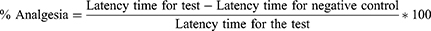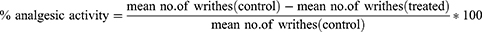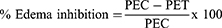Back to Journals » Journal of Experimental Pharmacology » Volume 15
Evaluation of Analgesics and Anti-Inflammatory Activity of the Root Extract of Impatiens rothii (Balsaminaceae) in Rodents
Authors Ashagrie G , Girmaw F , Tarekegn A , Baye T , Dagne A
Received 16 March 2023
Accepted for publication 18 April 2023
Published 20 April 2023 Volume 2023:15 Pages 207—214
DOI https://doi.org/10.2147/JEP.S410024
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Junmin Zhang
Getachew Ashagrie,1,2 Fentaw Girmaw,1 Abebe Tarekegn,1 Tenaw Baye,1 Abebe Dagne3
1Department of Pharmacy, College of Health Science, Woldia University, Woldia, Ethiopia; 2Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Pharmacy, College of Health science, Debre Markos University, Debre Markos, Ethiopia
Correspondence: Getachew Ashagrie, Email [email protected]
Background: The roots of Impatiens rothii has been used as a traditional remedy for painful conditions, rheumatism, isthmus and crural aches. However, the analgesic and anti-inflammatory properties of this plant have yet to be scientifically confirmed. The purpose of this study was to explore possible analgesic and anti-inflammatory activities 80% methanolic root extract of Impatiens rothii.
Methods: To obtain the crude extract, the roots of Impatiens rothii that had been dried and ground up were macerated in 80% methanol. The analgesic activity was determined using acetic acid-induced writhing and hot plate tests in mice, whereas the anti-inflammatory activity was analyzed using carrageenan-induced paw edema model in rats. The extract was orally administered at a dose of 100, 200 and 400 mg/kg.
Results: All tested doses of Impatiens rothii extract showed significant analgesic activity (p< 0.05) at observations of 30 to 120 minutes compared to the negative control in the hot plate test. In acetic acid-induced writhing test all tested doses of the 80% methanol extract of Impatiens rothii significantly (p < 0.001) reduced the number of writhing. In comparison to the control group, all tested doses displayed a significant decrease in paw edema, which appeared 2– 5 hours after induction (p< 0.05).
Conclusion: From the results of this study, it can be stated that 80% methanolic extract of Impatiens rothii possessed substantial analgesic and anti-inflammatory activities, hence providing scientific basis for the use of this plant in the treatment of pain and inflammatory diseases.
Keywords: Impatiens rothii, analgesic, hot plate, anti-inflammatory, carrageenan- induced paw edema
Introduction
Overview of Pain and Inflammation
The international association for the study of pain defined pain as an unpleasant sensation and emotional experience brought on by actual or probable tissue injury or something that is equivalent of such damage.1 Although pain is unpleasant, it also serves as a valuable mechanism for promoting healing since it compels the sufferer to rest the injured area and seek medical attention.2 Thirty percent of adult population is influenced by pain and inflammation, which are the most difficult and debilitating health problems worldwide.3 Pain can be mild, moderate, severe, sharp, burning, transient, intermittent, persistent and referred.4 The prevalence of chronic pain in the general population of developing countries was found to be 18%.5 Opioids, non-opioids (mainly non-steroidal anti-inflammatory drugs (NSAIDs)), anticonvulsants, antidepressants, cannabinoids, and topical agents are medications used to treat both acute and chronic pain.6
Inflammation is a dynamic process of cellular and plasma-derived events associated with infection and tissue injury. Redness, swelling, heat and hyperalgesia are the common signs of inflammation.7 It is the body’s biological defense mechanism to cell damage and tissue injury. Many pro-inflammatory mediators lead to pain and edema during inflammation at the site of injury.8 Increased vascular permeability, capillary infiltration, emigration of leukocytes, infiltration of mononuclear immune cells, macrophages, monocytes, neutrophils, fibroblast activation, proliferation and fibrosis leads to inflammation.7 NSAIDs can help to reduce the negative consequences of inflammation.9 Many inflammatory diseases, such as inflammatory arthritis, systemic lupus, sarcoidosis, and asthma, are treated with the use of glucocorticoids.10
The long-term use of NSAIDs is associated with cardiovascular, gastrointestinal, respiratory, and renal toxicities.11 They also caused hepatic injury, platelet inhibition, hormonal disturbance, physical dependence, tolerance, and addiction problems.11 The use of corticosteroids also leads to hyperglycemia, hypertension, osteoporosis, and growth arrest.12 Only 50% of individuals can have complete pain relief with analgesics, especially opioids and NSAIDs.13 As a result, it is essential to intensify research on medicinal plants that might be used to treat painful and inflammatory conditions.
Plants have long been used as traditional medicine for many years.14 Plants are medicinally useful due to the presence of secondary metabolites.15 Prototype molecules are derived from plants for possible development into conventional drugs by the pharmaceutical industry. However, few types of plants have been investigated scientifically so far, but, human kind has already got different benefits from them.16 Medicinal plants have been used for the management and prevention of different diseases along with epidemics starting from the Vedic era up to now. Most of them have anti-oxidant, anti-inflammatory, insecticidal, anti-parasitic, antibiotic, anti-hemolytic properties due to secondary metabolites.17 World Health Organization reports show that 80% of the population in the developing countries depends on traditional medicines for their primary health care needs.18 This is due to the availability of traditional healers and local pharmacopeias, the relative affordability of herbal medicines, and the lack of access to modern medical facilities.19
The genus Impatiens is perennial and herbs with an average of 20–40 cm in length. The stems are succulent-fleshy and flowers are white, pink, orange and purple colors.20 Leaves are usually alternately arranged along the stem. The seeds are scattering because the ripe seedpod bursts upon slight pressure.21 The genus Impatiens used as a traditional medicine for painful conditions, rheumatism, fractures, superficial infections, bruises, beriberi and fingernail inflammation.22,23 The stems and roots of Impatiens treat lumbago, neuralgia, burns and scalds in traditional Chinese medicine.24 Asian people used this genus for the prevention of rheumatism, inflammation of nails and treatment of fractures.20 In Ethiopia, it is locally known as Buri (Afan Oromo) and Gesherit (Amharic). It is used for stomach problems,25 fire burn,19 inflammation,26 cellulitis27 and wound.28 Anthraquinones, naphthoquinone, triterpenoid saponins, phytosteroids, flavonoids, coumarins, phenolic acids, anthocyanins and volatile oils were isolated from the genus Impatiens.20,22,23,29 Even if the traditional healers have asserted roots of Impatiens rothii has analgesic and anti-inflammatory effects, but to the best of our knowledge, no studies have been done on its anti-nociceptive and anti-inflammatory properties. Consequently, it is essential to perform scientific research on the analgesic and anti-inflammatory effects of this plant.
Materials and Methods
Materials and Equipment
Rotary evaporator (Heidolph, Germany), digital plethysmometer (Ugo Basile - Cat no 7140, Italy), analgesiometer, tissue drying oven, electronic balance (KERN-ALJ 220-4, Germany), cotton swab, oral gavage, hot plate (Ugo Basile 720), lyophilizer (OPERON, OPR-FDU-5012, Korea), and whatman filter paper no.1 were used in the experiment.
Drugs and Chemicals
Analytical chemicals and drugs used in the study was aspirin, morphine, indomethacin (Cadila, Ethiopia), methanol (Carlo Erba, Italy), carrageenan (Sigma Aldrich, Germany), acetic acid solution (Basell, India), chloroform, distilled water (DW), 2% tween 80 (Sigma Aldrich, Germany), and normal saline.
Plant Materials Collection and Extract Preparation
The roots of Impatiens rothii were collected from Machakel woreda, East Gojjam Zone, Amhara regional State, around 327 km North of Addis Ababa. Taxonomic identifications were established by Mr Melaku Wondaferash at the National Herbarium, Department of Plant Biology and Biodiversity Management, Addis Ababa University and specimens were deposited with a voucher number AD 002.
The roots were scrubbed clean of dust and debris, gently washed with water, and reduced to a coarse powder and air dried under shade at room temperature for two weeks. The dried root materials were then ground in to powder using mortar pestle. The dried root powder (200g) was macerated in 80% methanol with occasional shaking using mini orbital shaker at 120 rpm three times for 72 h. The mixture was filtered first using nylon cloth and then by Whatman filter paper no.1. Then the filtrates were evaporated under reduced pressure set at 40 °C in rotary evaporator 45 rpm and 40 °C. Using a lyophilizer, the extract was further concentrated to dryness at −40 °C. Then, the crude extract was weighed and stored in a refrigerator with air tight plastic containers until used.
Experimental Animals
Healthy Swiss albino mice (20–30g), rats (180–300 g), aged 6–8 weeks were used for analgesics and anti-inflammatory study, respectively. Both male and female mice and rats were used for the main study. Only female mice were used for the acute toxicity study. Mice were obtained from the animal house of the department Pharmacology and clinical Pharmacy, School of Pharmacy, Addis Ababa University while rats from the animal house of Ethiopian public health institute (EPHI). The animals were housed in plastic cages at room temperature on a 12 h light–dark cycle with free access to pellet food and water ad libitum. They were acclimatized for a week before the start of the experiment. All studies were carried out in agreement with international guideline30 and approval was done by ethical review committee of School of Pharmacy, Addis Ababa University.
Animal Grouping and Dosing
For the analgesic and anti-inflammatory activity test, mice and rats of both sexes were randomly allocated into five groups, each consisting of six animals; one negative control, one positive control, and three test groups. Distilled water (DW), 10 mL/kg in volume, was given to the first group as a negative control. The second group was designated as the positive control and given standard medications (morphine 20 mg/kg for the hot plate method, aspirin 150 mg/kg for the acetic acid-induced writhing, and indomethacin 10 mg/kg for the carrageenan-induced paw edema model). The other three groups (test groups) were given oral doses of the 80% crude extract at varying doses (100, 200, and 400 mg/kg).
Acute Toxicity Study
Acute oral toxicity study was conducted in accordance with the internationally recognized protocol of Organization for Economic Cooperation and Development (OECD) Guideline 425.30 Fasted female albino mice that were between 6 and 8 weeks old were used for the toxicity study. In the initial screening test, a single female mouse was administered 2000 mg/kg of the extract as a single dose to determine the starting dose. Four additional mice received the same dose of extract as there was no death within the first 24 hours. The animals were observed for the first four hours at 30 minutes interval, and then for the next 14 days at intervals of 24 hours. They were observed for general toxic signs and symptoms, such as changes in skin and fur, somatomotor activities and behavioral patterns, eyes and mucous membranes, diarrhea and salivation, convulsions and tremor, food and water intake, weight loss, lethargy, paralysis and mortality.
Evaluation of Analgesic Activity of the Extract
Hot Plate Method
This test was carried out to evaluate the central analgesic activities of Impatiens rothii root extract. Pain was induced by placing the mice on a hot plate maintained at 55 ±1 °C. The latency time was determined when the mice reacted to the thermal pain by licking their paws or jumping. The reaction time was measured at 0 min, 30, 60, 90 and 120 min after the administration of the extracts. The cut-off time was 15 sec to avoid any injury to the tissues of the paws. The percentage analgesia was calculated as follows:13
Acetic Acid-Induced Writhing Method
The peripheral analgesic activity of root extract of Impatiens rothii was determined by the acetic acid-induced writhing test. Mice of either sex were given varying doses of the crude extract, a vehicle (negative control) and aspirin 150 mg/kg (positive control). One hour later, all of the animals in their respective groups received intraperitoneal injections of 0.6% acetic acid (10 mL/kg). The analgesic activities of the crude extract was measured five minutes after the acetic acid injection by counting the numbers of writhing, which is characterized by contraction of the abdominal muscle together with stretching of the hind limbs for 30 minutes. The percent reduction in the number of writhes relative to the control group was used as an index of analgesia and calculated by the following formula;31
Evaluation of Anti-Inflammatory Activity of the Extract
Carrageenan-Induced Mice Paw Edema
Carrageenan-induced rat hind paw edema was used as the animal model of acute inflammation. Carrageenan (1% w/v carrageenan in normal saline, 100 µL) was injected into the right hind paw of the rat to produce acute inflammation. Before induction of inflammation, the paw was labeled with ink at the lateral malleolus. Carrageenan (1%, 0.1mL) was injected into sub-plantar tissue of the right hind paw of each rats one hour after oral administration of the crude extract, the vehicle and the standard drug. The paw volume was measured using plethysmometer immediately after injection of carrageenan at 0, 1, 2, 3, 4 and 5h. The percent inhibition of edema was calculated in comparison to the control rats using the formula below.31
Where; PEC paw edema in control group, PET paw edema in test group
Statistical Analysis
Data were analyzed using statistical package for social science (SPSS) software version 25. The results were presented as mean ± standard error of the mean. Means of all parameters among groups and within a group were compared using one-way ANOVA followed by Tuckey’s post hoc multiple comparison test. P-values < 0.05 were considered statistically significant. The analyzed data were then presented by tables and graph as necessary.
Results
Acute Toxicity Study
The acute oral toxicity test of 80% methanolic root extract of Impatiens rothii at a dose of 2000 mg/kg revealed no gross behavioral alterations, toxic effects or mortality within 24 h and in the next 14 days. In accordance with the Limit Test at 2000 mg/kg‖ ofOECD guideline 425,30 it can be determined that the oral LD50 of 80% methanolic root extract is higher than 2000 mg/kg in mice. As a result, the finding indicates that the extract is safe.
Analgesic Activity of the Crude Extract of Impatiens Rothii
Hot Plate Model
From 30 to 120 minutes of observation, all tested dosages of Impatiens rothii extract exhibited a significant analgesic effect (p < 0.05) when compared to the negative control. The extract’s higher dose (400 mg/kg) had a greater effect than the lower dose (100 mg/kg). At all observational intervals, the latency delayed by the three doses of the extract was significantly smaller (p < 0.05) than that of Morphine (20 mg/kg). The crude extract of Impatiens rothii at 100, 200, and 400 mg/kg doses showed the maximum analgesic effect at 120 minutes, with values of 53.03%, 64.3%, and 68.2%, respectively (Table 1)
 |
Table 1 Analgesic Effect of 80% Methanol Extracts of Impatiens Rothii in Hot Plate Test |
Acetic Acid-Induced Writhing Method
All the tested doses of 80% methanolic root extract of Impatiens rothii significantly (p < 0.001) lowered the number of acetic acid-induced writhing compared to the negative control. The higher dose of the extract significantly (p<0.001) had greater protection of writhing in mice than the lower and medium doses of the crude extract. Acetyl salicylic acid significantly (p<0.05) reduced the number of acetic acid induced writhes compared to all extracts (Table 2).
 |
Table 2 Analgesic Activities of 80% Methanolic Root Extract of Impatiens Rothii in Acetic Acid-Induced Writhing Test |
Anti-Inflammatory Activity of the Crude Extract of Impatiens Rothii
Carrageenan-Induced Paw Edema
The crude extract of Impatiens rothii at all tested doses showed a significant reduction in paw edema that starts from 2 to 5 hr after induction (p < 0.05) as compared to the negative control in carrageenan-induced paw edema model. Indomethacin significantly (p<0.001) reduced the paw volume at 2, 3, 4 and 5 hrs as compared to the negative control and at the later time with the lower dose of the crude extract (100 mg/kg). The medium (200 mg/kg) and higher (400mg/kg) doses of the extract had comparable anti-inflammatory activity to positive control at all observations. The maximum edema inhibition of 100, 200, and 400 mg/kg Impatiens rothii was accomplished 5 hours after induction, with respective values of 23.8%, 31.9%, and 32.5%. (Table 3)
 |
Table 3 Anti-Inflammatory Effect of 80% Methanolic Root Extracts of Impatiens Rothii on Carrageenan-Induced Paw Edema in Rats |
Discussion
The aim of this research was to determine the analgesic and anti-inflammatory effects of 80% methanolic root extract of Impatiens rothii. In the current study, the higher dose of the crude extract exhibited greater analgesic and anti-inflammatory activity compared to the negative control.
An intraperitoneal injection of acetic acid results in an increase in prostaglandin E2 and prostaglandin F2a levels in the peritoneal fluid, which causes peritoneal inflammation characterized by writhing.32 The acetic acid induced writhing test simulates visceral pain, and the writhing that occurs is often accompanied by abdominal muscular contraction, forelimb expansion, and body lengthening.33 The oral administration of all test dosages of 80% methanolic root extract greatly reduced the abdominal writhing reactions following acetic acid injection. These data provide evidence that the 80% methanolic root extract of Impatiens rothii inhibit lipoxygenase and/or cyclooxygenase enzymes in peripheral tissues, consequently interfering with the mechanism of transduction in the primary afferent nociceptor. Similar with this study, hydroethanolic extracts of Impatiens glandulifera and Impatiens parviflora presented an anti-nociceptive effect in acetic acid induced writhing test.23
Due to its sensitivity to potent analgesics and low tissue damage, the hot plate test is often utilized in elucidating the centrally mediated anti-nociceptive effects of the test drugs. A cutoff time of 15 seconds is generally used to limit the time that the mouse is exposed to the hot plate.34 Jumping and paw licking are considered supraspinally integrated behavioral responses, and the time of the latency to the onset of this reaction following injection is a measure of the analgesic activity.35 The result demonstrated that the 80% methanolic root extract of Impatiens rothii dramatically enhanced the nociceptive threshold significantly that was determined by increased latencies. During the hot-plate test, animals administered 80% methanolic root extract of Impatiens rothii had a longer latency time than those in the control group. Based on the increased mice response times in the hot-plate test, it may be concluded that the 80% methanolic root extract of Impatiens rothii has a central analgesic activity.
It is well recognized that carrageenan-induced paw edema is main assay for evaluating possible new anti-inflammatory drugs and medicinal plants and is biphasic. The initial phase occurs within 1–2 hours of carrageenan injection and is caused by the release of serotonin, histamine, and bradykinin from mast cells into surrounding damaged tissues.36 The second phase, which begins 3–6 hours after carrageenan injection, is related with the generation and release of prostaglandins, leukotrienes, and different cytokines, such as IL-1β, IL-6, IL-10, and TNF-α.36 At all observations, all test dosages of 80% methanolic root extract of Impatiens rothii revealed a significant (p < 0.05) anti-inflammatory effect. The higher doses of 80% methanol extract of Impatiens rothii exhibited maximum percentage inhibition of edema (32.5%) at 5 hours post-induction. Similar with the effects of non-steroidal anti-inflammatory medications, such as indomethacin, the observed edema inhibition was higher in the later stages of inflammation, implying that the anti-inflammatory activity is mediated by inhibition of the cyclooxygenase enzyme. In line with this study, ethanol extract of Impatiens balsamina roots displayed significant anti-inflammatory activity against carrageenan-induced paw edema model.37 The presence of secondary metabolites, such as polyphenols, flavonoids, coumarins, and phenolic acids that have been reported in the root of Impatiens rothii may be accountable for the analgesic and anti-inflammatory effects.
Conclusion
Based on the findings of this study, 80% methanolic extract of Impatiens rothii displayed significant analgesic and anti-inflammatory activities, contributing greatly to the plant’s traditional use in the management of a variety of painful and inflammatory disorders. The plant extract had both peripheral analgesic and central pain inhibiting activities. It also possessed anti-inflammatory properties in the acute phase of inflammation.
Abbreviations
NSAID, Non-steroidal anti-inflammatory drugs; COX, Cyclooxygenase; IL, Interleukin; OECD, Organization for Economic Cooperation and Development.
Availability of the Data
The data is in the hands of the corresponding author and is available upon reasonable request.
Ethics Approval and Consent to Participate
The ethical review committee at the school of pharmacy, College of Health Sciences, Addis Ababa University approved this work. For the care of the experimental animals, OECD guideline 25 was implemented.
Acknowledgments
The authors would like to express their gratitude to Ethiopian Public Health Institute (EPHI) and Addis Ababa University’s Department of Pharmacology and Clinical Pharmacy for donating laboratory animals and chemicals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Addis Abeba University is funding the study.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Raja SN, Carr DB, Cohen M, et al. The revised IASP definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976. doi:10.1097/j.pain.0000000000001939
2. Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37(1):29–42. doi:10.1007/s00296-016-3481-8
3. Javed F, Jabeen Q, Aslam N, Awan AM. Pharmacological evaluation of analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of Indigofera argentea Burm. F. J Ethnopharmacol. 2020;259:112966. doi:10.1016/j.jep.2020.112966
4. Mamun-Or-Rashid M, Islam A, Amran MS, Hossain MA. Evaluation of analgesic activity by acetic acid induced writhing method of crude extracts of acacia nilotica. Sch Acad J Pharm. 2017;6(4):126–128.
5. Sá KN, Moreira L, Baptista AF, et al. Prevalence of chronic pain in developing countries: systematic review and meta-analysis. Pain Rep. 2019;4(6):e779. doi:10.1097/PR9.0000000000000779
6. Beal BR, Wallace MS. An overview of pharmacologic management of chronic pain. Med Clin. 2016;100(1):65–79. doi:10.1016/j.mcna.2015.08.006
7. Kumar S, Bajwa B, Kuldeep S, Kalia A. Anti-inflammatory activity of herbal plants: a review. Int J Adv Pharm Biol Chem. 2013;2(2):272–281.
8. Gupta AK, Parasar D, Sagar A, et al. Analgesic and anti-inflammatory properties of gelsolin in acetic acid induced writhing, tail immersion and carrageenan induced paw edema in mice. PLoS One. 2015;10(8):e0135558. doi:10.1371/journal.pone.0135558
9. Haley RM, von Recum HA. Localized and targeted delivery of NSAIDs for treatment of inflammation: a review. Exp Biol Med. 2019;244(6):433–444. doi:10.1177/1535370218787770
10. Pahwa R, Goyal A, Jialal I. Chronic Inflammation. StatPearls; 2021.
11. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180:114147. doi:10.1016/j.bcp.2020.114147
12. Uwaifo GI, Hura DE. Hypercortisolism. In: StatPearls. StatPearls Publishing; 2022.
13. Fan S-H, Ali NA, Basri DF. Evaluation of analgesic activity of the methanol extract from the galls of Quercus infectoria (Olivier) in rats. Evid Based Complementary Altern Med. 2014;2014:1–6. doi:10.1155/2014/976764
14. Karunamoorthi K, Jegajeevanram K, Vijayalakshmi J, Mengistie E. Traditional medicinal plants: a source of phytotherapeutic modality in resource-constrained health care settings. J Evid Based Complementary Altern Med. 2013;18(1):67–74. doi:10.1177/2156587212460241
15. Nigussie D, Legesse BA, Davey G, Fekadu A, Makonnen E. Ethiopian medicinal plants used for their anti-inflammatory, wound healing or anti-infective activities: protocol for systematic literature review and meta-analysis. BMJ Open Sci. 2020;4(1):e100064. doi:10.1136/bmjos-2020-100064
16. Chekole G. Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia. J Ethnobiol Ethnomed. 2017;13(1):1–29. doi:10.1186/s13002-017-0182-7
17. Bamola N, Verma P, Negi C. A review on some traditional medicinal plants. Int J Life Sci Res. 2018;4(1):1550–1556. doi:10.21276/ijlssr.2018.4.1.7
18. Khan MSA, Ahmad I. Herbal medicine: current trends and future prospects. In: New Look to Phytomedicine. Elsevier; 2019:3–13.
19. Seble WY, Zemede A, Ensermu K. Ethnobotanical study of medicinal plants used by local people in menz gera midir district, north Shewa zone, Amhara regional state, Ethiopia. J Med Plant Res. 2018;12(21):296–314. doi:10.5897/JMPR2018.6616
20. Pires Jr. EO
21. Rewicz A, Myśliwy M, Adamowski W, Podlasiński M, Bomanowska A. Seed morphology and sculpture of invasive Impatiens capensis Meerb. from different habitats. Peer J. 2020;8:e10156. doi:10.7717/peerj.10156
22. Singh P, Singh R, Sati N, Ahluwalia V, Sati OP. Phytochemical and pharmacological significance of genus: impatiens. Int J Life Sci Res. 2017;3(1):868–881. doi:10.21276/ijlssr.2017.3.1.20
23. Szewczyk K, Orzelska-Górka J, Polakowska M, Biała G. Antinociceptive and antianxiety activity of hydroethanolic extracts of three Impatiens species in mice. Acta Pol Pharm. 2018;75(4):989–1001. doi:10.32383/appdr/80855
24. Luo C, Huang W, Li Y, et al. The complete chloroplast genome sequence of horticultural plant, impatiens hawkeri (Sect. Balsaminacea, impatiens). Mitochondrial DNA B. 2020;5(1):119–120. doi:10.1080/23802359.2019.1698339
25. Abdela G, Sultan M, Amano T. Ethnobotanical study of medicinal plants in Heban Arsi District, Oromia, South Eastern Ethiopia. Adv Life Sci Technol. 2018;68:27–45.
26. Amare F, Getachew G. An ethnobotanical study of medicinal plants in chiro district, West Hararghe, Ethiopia. Afr J Plant Sci. 2019;13(11):309–323. doi:10.5897/AJPS2019.1911
27. Birhan YS, Kitaw SL, Alemayehu YA, Mengesha NM. Ethnobotanical study of medicinal plants used to treat human diseases in Enarj Enawga district, East Gojjam zone, Amhara region, Ethiopia. SM J Med Plant Stud. 2017;1(1):1–20. doi:10.36876/smjmps.1006
28. Bitew H, Gebregergs H, Tuem KB, Yeshak MY. Ethiopian medicinal plants traditionally used for wound treatment: a systematic review. Ethiop J Health Dev. 2019;33(2):1.
29. Miazga-Karska M, Szewczyk K, Klimek K, Ginalska G. In vitro activity of peptide fractions from impatiens glan-dulifera against caries causing bacteria. Acta Pol Pharm. 2017;74(2):710–714.
30. Couto M, Cates C. Laboratory guidelines for animal care. In: Vertebrate Embryogenesis. Springer; 2019:407–430.
31. Demsie DG, Yimer EM, Berhe AH, Altaye BM, Berhe DF. Anti-nociceptive and anti-inflammatory activities of crude root extract and solvent fractions of Cucumis ficifolius in mice model. J Pain Res. 2019;12:1399. doi:10.2147/JPR.S193029
32. Tatiya AU, Saluja AK, Kalaskar MG, Surana SJ, Patil PH. Evaluation of analgesic and anti-inflammatory activity of Bridelia retusa (Spreng) bark. J Tradit Complement Med. 2017;7(4):441–451. doi:10.1016/j.jtcme.2016.12.009
33. Yasmen N, Aziz M, Tajmim A, Akter M, Hazra AK, Rahman S. Analgesic and anti-inflammatory activities of diethyl ether and n-Hexane extract of polyalthia suberosa leaves. Evid Based Complementary Altern Med. 2018;2018:1–8. doi:10.1155/2018/5617234
34. Jan S, Khan MR. Antipyretic, analgesic and anti-inflammatory effects of Kickxia ramosissima. J Ethnopharmacol. 2016;182:90–100. doi:10.1016/j.jep.2016.02.020
35. Verdam MCDS, Guilhon-Simplicio F, Andrade KCD, et al. Analgesic, anti-inflammatory, and antioxidant activities of byrsonima duckeana W. R. Anderson (Malpighiaceae). Sci World J. 2017;2017:1–8. doi:10.1155/2017/8367042
36. Karbab A, Mokhnache K, Ouhida S, et al. Anti-inflammatory, analgesic activity, and toxicity of Pituranthos scoparius stem extract: an ethnopharmacological study in rat and mouse models. J Ethnopharmacol. 2020;258:112936. doi:10.1016/j.jep.2020.112936
37. Neevashnhi N, Anandarajagopal K, Sunilson JAJ. Anti-inflammatory activity of impatiens balsamina roots and stem. Sch Acad J Pharm. 2017;6(8):368–371.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.