation’s leading position in research is inseparable from experienced institutions.
Status and Quality of Authors, Journals, and Reference
According to the authors analysis, Inoue Kazuhide was the most prolific author. He and his colleagues (Tsuda Makoto, top2) contributed to investigating the role of purinergic receptors on microglia in the state of neuropathic pain.10,39–42 Ji, Ru-Rong was the third prolific writer dedicated to studying the role of MAP kinase in pain.16,26,43–45 Watkins Linda R. was the fourth prolific author. He worked on the effects of toll-like receptors on glia46–49 and opioid-induced pro-inflammatory actions.50–53 As the fifth prolific author, Wang Yongxiang was committed to studying the analgesic mechanism of aconitine and other herbal extracts and believed that the endogenous opioid peptides released by spinal cord microglia may participate in the analgesic process.54–58 The analysis of the author collaboration network shows that the collaborative relationship between authors was limited to a specific research team, institution, or country, which indicates that scholars need to strengthen academic exchanges and cooperation to promote the progress of this field.
Despite concerns, the impact factor remains the most common method for assessing the quality and impact of journals.59 Among the top ten journals, Brain Behavior And Immunity had the highest influence factor (19.227) and the third citation number (4460). The journal is not only devoted to neuroscience but also the research of psychology and psychiatry, indicating that more interdisciplinary scholars have begun to pay attention to the study in this area. We also note that the top ten journals belong to the United States, the United Kingdom, the Netherlands, and Switzerland, respectively. The reason may be that the above European and North American countries are the main forces of international cooperation,60 suggesting that Asian countries are supposed to strengthen the advancement of international journals61 and introduce more and better articles to promote the development of high-quality journals.62
Of the 83,045 cited references, 75 co-cited references were cited at least 100 times. The most co-cited reference was produced by Chaplan et al9 from Journal Of Neuroscience Methods in 1994. This article confirmed previous research63 on measuring mechanical pain behavior, and many other experimental studies have adopted it as the standard method of measurement. It is worth noting that seven of the top ten references with the strongest citation burst were reviews, and only three were original studies. The first original study applied different models of pain to prove that microglia are necessary to mediate mechanical hypersensitivity in male mice rather than female mice.31 Another original study applied the spinal nerve ligation (SNL) model to demonstrate that inhibiting spinal microglia activation can control hypersensitivity progress but has no significant improvement on the formed hypersensitivity.12 The third original study, published in Nature, first clarified the role of the microglial P2X4 receptors as a key factor in neuropathic pain.64
Research Hotspots and Frontiers
The author keywords analysis can reflect the academic frontier of the discipline and help scholars grasp the development trend in this area. According to our study, microglia (1052, top1), neuropathic pain (715, top2), pain (365, top3), astrocyte (328, top4), and spinal cord (272, top5) were the five most frequent author keywords, indicating that interactions between microglia and astrocyte in the spinal cord play a crucial role in the pathogenesis of pain.65
The author keywords cluster analysis revealed seven different research directions.
Cluster 1 was mainly composed of author keywords of the microglia activation pathways. The primary keywords were microglia activation, TLR4, MAPK, NF-kappa B, opioids, and NLRP3 inflammasome. As “gatekeepers” of the immune system in humans and other animals,66 TLR4 is considered to be necessary for microglia activation and spinal sensitization,5 and participates in MAPK,67 NF-kappa B,68 and opioid receptor pathways69 to regulate neuroinflammation caused by microglia activation. The latest research70 showed that the activation of the TLR4/NF-κB/NLRP3 pathway might promote pyroptosis in neurons but also aggravate M1-type polarization of microglia, which provides a new direction for pain research.
Cluster 2 mainly focused on pain treatment research, such as minocycline, acupuncture, and cannabinoids. Minocycline is a second-generation semi-synthetic tetracycline that can act on the central nervous system through the blood-brain barrier.71 It has anti-apoptotic, antioxidant, and anti-inflammatory properties and is often used as a microglia activation inhibitor to relieve pain progression. However, a systematic review72 pointed out that more highly therapeutic clinical research is required to prove the analgesic effect of minocycline, which means the clinical researches about minocycline need to be more comprehensive. Acupuncture is an essential component of Traditional Chinese Medicine and is often applied to relieve pain. Meta-analysis showed that acupuncture could significantly inhibit the activity of microglia73 and can offer a similar microglial inhibition effect of minocycline.74 Recent research found that neuron GRK2 can specifically mediate the inhibitory effect of acupuncture on microglia activation and contribute to the analgesia of neuropathic pain75 and inflammatory pain.76
Cluster 3 contained mental symptoms of chronic pain, including depression and anxiety. Chronic pain can lead to depression and the hippocampus (HP) play a key role in this process.77 A previous study showed that the chronic constriction injury (CCI) rat model developed depression symptoms eight weeks later with microglial proliferation in the amygdala, medial prefrontal cortex (mPFC), and HP.78 The transcription level of TNF-α, CD11b, and other genes related to microglia activation or depression were also upregulated in these regions.78 Clinical research showed that [11C]PBR28 signal increased in the anterior cingulate cortex (ACC) and was positively correlated with Beck Depression Inventory (BDI) scores in chronic pain,79 indicating neuroinflammation is one of the mechanisms of depression and neuropathic pain.
Cluster 4 was mainly related to central nervous system research with a significant focus on purinergic receptors. As a result of inflammation, ATP will be released from damaged cells and act on P2 receptors.80 P2X4R is widely distributed in the microglia of the central nervous system and is a highly sensitive purinergic receptor, approximately 1000 times more than P2X7R.81 The massive release of ATP activates P2X4R and the opening of ion channels leads to the influx of calcium ions, promotes microglia activation, and releases pro-inflammatory cytokines. Meanwhile, P2X4R activation leads to BDNF release in microglia, further activating TrkB in lamina I neurons, down-regulating the expression of KKC2, finally reversing the polarity of GABA and glycine-activated current, and causing depolarization in neurons.11,82
Cluster 5 exhibited immune cells and cytokines research associated with pain, such as microglia, astrocyte, T-cells, TNF-α, IL-6, IL-1β, and IL-10. Healthy development and functioning of the nervous system require balanced communication between different subtypes of glia. Similarly, astrocytes and microglia have a synergistic effect on pain. In the chronic post-ischemic pain model, astrocytes release CSF1, which activates CSF1R of microglia, to facilitate BDNF production and the excitability of dorsal horn.83 In addition, microglia release TNF to activate astrocyte through JNK/CXCL1 pathway84 and promote astrocyte polarization through CXCR1/PI2K/Akt pathway.85 It is worth mentioning that both microglia and astrocyte have the characteristic of sequential alteration86 and activation,24 indicating these two types of glia perform distinct temporal roles in pain.
As for Cluster 6 (mechanism research), the main keywords were autophagy, apoptosis, and glial activation. Apoptosis and autophagy are critical processes of cell stress or damage.87,88 Take autophagy as an example; it might regulate neuroinflammation at the beginning of neuron injury89 and has a dual regulatory effect on neuropathic pain. On the one hand, there is increasing evidence that shows the upregulation of autophagy can suppress pain-related neuroinflammation by suppressing microglial activation,90 polarization,91 and pyroptosis.92 On the other hand, autophagy may also function as a pain enhancer.93 This heterogeneous phenomenon may be due to the fact that autophagy expression in the dorsal horn of the spinal cord is influenced by differences in the type of pain model and the experimental setting.94
Cluster 7 is mainly related to peripheral nervous system research, and the main keywords were dorsal root ganglion (DRG), satellite glial cells (SGCs) and neuron. It has been found that DRG is regulated by SGCs in neuroinflammation,33 and there is “cross-organ” sensitization between the spinal cord and DRG.95 A recent study has shown that upregulation of GPR151 in DRG after nerve injury promotes the release of CSF1 and ultimately activates microglia.96 In turn, microglia-derived BDNF promotes the extension of CGRP sensory fibers into the uninjured spinal segments to promote cross-sensitization,3 indicating microglia communicate with the peripheral nervous system in the process of pain.
Future Research Trends
According to the timeline analysis of keywords, the research direction of microglia associated with pain has gradually shifted from inflammatory pain and bone cancer pain to migraine and visceral pain; meanwhile, in the field of neuropathic pain, diabetic neuropathic pain is more prevalent in recent year, suggesting the research in this area will be more comprehensive and in-depth in the future. Regarding mechanism studies, NLRP3 inflammasome has been more prevalent in recent years. Combined with the author keywords occurrence analysis above, it can be speculated that programmed cell death (autophagy, pyroptosis, etc.) will be a focus of future research. When it comes to specific receptors and pathways, our study showed that MAPK, ATP, and purinergic signals have been studied for ten years, implying MAPK pathway and microglial purinergic receptors will continue to receive attention.
In summary, our study described the characteristics of publications, countries, institutions, scholars, journals, highly co-cited references, and author keywords analysis of pain-related microglia research and made predictions for future research trends.
Limitations
Several limitations should be taken into account. Firstly, the articles included in this study were sourced from the WosCC database only, which may have led to bias in results as articles from other medical databases were not included. However, many bibliometric studies are based on the WoSCC database, illustrating its well-recognized literature integrity.97 Secondly, in the keywords analysis, we only refer to the author keywords but not the keywords plus, mainly considering that the author keywords are the main ideas provided by the author according to the theme and concern of an article, which is more consistent with the research preferences of an author.37 Thirdly, this study only included articles in English, which may have resulted in search omissions.
Conclusion
In conclusion, studies on pain-related microglia have been on the rise over the past two decades. China and the United States contributed the most in publications and citations, respectively. The research direction in this field can be summarized into seven major clusters. Last but not least, autophagy and pyroptosis may become new directions in the future.
Abbreviations
ACC, anterior cingulate cortex; ACPP, the average citation per paper; BDI, Beck Depression Inventory; BDNF, brain-derived neurotrophic factor; CCI, chronic constriction injury; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion; HP, hippocampus; IASP, International Association for the Study of Pain; MPFC, medial prefrontal cortex; NC, the number of citations; NP, the number of publications; SCIE, the Science Citation Index-expanded; SGCs, satellite glial cells; SSCI, the Social Sciences Citation Index; WosCC, Web of Science core collection.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article.
Funding
This study was funded by a grant from the National Natural Science Foundation of China (No. 81473759).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Raja SN, Carr DB, Cohen M, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
2. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi:10.15585/mmwr.mm6736a2
3. Zhou LJ, Peng J, Xu YN, et al. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Rep. 2019;27(13):3844–3859.e3846. doi:10.1016/j.celrep.2019.05.087
4. Tansley S, Gu N, Guzmán AU, et al. Microglia-mediated degradation of perineuronal nets promotes pain. Science. 2022;377(6601):80–86. doi:10.1126/science.abl6773
5. Schaible HG, König C, Ebersberger A. Spinal pain processing in arthritis: neuron and glia (inter)actions. J Neurochem. 2022. doi:10.1111/jnc.15742
6. Tsuda M, Masuda T, Kohno K. Microglial diversity in neuropathic pain. Trends Neurosci. 2023;46(7):597–610. doi:10.1016/j.tins.2023.05.001
7. Bossuyt J, Van Den Herrewegen Y, Nestor L, Buckinx A, De Bundel D, Smolders I. Chemogenetic modulation of astrocytes and microglia: state-of-The-art and implications in neuroscience. Glia. 2023;71:2071–2095. doi:10.1002/glia.24390
8. Sabe M, Pillinger T, Kaiser S, et al. Half a century of research on antipsychotics and schizophrenia: a scientometric study of hotspots, nodes, bursts, and trends. Neurosci Biobehav Rev. 2022;136:104608. doi:10.1016/j.neubiorev.2022.104608
9. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
10. Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi:10.1038/nature01786
11. Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi:10.1038/nature04223
12. Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306(2):624–630. doi:10.1124/jpet.103.052407
13. Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–1368. doi:10.1038/nn1992
14. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi:10.1038/nrn2533
15. Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28(2):101–107. doi:10.1016/j.tins.2004.12.002
16. Jin SX, Zhuang ZY, Woolf CJ, Ji RR. P38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23(10):4017–4022. doi:10.1523/JNEUROSCI.23-10-04017.2003
17. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi:10.1016/0304-3959(88)90209-6
18. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi:10.1016/0304-3959(83)90201-4
19. Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–455. doi:10.1016/S0166-2236(00)01854-3
20. Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2(12):973–985. doi:10.1038/nrd1251
21. Milligan ED, Twining C, Chacur M, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23(3):1026–1040. doi:10.1523/JNEUROSCI.23-03-01026.2003
22. Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45(1):89–95. doi:10.1002/glia.10308
23. Ledeboer A, Sloane EM, Milligan ED, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1–2):71–83. doi:10.1016/j.pain.2005.02.009
24. Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114(1–2):149–159. doi:10.1016/j.pain.2004.12.022
25. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6(7):521–532. doi:10.1038/nrn1700
26. Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60(1):135–148. doi:10.1016/j.brainresrev.2008.12.011
27. Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(1):S10–s28. doi:10.1016/j.pain.2013.06.022
28. Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol. 2013;716(1–3):106–119. doi:10.1016/j.ejphar.2013.01.072
29. Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. doi:10.1038/nri3621
30. Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13(7):533–548. doi:10.1038/nrd4334
31. Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081–1083. doi:10.1038/nn.4053
32. Guan Z, Kuhn JA, Wang X, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19(1):94–101. doi:10.1038/nn.4189
33. Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–577. doi:10.1126/science.aaf8924
34. Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19(3):138–152. doi:10.1038/nrn.2018.2
35. Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100(6):1292–1311. doi:10.1016/j.neuron.2018.11.009
36. Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018;129(2):343–366. doi:10.1097/ALN.0000000000002130
37. Guo M, Li J, Sheng C, Xu J, Wu L. A review of wetland remote sensing. Sensors. 2017;17(4):1.
38. Luo H, Cai Z, Huang Y, et al. Study on pain catastrophizing from 2010 to 2020: a bibliometric analysis via CiteSpace. Front Psychol. 2021;12:759347. doi:10.3389/fpsyg.2021.759347
39. Biber K, Tsuda M, Tozaki-Saitoh H, et al. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 2011;30(9):1864–1873. doi:10.1038/emboj.2011.89
40. Masuda T, Iwamoto S, Yoshinaga R, et al. Transcription factor IRF5 drives P2X4R(+)-reactive microglia gating neuropathic pain. Nat Commun. 2014;5:11. doi:10.1038/ncomms4771
41. Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y(12) receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28(19):4949–4956. doi:10.1523/JNEUROSCI.0323-08.2008
42. Tsuda M, Toyomitsu E, Komatsu T, et al. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56(5):579–585. doi:10.1002/glia.20641
43. Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:9. doi:10.1186/1744-8069-3-33
44. Taves S, Berta T, Liu DL, et al. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2016;55:70–81. doi:10.1016/j.bbi.2015.10.006
45. Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21(5):642–651. doi:10.1016/j.bbi.2006.11.003
46. Hutchinson MR, Zhang YN, Brown K, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci. 2008;28(1):20–29. doi:10.1111/j.1460-9568.2008.06321.x
47. Lacagnina MJ, Watkins LR, Grace PM. Toll-like receptors and their role in persistent pain. Pharmacol Ther. 2018;184:145–158. doi:10.1016/j.pharmthera.2017.10.006
48. Lewis SS, Hutchinson MR, Rezvani N, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1 beta. Neuroscience. 2010;165(2):569–583. doi:10.1016/j.neuroscience.2009.10.011
49. Wang X, Zhang Y, Peng Y, et al. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol. 2016;173(5):856–869. doi:10.1111/bph.13394
50. Grace PM, Strand KA, Galer EL, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci USA. 2016;113(24):E3441–E3450. doi:10.1073/pnas.1602070113
51. Hutchinson MR, Zhang YN, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24(1):83–95. doi:10.1016/j.bbi.2009.08.004
52. Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21(2):131–146. doi:10.1016/j.bbi.2006.10.011
53. Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. 2007;56(1):148–169. doi:10.1016/j.brainresrev.2007.06.006
54. Fan H, Li TF, Gong N, Wang YX. Shanzhiside methylester, the principle effective iridoid glycoside from the analgesic herb Lamiophlomis rotata, reduces neuropathic pain by stimulating spinal microglial beta-endorphin expression. Neuropharmacology. 2016;101:98–109. doi:10.1016/j.neuropharm.2015.09.010
55. Huang Q, Mao XF, Wu HY, et al. Cynandione A attenuates neuropathic pain through p38 beta MAPK-mediated spinal microglial expression of beta-endorphin. Brain Behav Immun. 2017;62:64–77. doi:10.1016/j.bbi.2017.02.005
56. Li TF, Fan H, Wang YX. Aconitum-derived bulleyaconitine A exhibits antihypersensitivity through direct stimulating dynorphin A Expression in spinal microglia. J Pain. 2016;17(5):530–548. doi:10.1016/j.jpain.2015.12.015
57. Li TF, Gong N, Wang YX. Ester hydrolysis differentially reduces aconitine-induced anti-hypersensitivity and acute neurotoxicity: involvement of spinal microglial dynorphin expression and implications for aconitum processing. Front Pharmacol. 2016;7:13. doi:10.3389/fphar.2016.00367
58. Sun ML, Ao JP, Wang YR, et al. Lappaconitine, a C18-diterpenoid alkaloid, exhibits antihypersensitivity in chronic pain through stimulation of spinal dynorphin A expression. Psychopharmacology. 2018;235(9):2559–2571. doi:10.1007/s00213-018-4948-y
59. Mohamed NS, Gwam CU, Etcheson JI, et al. Impact factors of orthopaedic journals between 2010 and 2016: trends and comparisons with other surgical specialties. Ann Transl Med. 2018;6(7):114. doi:10.21037/atm.2018.03.02
60. Tian H, Chen J. A bibliometric analysis on global eHealth. Digit Health. 2022;8:20552076221091352. doi:10.1177/20552076221091352
61. Wu Z, Cheng K, Shen Z, et al. Mapping knowledge landscapes and emerging trends of sonodynamic therapy: a bibliometric and visualized study. Front Pharmacol. 2022;13:1048211. doi:10.3389/fphar.2022.1048211
62. Hong ST, Youn HS. Status of editing and publishing of scholarly journals by academic societies of science and technology in Korea. J Korean Med Sci. 2020;35(25):e208. doi:10.3346/jkms.2020.35.e208
63. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi:10.1146/annurev.pa.20.040180.002301
64. McCleskey EW. Neurobiology: new player in pain. Nature. 2003;424(6950):729–730. doi:10.1038/424729a
65. Donnelly CR, Andriessen AS, Chen G, et al. Central nervous system targets: glial cell mechanisms in chronic pain. Neurotherapeutics. 2020;17(3):846–860. doi:10.1007/s13311-020-00905-7
66. Wang Y, Zhang S, Li H, et al. Small-molecule modulators of toll-like receptors. Acc Chem Res. 2020;53(5):1046–1055. doi:10.1021/acs.accounts.9b00631
67. Huang J, Chai X, Wu Y, et al. β-Hydroxybutyric acid attenuates heat stress-induced neuroinflammation via inhibiting TLR4/p38 MAPK and NF-κB pathways in the hippocampus. FASEB J. 2022;36(4):e22264. doi:10.1096/fj.202101469RR
68. Chang S, Li X, Zheng Y, et al. Kaempferol exerts a neuroprotective effect to reduce neuropathic pain through TLR4/NF-ĸB signaling pathway. Phytother Res. 2022;36(4):1678–1691. doi:10.1002/ptr.7396
69. Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S. Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front Immunol. 2020;11:1455. doi:10.3389/fimmu.2020.01455
70. Luo L, Liu M, Fan Y, et al. Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFκB/NLRP3 signaling pathway in cerebral ischemic mice. J Neuroinflammation. 2022;19(1):141. doi:10.1186/s12974-022-02501-2
71. Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169(2):337–352. doi:10.1111/bph.12139
72. Shin DA, Kim TU, Chang MC. Minocycline for controlling neuropathic pain: a systematic narrative review of studies in humans. J Pain Res. 2021;14:139–145. doi:10.2147/JPR.S292824
73. Yan B, Tang S, Zhang Y, Xiao X. The role of glia underlying acupuncture analgesia in animal pain models: a systematic review and meta-analysis. Pain Med. 2023;24(1):11–24. doi:10.1093/pm/pnac115
74. Pei P, Cui S, Zhang S, Hu S, Wang L, Yang W. Effect of electroacupuncture at fengchi on facial allodynia, microglial activation, and microglia-neuron interaction in a rat model of migraine. Brain Sci. 2022;12(8):1100. doi:10.3390/brainsci12081100
75. Ma X, Chen Y, Li XC, et al. Spinal neuronal GRK2 contributes to preventive effect by electroacupuncture on cisplatin-induced peripheral neuropathy in mice. Anesth Analg. 2022;134(1):204–215. doi:10.1213/ANE.0000000000005768
76. Chen Y, Zhou Y, Li XC, et al. Neuronal GRK2 regulates microglial activation and contributes to electroacupuncture analgesia on inflammatory pain in mice. Biol Res. 2022;55(1):5. doi:10.1186/s40659-022-00374-6
77. Fiore NT, Austin PJ. Glial-cytokine-neuronal adaptations in the ventral hippocampus of rats with affective behavioral changes following peripheral nerve injury. Neuroscience. 2018;390:119–140. doi:10.1016/j.neuroscience.2018.08.010
78. Barcelon EE, Cho WH, Jun SB, Lee SJ. Brain microglial activation in chronic pain-associated affective disorder. Front Neurosci. 2019;13:213. doi:10.3389/fnins.2019.00213
79. Albrecht DS, Kim M, Akeju O, et al. The neuroinflammatory component of negative affect in patients with chronic pain. Mol Psychiatry. 2021;26(3):864–874. doi:10.1038/s41380-019-0433-1
80. Inoue K. Microglial activation by purines and pyrimidines. Glia. 2002;40(2):156–163. doi:10.1002/glia.10150
81. Suurväli J, Boudinot P, Kanellopoulos J, Rüütel boudinot S. P2X4: a fast and sensitive purinergic receptor. Biomed J. 2017;40(5):245–256. doi:10.1016/j.bj.2017.06.010
82. Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29(11):3518–3528. doi:10.1523/JNEUROSCI.5714-08.2009
83. Tang Y, Liu L, Xu D, et al. Interaction between astrocytic colony stimulating factor and its receptor on microglia mediates central sensitization and behavioral hypersensitivity in chronic post ischemic pain model. Brain Behav Immun. 2018;68:248–260. doi:10.1016/j.bbi.2017.10.023
84. Zhang R, Xu B, Zhang N, et al. Spinal microglia-derived TNF promotes the astrocytic JNK/CXCL1 pathway activation in a mouse model of burn pain. Brain Behav Immun. 2022;102:23–39. doi:10.1016/j.bbi.2022.02.006
85. Li T, Liu T, Chen X, et al. Microglia induce the transformation of A1/A2 reactive astrocytes via the CXCR7/PI3K/Akt pathway in chronic post-surgical pain. J Neuroinflammation. 2020;17(1):211. doi:10.1186/s12974-020-01891-5
86. Blaszczyk L, Maître M, Lesté-Lasserre T, et al. Sequential alteration of microglia and astrocytes in the rat thalamus following spinal nerve ligation. J Neuroinflammation. 2018;15(1):349. doi:10.1186/s12974-018-1378-z
87. Peng D, Wei J, Gan Y, et al. Testis developmental related gene 1 regulates the chemosensitivity of seminoma TCam-2 cells to cisplatin via autophagy. J Cell Mol Med. 2019;23(11):7773–7784. doi:10.1111/jcmm.14654
88. Liu X, Wang S, Zheng H, et al. Epimedokoreanin C, a prenylated flavonoid isolated from Epimedium koreanum, induces non-apoptotic cell death with the characteristics of methuosis in lung cancer cells. Am J Cancer Res. 2021;11(7):3496–3514.
89. Su SH, Wu YF, Lin Q, Wang DP, Hai J. URB597 protects against NLRP3 inflammasome activation by inhibiting autophagy dysfunction in a rat model of chronic cerebral hypoperfusion. J Neuroinflammation. 2019;16(1):260. doi:10.1186/s12974-019-1668-0
90. Wang Y, Shi Y, Huang Y, et al. Resveratrol mediates mechanical allodynia through modulating inflammatory response via the TREM2-autophagy axis in SNI rat model. J Neuroinflammation. 2020;17(1):311. doi:10.1186/s12974-020-01991-2
91. Bai J, Geng B, Wang X, et al. Exercise facilitates the M1-to-M2 polarization of microglia by enhancing autophagy via the BDNF/AKT/mTOR pathway in neuropathic pain. Pain Phys. 2022;25(7):E1137–e1151.
92. Shao S, Xu CB, Chen CJ, et al. Divanillyl sulfone suppresses NLRP3 inflammasome activation via inducing mitophagy to ameliorate chronic neuropathic pain in mice. J Neuroinflammation. 2021;18(1):142. doi:10.1186/s12974-021-02178-z
93. Cai W, Zhang Y, Su Z. ciRS-7 targeting miR-135a-5p promotes neuropathic pain in CCI rats via inflammation and autophagy. Gene. 2020;736:144386. doi:10.1016/j.gene.2020.144386
94. Liao MF, Lu KT, Hsu JL, Lee CH, Cheng MY, Ro LS. The role of autophagy and apoptosis in neuropathic pain formation. Int J Mol Sci. 2022;23(5):2685. doi:10.3390/ijms23052685
95. Qiao LY, Tiwari N. Spinal neuron-glia-immune interaction in cross-organ sensitization. Am J Physiol Gastrointest Liver Physiol. 2020;319(6):G748–g760. doi:10.1152/ajpgi.00323.2020
96. Xia LP, Luo H, Ma Q, et al. GPR151 in nociceptors modulates neuropathic pain via regulating P2X3 function and microglial activation. Brain. 2021;144(11):3405–3420. doi:10.1093/brain/awab245
97. Song L, Liang J, Wang W, et al. Global trends in research of mitochondrial biogenesis over past 20 years: a bibliometric analysis. Oxid Med Cell Longev. 2023;2023:7291284. doi:10.1155/2023/7291284
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

