Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Exacerbation Burden in COPD and Occurrence of Mortality in a Cohort of Italian Patients: Results of the Gulp Study
Authors Santus P, Di Marco F , Braido F , Contoli M , Corsico AG , Micheletto C, Pelaia G , Radovanovic D , Rogliani P , Saderi L, Scichilone N , Tanzi S, Vella M, Boarino S, Sotgiu G, Solidoro P
Received 26 October 2023
Accepted for publication 28 January 2024
Published 1 March 2024 Volume 2024:19 Pages 607—618
DOI https://doi.org/10.2147/COPD.S446636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Pierachille Santus,1 Fabiano Di Marco,2 Fulvio Braido,3 Marco Contoli,4 Angelo Guido Corsico,5 Claudio Micheletto,6 Girolamo Pelaia,7 Dejan Radovanovic,1 Paola Rogliani,8 Laura Saderi,9 Nicola Scichilone,10 Silvia Tanzi,11 Manlio Vella,11 Silvia Boarino,11 Giovanni Sotgiu,9 Paolo Solidoro12
1Department of Biomedical and Clinical Sciences (DIBIC), Università degli Studi di Milano, Division of Respiratory Diseases, Ospedale L. Sacco, ASST Fatebenefratelli-Sacco, Milano, Italy; 2Department of Health Sciences, Università degli Studi di Milano Pneumology, ASST Papa Giovanni XXIII, Bergamo, Italy; 3Department of Internal Medicine (DiMI), Respiratory Unit for Continuity of Care, IRCCS Ospedale Policlinico San Martino, University of Genova, Genova, Italy; 4Department of Translational Medicine, Respiratory Section, University of Ferrara, Ferrara, Italy; 5Department of Medical Sciences and Infective Diseases, Unit of Respiratory Diseases, IRCCS Policlinico San Matteo Foundation and University of Pavia Medical School, Pavia, Italy; 6Cardio-Thoracic Department, Respiratory Unit, University Integrated Hospital, Verona, Italy; 7Dipartimento di Scienze della Salute, Università Magna Graecia, Catanzaro, Italy; 8Department of Experimental Medicine, Unit of Respiratory Medicine, University of Rome ”Tor Vergata”, Division of Respiratory Medicine, University Hospital ”Tor Vergata”, Rome, Italy; 9Department of Medicine, Surgery and Pharmacy, University of Sassari, Sassari, Italy; 10Biomedical Department of Internal and Specialist Medicine, University of Palermo, Palermo, Italy; 11AstraZeneca Italia, Milan, Italy; 12Department of Medical Sciences, University of Turin, S.C. Pneumologia, Azienda Ospedaliero Universitaria Città della Salute e della Scienza, Torino, Italy
Correspondence: Pierachille Santus, Università degli Studi di Milano, Via G.B. Grassi 74, Milano, 20157, Italy, Tel +39 0239042801, Fax +39 0239042473, Email [email protected]
Objective: To describe the burden of moderate to severe exacerbations and all-cause mortality; the secondary objectives were to analyze treatment patterns and changes over follow-up.
Design: Observational, multicenter, retrospective, cohort study with a three year follow-up period.
Setting: Ten Italian academic secondary- and tertiary-care centers.
Participants: Patients with a confirmed diagnosis of COPD referring to the outpatient clinics of the participating centers were retrospectively recruited.
Primary and Secondary Outcome Measures: Annualized frequency of moderate and severe exacerbations stratified by exacerbation history prior to study enrollment. Patients were classified according to airflow obstruction, GOLD risk categories, and divided in 4 groups: A = no exacerbations; B = 1 moderate exacerbation; C = 1 severe exacerbation; D = ≥ 2 moderate and/or severe exacerbations. Overall all-cause mortality stratified by age, COPD category, and COPD therapy. A logistic regression model assessed the association of clinical characteristics with mortality.
Results: 1111 patients were included (73% males), of which 41.5% had a history of exacerbations. As expected, the proportion of patients experiencing ≥ 1 exacerbation during follow-up increased according to pre-defined study risk categories (B: 79%, C: 84%, D: 97.4%). Overall, by the end of follow-up, 45.5% of patients without a history of exacerbation experienced an exacerbation (31% of which severe), and 13% died. Deceased patients were significantly older, more obstructed and hyperinflated, and more frequently active smokers compared with survivors. Severe exacerbations were more frequent in patients that died (23.5%, vs 10.2%; p-value: 0.002). Chronic heart failure and ischemic heart disease were the only comorbidities associated with a higher odds ratio (OR) for death (OR: 2.2, p-value: 0.001; and OR: 1.9, p-value: 0.007). Treatment patterns were similar in patients that died and survivors.
Conclusion: Patients with a low exacerbation risk are exposed to a significant future risk of moderate/severe exacerbations. Real life data confirm the strong association between mortality and cardiovascular comorbidities in COPD.
Keywords: pulmonary disease chronic obstructive, heart failure, ischaemic heart disease, respiratory medicine, public health
Introduction
Chronic obstructive pulmonary disease (COPD) is a treatable but debilitating medical condition associated with persistent symptoms and chronic airflow obstruction.1 Despite the availability of multiple therapeutic options, COPD is the third leading cause of death worldwide and has a substantial socioeconomic impact.2,3 COPD is diagnosed when patients present with respiratory symptoms and/or history of exposure to risk factors, having bronchial obstruction confirmed by spirometry.2 However, even mild obstruction hides a significant loss of small airways4 making a timely diagnosis and a prompt treatment initiation of great importance to reduce morbidity and mortality.5 Greater understanding of individual variability of COPD progression through multidimensional evaluation may help recommend tailored interventions.6–9 Patients with COPD are susceptible to exacerbations, in fact, 30%-50% of patients experience at least one exacerbation per year.9 Exacerbations are associated with disease severity and history of previous exacerbations itself is considered the most reliable predictor of future exacerbations.10 Nevertheless, patients with mild airflow obstruction and symptoms that may not yet affect activities of daily living can still experience frequent or severe exacerbations.11 Also, mild and moderate exacerbations can increase the risk of future exacerbations, accelerating lung function decline, promoting cardiovascular complications, and increasing mortality.12–14
A Canadian study showed that severe exacerbations leading to hospitalization may increase the risk of a second severe event by 3-fold and may increase mortality up to 50% after 3.6 years of follow-up after a first hospitalization.15 Moreover, after a severe exacerbation, patients are at greater risk of cardiovascular events,16,17 putting pharmacological and non-pharmacological preventive strategies the highest priority in the management of the disease.18 Pharmacological options include long-acting β2 agonists (LABA) and/or long-acting antimuscarinic agents (LAMA), in combination or without inhaled corticosteroids (ICS), that decrease airway inflammation and reduce the rate of exacerbations.19,20
The estimated prevalence of COPD in Italy ranges from 2.6%, assessed via patient-directed survey,21 to 3.01% in primary care,22 thus affecting up to 3.5 million adults and representing the sixth most prevalent chronic disease. It also has large impact on the national healthcare system: the mean annual cost per patient was €3291 in 2015, with the major cost component being hospitalizations following exacerbations.23 Considering the overall socio-economic and healthcare burden of the disease, a detailed clinical profile of COPD patients in Italy appears desirable, but unfortunately to date, real life data are lacking. The present real life study is aimed at describing the clinical and functional characteristics, treatment patterns, impact of exacerbations and comorbidities and their association with mortality in a large cohort of Italian patients with COPD.
Materials and Methods
Study Design
The DescribinG bUrden of COPD and occurrence of mortaLity in a cohort of Italian Patients (GULP) study, part of AstraZeneca’s European AvoidEX program, was an observational, multicenter, retrospective cohort study based on a multicenter database, recently approved as the Italian COPD Registry (Ethics Committee protocol n. 20–27 Sept 2023), conducted in ten Italian academic secondary- and tertiary-care centers: Division of Respiratory Diseases of L. Sacco University Hospital (Università degli Studi di Milano, Milano), Pneumology unit, ASST Papa Giovanni XXIII (Università degli Studi di Milano, Bergamo), Respiratory Unit for Continuity of Care, IRCCS Ospedale Policlinico San Martino (University of Genova, Genova), Respiratory Section, Department of Translational Medicine (University of Ferrara, Ferrara), Unit of Respiratory Diseases, IRCCS Policlinico San Matteo Foundation (University of Pavia Medical School, Pavia), Respiratory Unit, Cardio-Thoracic Department (University Integrated Hospital, Verona), Pulmonary Unit, Dipartimento di Scienze della Salute (Università Magna Graecia, Catanzaro), Unit of Respiratory Medicine, Department of Experimental Medicine (University of Rome “Tor Vergata”, Rome), Biomedical Department of Internal and Specialist Medicine (University of Palermo, Palermo), Pulmonary Unit, Azienda Ospedaliero Universitaria Città della Salute e della Scienza (University of Turin, Torino). The study was carried out according to the amended Declaration of Helsinki, ICH GCPs, GPP, and the legislation on non-interventional studies and/or observational studies (AIFA guidelines, 20/Mar/2008) and approved by the ethics committee of each participating site. All participants gave written informed consent.
Data protection and privacy legislation compliance were ensured. The dataset covered a period of 365 days prior to the index date and a minimum of 365 days post index date up to three years of follow up. The index date was the date of the study entry, i.e. the date when the patient entered the database with a record of a COPD diagnosis.
Study Objectives
The primary objective was to describe the burden of moderate to severe COPD exacerbations. Rates of moderate and severe exacerbations, as well as all-cause mortality were collected and analyzed.
The secondary objective was to describe treatment patterns at baseline and eventual treatment changes.
The pharmacological inhaled treatments considered were: LAMA or LABA monotherapies or fixed combinations thereof, ICS and a LABA and/or a LAMA or their fixed combinations.
Mortality was assessed at 3 years. Patients were stratified in two groups according to survival status and the following variables were assessed: demographic and clinical characteristics, baseline exacerbations, relationship between mortality and clinical characteristics.
Study Subjects
Electronic records of patients aged ≥40 years with an established diagnosis of COPD between January 1, 2015, and December 31, 2017, and referring to the outpatient clinics of the participating centers were retrospectively reviewed. COPD diagnosis was considered if having age ≥ 40 years old, a smoking history > 20 pack years and a post-bronchodilator forced expiratory volume in one second to slow vital capacity ratio (FEV1/VC) < the lower limit of normal (LLN) criteria.11 Severity of disease was graded using three different classifications proposed by GOLD over time: airflow obstruction (GOLD stages 1 to 4);24 airflow obstruction, exacerbations and respiratory symptoms25 or exacerbations and respiratory symptoms (GOLD A, B, C, or D).26 Patients were excluded if had a current asthma diagnosis or clinically significant alternative respiratory diseases such as interstitial lung disease or bronchiectasis.
Clinical phenotypes of the enrolled patients were obtained following the multifactorial model proposed by Pistolesi et al.27 The presence of chronic cough, sputum, and sputum purulence, adventitious sounds and hyper-resonance at physical examination, chest X-ray parameters, such as increased vascular markings, bronchial wall thickening, increased lung volume and reduced lung density, together with the FEV1/FVC ratio were registered. These parameters were included in the web-based estimation model28 that allowed the assessment of the predominant clinical phenotype: airways obstructive (chronic bronchitis), parenchymal destructive (emphysema), or intermediate. At enrollment, patients were assigned to one of 6 groups based on the ongoing therapy:
- LAMA or LABA monotherapy
- Combinations of LABA + LAMA
- ICS without LABA or LAMA
- Combinations of ICS + LABA or ICS + LAMA
- Combinations ICS + LABA + LAMA
- None of the above
Patients treated with more than one pharmacological class were considered as exposed to combination therapy if they had taken the medications for at least 14 days prior to the index date.
Outcomes and Variables
According to the history of exacerbations in the year before the index date,29 patients were grouped into one of four categories:
- Category A: no exacerbations
- Category B: 1 moderate exacerbation (symptomatic deterioration requiring antibiotic therapy or Medium to high-dose systemic corticosteroids)
- Category C: 1 severe exacerbation (exacerbation requiring hospitalization or emergency visits)
- Category D: ≥2 moderate and/or severe exacerbations
Moderate exacerbations were defined as claims for courses of oral corticosteroids and/or respiratory antibiotics. Severe exacerbations were defined as need for hospitalization. If more than one of the episodes occurred within a 2-week window, a single exacerbation was considered. If a moderate and a severe exacerbations occurred concurrently within a 2-week window, the episode was considered as a severe exacerbation.
Patient and Public Involvement
Due to the study design, patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Statistical Analysis
Categorical variables were summarized with absolute and relative frequencies. Continuous variables were summarized with central tendency (i.e. medians) and variability (i.e. interquartile ranges, IQR) indicators. Statistical differences were evaluated using chi-square or Mann–Whitney tests, as appropriate. COPD exacerbations are described overall and in selected strata. All-cause mortality is described overall and stratified by age (<65, 65–75, >75), COPD category, and COPD therapy. A logistic regression model was used to evaluate association of covariates at enrollment with mortality. Missing data were not imputed. All statistical analyses were performed using the statistical software STATA version 16 (StatsCorp, Texas, USA).
Results
Characteristics of the Study Population
The study included 1111 COPD patients (Table 1). Patients were predominately male (72.9%) with a median (IQR) age of 76 (70–82) years and body mass index of 26.5 (23.4–29.4) Kg/m2. Most participants were current smokers (70.1%) with a median (IQR) smoking history of 40 (30–60) pack-years. 56.8% of patients had emphysema and 14.9% had chronic bronchitis, whereas 28.4% had a mixed phenotype. Most patients had moderate to severe airflow obstruction (GOLD 2 and GOLD 3: 44.8% and 28.1%, respectively). By GOLD 2016 and GOLD 2017 criteria, the highest proportion of patients was classified as GOLD D (46.4% and 41.4%, respectively) (Table 2). Among the COPD therapies, the most widely prescribed were LABA+LAMA (21% in 2015; 25.9% in 2016, and 28.7% in 2017) and combination therapy with ICS +LABA+LAMA (42.4% in 2015; 44.5% in 2016, and 46.6% in 2017). 13.3% of patients died within three years of follow-up.
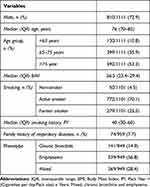 |
Table 1 Patients’ Characteristics at Enrollment |
 |
Table 2 Exacerbations and Treatment Patterns During the Follow-Up |
Exacerbation Patterns in COPD Patients
Prior to the index date, 41.5% (461/1111) of patients had a history of exacerbations. During follow up the majority of patients experienced moderate exacerbations (37.3%, 41.4%, and 40% for each year of follow up, respectively) (Table 2), while the proportion of patients experiencing severe exacerbations was lower though stable over the follow up period (17.8%, 18.9%, 15.3%).
Among patients without prior history of exacerbations (category A), 45.5% experienced an exacerbation during the follow up, 30.6% of which were severe. The proportion of patients with an exacerbation during the follow up period increased in categories B, C and D (60.7%, 83.7%, 97.4%, respectively). Accordingly, the proportion of moderate exacerbations during follow up increased with increasing exacerbation risk from category A to D (28.4%, 60.7%, 58.2%, 90.6%, respectively). Patients that were frequent exacerbators in the year before entering the study (group D) experienced the highest median number of moderate exacerbations during follow up (4 (2–5); p<0.001 compared with other groups). 18.5% of patients with a history of one moderate exacerbation in the previous year (category B) had a severe exacerbation during the follow up. The highest proportion of severe exacerbations was observed in patients with a single severe (category C, 51%) and frequent exacerbators (category D, 34.6%) (Table 3).
 |
Table 3 Exacerbations Over 3 Years in a, B, C and D Categories |
COPD Treatments and Therapeutic Switch
A significant percentage of patients switched inhaled therapy by the end of the follow up period (Table 4). Patients on a bronchodilator monotherapy most frequently switched to a LABA/LAMA combination (38.7% of patients previously on a LABA and 22% of patients on a LAMA) (Table 4). The proportion of patients already on LABA/LAMA and on LABA/ICS that switched to a triple combination therapy (ICS/LABA/LAMA) was 14.8% and 28.2% respectively, while 81.8% of patients treated with LABA/LAMA continued the same therapy, a proportion that increased to 89% in patients treated with ICS/LABA/LAMA. ICS were withdrawn in 21.8% of cases in patients treated with ICS/LABA, while this percentage was reduced to 11.1% in patients on ICS/LABA/LAMA, the majority of which (8.7%) were switched to a LABA/LAMA combination (Table 4).
 |
Table 4 Pharmacological Therapy for COPD, 2015 Vs 2017 |
Characteristics of Deceased Patients
Compared to patients alive at the end of the follow up, patients who died were significantly older, more frequently active smokers, and were significantly more obstructed and hyperinflated (Supplementary Table 1). The proportion of patients that experienced at least one exacerbation during follow up did not differ between groups, but the proportion of patients experiencing moderate exacerbations tended to be less (13.7% vs 16.8%) while severe exacerbations were significantly more frequent in patients that did not survive (23.5%, vs 10.2%; p-value: 0.002) (Supplementary Table 1). Frequent exacerbators were similar between groups (20.6% vs 23.2%). The distribution of treatment patterns at the end of follow up was not different in patients that died and those that survived, although the proportion of patients on ICS/LABA/LAMA tended to be higher in the former group (59.7% vs 47.9%). Cardiovascular comorbidities were the most frequently observed, being significantly more prevalent in deceased patients than in patients alive at the end of follow up (27.2% vs 14.2% for chronic heart failure, p-value: <0.0001; 28.1% vs 17.3% for ischemic heart disease, p-value: 0.006) (Supplementary Table 1). Chronic heart failure and ischemic heart disease were the only comorbidities/clinical characteristics associated with a significantly higher odds ratio (OR) for death (OR: 2.2, p-value: 0.001; and OR: 1.9, p-value: 0.007, respectively) (Figure 1). Mortality was significantly higher in patients with a history of one severe exacerbation (category C): 24.7% VS category A (10.7%), category B (10.4%) and category D (11.2%) (Supplementary Figure 1).
Discussion
The present study evaluated clinical characteristics, treatment patterns, rates of moderate and severe exacerbations, and survival of a cohort of Italian COPD patients.
The burden of exacerbations was almost constant during the study period: 45.5% of patients that had no exacerbations in the year before entering the study experienced at least one exacerbation over a 3-year follow-up period. Moreover, 79.3% of patients that already had a history of a moderate exacerbation had at least one subsequent event. This suggests that even patients perceived as low-risk should be adequately managed over time, since the absence of previous events in the majority of cases does not prevent the occurrence of future exacerbations, highlighting the importance of preventing exacerbation of any severity in order to reduce the risk of future events, and monitoring progression and preventing worsening of disease represent crucial goals, considering that moderate exacerbations correlate with a high risk of severe exacerbations and increased mortality.15 Indeed, in the present study, mortality was associated with severe exacerbations, which confirms the importance of exacerbation events in prognosis.
COPD mostly affects older adults, and the development of multimorbidity may complicate COPD management.30 People living with COPD have almost twice the risk of heart failure and myocardial infarction when compared with those without COPD belonging to the same age, sex, race, and education level.30 Even COPD patients with no history of cardiovascular disease have a higher risk of cardiovascular complications, such as myocardial infarction and stroke, following a moderate exacerbation.14 We showed that cardiovascular comorbidities are a major risk factor for death in Italian patients with COPD. In fact, among all comorbidities, only chronic heart failure and ischemic heart disease were associated with a significantly higher risk of death, independent of the severity of airflow obstruction or hyperinflation. Apparently, frequent exacerbators (group D) were exposed to a lower risk of hospitalizations compared with group C during follow up. Considered the higher mortality in group C and the proportion of patients with cardiovascular comorbidities among patients that died, it could be speculated that patients with frequent exacerbations, irrespective of the severity of exacerbations, could be exposed to a stricter pulmonary outpatient monitoring and therefore with a higher chance of being managed outside the hospital setting in case of an exacerbation. On the other hand, patients in group C might have had a higher risk of being hospitalized for an acute event, with an increased overall mortality risk secondary to the higher prevalence of cardiovascular risk factors.
Current treatments for COPD foresee escalation of therapy from monotherapy to dual/triple therapy based on symptoms and number and severity of exacerbations, and is usually recommended in symptomatic patients with a history of frequent and/or severe exacerbations.2 ICS/LABA/LAMA fixed-dose combinations improve respiratory function, symptoms, health status, and reduce exacerbations compared to dual therapies.31,32 Triple therapy also demonstrated a significant impact on mortality and frequency of moderate or severe exacerbations compared with LABA/LAMA.2,33,34 Our observations showed that patients treated with triple therapy remained on triple therapy throughout the study, whereas patient prescribed ICS/LABA were often stepped up to triple therapy. In spite of the recommendation for ICS treatment only in patients that experience exacerbations,2 in our real life study we observed that the prevalence of ICS prescription in clinical practice reaches 50% of the patients enrolled, suggesting the possibility of overtreatment or inadequate disease control despite maximized bronchodilation in a proportion of patients.
Our work demonstrated a high prevalence of cardiovascular comorbidities in patients with COPD, confirming previous observations.35,36 Furthermore, our analysis showed that after three years of follow up a notable percentage of patients died (13.3%) and only chronic heart failure and ischemic heart disease were associated with higher odds of mortality. Patients that died during the follow up had poorer lung function (lower FEV1 and inspiratory capacity) and had more frequently a history of a severe exacerbation before entering the study, thus justifying the higher proportion treated with triple therapy, but also suggesting that triple therapy is initiated late in the clinical history pf COPD patients. These observations confirm the need for increased alertness on pharmacological optimization and careful patients’ assessment in terms of exacerbations and mortality, and on the connection between chronic cardiac and lung diseases, in order to improve both patients’ quality and quantity of life.
The present study has several limitations. First, patients were enrolled from secondary and tertiary care hospitals, thus the study might suffer from a selection bias, making results not fully generalizable in terms of severity of disease and mortality. Second, in the last years prescription patterns have changed over time due to the market introduction of triple fixed dose combination therapies, therefore switching patterns might have evolved differently than described. Third, the cause of death was not registered therefore any consideration about the possible causative role of cardiovascular comorbidities or exacerbations in the risk of death could not be drawn. Finally, adverse drug effects and major cardiovascular events were not studied and the cause of therapeutic switch was not assessed. Indeed, the study has strengths, mainly represented by the real life setting, the multicenter study and by the length of the follow up period.
Conclusion
In conclusion, this study provided for the first time a detailed clinical overview of the exacerbation burden in patients with COPD in Italy, highlighting from real life data that even patients with a low exacerbation risk are exposed to a significant future risk of moderate to severe exacerbations. The study also confirmed the existence of a strong association between mortality and cardiovascular comorbidities in COPD, in particular with heart failure and ischemic heart disease. Despite the overall exacerbation and mortality burden, a lower than expected number of patients were treated with triple therapy with ICS//LABA/LAMA. The study should represent a starting point and gives the rationale for continuing the implementation of large shared national databases as a source of patients’ characterization and as monitoring tools for preventive pharmacological and non-pharmacological strategies.
Data Sharing Statement
The anonymized dataset will be available upon reasonable request by the Corresponding Author.
Acknowledgments
Medical writing and editorial assistance were provided by Maria Vittoria Verga Falzacappa, PhD (EDRA S.p.A., Milan, Italy) and funded by AstraZeneca.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Astra Zeneca.
Disclosure
PSa has received lectures fees at national and international meetings and consultancy fees from Boehringer Ingelgheim, Chiesi Farmaceutici, Astra Zeneca, Berlin-Chemie, Edmondpharma, Guidotti, Neopharmed, Novartis, Valeas, GlaxoSmithKline, Alfasigma, Zambon and Sanofi; research grants from Air Liquide, Almirall, Boehringer Ingelgheim, Chiesi Farmaceutici, Pfizer, Edmondpharma. FB declares participation in a company sponsored speaker’s bureau: Astra Zeneca, GSK, Novartis, Boehringer Ingelgheim, Chiesi, MSD, Menarini, Malesci, Guidotti, Sanofi and support for research: Chiesi, Vitalair. M.C. declares grants for research, personal fees and non-financial support from Chiesi and GlaxoSmithKline, personal fees and non-financial support from AstraZeneca, Boehringer Ingelheim, Alk-Abello, and Novartis, and research grants from the University of Ferrara, Italy. FDM has received lectures fees at national and international meetings and consultancy fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Dompe, Guidotti/Malesci, GlaxoSmithKline, Menarini, Novartis, and Zambon; CM received fees as a speaker from Astrazeneca, GSK, Sanofi, Chiesi, Menarini, Guidotti, Novartis, Zambon, Boehringer. GP has received lecture fees and consultancy fees from Alfasigma, AstraZeneca, Chiesi, GlaxoSmithKline, Guidotti-Malesci, Menarini, Mundipharma, Novartis, Sanofi, Zambon. DR has received fees for lectures from Astra Zeneca, Berlin Chemie, Boehringer Ingelheim, Glaxo Smith Kline, Menarini; fees for consultancy from Damor Farmaceutic and honoraria for consulting and participation to advisory boards from Astra Zeneca, Boehringer Ingelheim. PR participated as a lecturer and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, MSD, Mundipharma, Novartis and Recipharm. Her department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, and Zambon. NS has received lectures fees at national and international meetings and consultancy fees from Astra Zeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline; research grants from Boehringer Ingelheim, Chiesi Farmaceutici, Sanofi. SB, ST, MV are AstraZeneca employees. PSo has participated as a lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship from Boehringer Ingelheim, Chiesi Farmaceutici, Astra Zeneca, Guidotti-Malesci, Novartis, Valeas, GlaxoSmithKline, Menarini, ABC Farmaceutici, Almirall, Dompè and Biotest. The authors report no other conflicts of interest in this work.
References
1. Viegi G, Pistelli F, Sherrill DL, et al. Definition, epidemiology and natural history of COPD. Eur Respir J. 2007;30(5):993–1013. doi:10.1183/09031936.00082507
2. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2022.Available from: https://goldcopd.org/2022-gold-reports-2/.
3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–2128. doi:10.1016/S0140-6736(12)61728-0
4. McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi:10.1056/NEJMoa1106955
5. Radovanovic D, Contoli M, Braido F, et al. Future perspectives of revaluating mild COPD. Respiration. 2022;101(7):688–696. doi:10.1159/000524102
6. Papaioannou AI, Loukides S, Gourgoulianis KI, et al. Global assessment of the COPD patient: time to look beyond FEV1? Respir Med. 2009;103(5):650–660. doi:10.1016/j.rmed.2009.01.001
7. Casanova C, de Torres JP, Aguirre-Jaime A, et al. The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med. 2011;184(9):1015–1021. doi:10.1164/rccm.201105-0831OC
8. Han MK, Wise R, Mumford J, et al. Prevalence and clinical correlates of bronchoreversibility in severe emphysema. Eur Respir J. 2010;35(5):1048–1056. doi:10.1183/09031936.00052509
9. Whittaker H, Rubino A, Mullerova H, et al. Frequency and severity of exacerbations of COPD associated with future risk of exacerbations and mortality: a UK routine health care data study. Int J Chron Obstruct Pulmon Dis. 2022;17:427–437. doi:10.2147/COPD.S346591
10. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
11. Radovanovic D, Contoli M, Di Marco F, et al. Clinical and functional characteristics of COPD patients across gold classifications: results of a multicenter observational study. COPD. 2019;16(3–4):215–226. doi:10.1080/15412555.2019.1659760
12. Alqahtani JS, Aquilina J, Bafadhel M, et al. Research priorities for exacerbations of COPD. Lancet Respir Med. 2021;9(8):824–826. doi:10.1016/S2213-2600(21)00227-7
13. Celli BR, Decramer M, Wedzicha JA, et al. An official American thoracic society/European respiratory society statement: research questions in COPD. Eur Respir J. 2015;45(4):879–905. doi:10.1183/09031936.00009015
14. Donaldson GC, Hurst JR, Smith CJ, et al. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi:10.1378/chest.09-2029
15. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi:10.1136/thoraxjnl-2011-201518
16. Hesse K, Bourke S, Steer J. Heart failure in patients with COPD exacerbations: looking below the tip of the iceberg. Respir Med. 2022;196:106800. doi:10.1016/j.rmed.2022.106800
17. Dransfield MT, Criner GJ, Halpin DMG, et al. Time-dependent risk of cardiovascular events following an exacerbation in patients with chronic obstructive pulmonary disease: post hoc analysis from the IMPACT trial. J Am Heart Assoc. 2022;11(18):e024350. doi:10.1161/JAHA.121.024350
18. Halpin DM, Miravitlles M, Metzdorf N, et al. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int J Chron Obstruct Pulmon Dis. 2017;12:2891–2908. doi:10.2147/COPD.S139470
19. Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–1303. doi:10.1136/bmj.320.7245.1297
20. Calverley PM, Wedzicha JA. Chronic obstructive pulmonary disease past, present and future. Thorax. 2007;62(12):1026–1027. doi:10.1136/thx.2007.092635
21. Ferrante G, Baldissera S, Campostrini S. Epidemiology of chronic respiratory diseases and associated factors in the adult Italian population. Eur J Public Health. 2017;27(6):1110–1116. doi:10.1093/eurpub/ckx109
22. Lupi L. Prevalenza della broncopneumopatia cronica ostruttiva e pattern di utilizzo vaccino antinfluenzale nei pazienti assistititi dalla Medicina Generale Italiana. Newsletter Health Search Istituto di Ricerca della S.I.M.G. (Società Italiana di Medicina Generale e delle Cure Primarie), 2020. Available from: https://www.healthsearch.it/documenti/documenti_ricercatori/Newsletter/2020/1_2020.pdf.
23. Dal negro RW. COPD: the annual cost-of-illness during the last two decades in Italy, and its mortality predictivity power. Healthcare. 2019;7(1):35. doi:10.3390/healthcare7010035
24. Strategia globale per la diagnosi, il trattamento e la prevenzione della broncopneumopatia cronica ostruttiva 2015; 2015. https://goldcopd.it/wpcontent/uploads/materiali/2015/GOLD_workshop_report_2015.pdf.
25. Strategia globale per la diagnosi, il trattamento e la prevenzione della broncopneumopatia cronica ostruttiva 2016; 2016. https://goldcopd.it/wpcontent/uploads/materiali/2016/GOLD_workshop_report_2016.pdf.
26. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2017; 2017. https://goldcopd.org/wp-content/uploads/2017/02/wms-GOLD-2017-FINAL.pdf.
27. Pistolesi M, Camiciottoli G, Paoletti M, et al. Identification of a predominant COPD phenotype in clinical practice. Respir Med. 2008;102(3):367–376. doi:10.1016/j.rmed.2007.10.019
28. Clinical Identification of Phenotypes in COPD. CLIP COPD 2022; 2022. http://www.clipcopd.com/.
29. Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s. doi:10.1183/09031936.03.00078002
30. Witt LJ, Wroblewski KE, Pinto JM, et al. Beyond the lung: geriatric conditions afflict community-dwelling older adults with self-reported chronic obstructive pulmonary disease. Front Med Lausanne. 2022;9:814606. doi:10.3389/fmed.2022.814606
31. Solidoro P, Albera C, Ribolla F, et al. Triple therapy in COPD: can we welcome the reduction in cardiovascular risk and mortality? Front Med Lausanne. 2022;9:816843. doi:10.3389/fmed.2022.816843
32. Calzetta L, Cazzola M, Matera MG, et al. Adding a LAMA to ICS/LABA therapy: a meta-analysis of triple combination therapy in COPD. Chest. 2019;155(4):758–770. doi:10.1016/j.chest.2018.12.016
33. Lai CC, Chen CH, Chen KH, et al. The impact of 52-week single inhaler device triple therapy versus dual therapy on the mortality of COPD patients: a systematic review and meta-analysis of randomized controlled trials. Life. 2022;12(2):173. doi:10.3390/life12020173
34. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
35. Rogliani P, Ritondo BL, Laitano R, Chetta A, Calzetta L. Advances in understanding of mechanisms related to increased cardiovascular risk in COPD. Expert Rev Respir Med. 2021;15(1):59–70. doi:10.1080/17476348.2021.1840982
36. Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1(1):73–83. doi:10.1016/S2213-2600(12)70060-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

