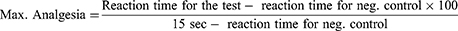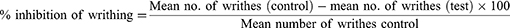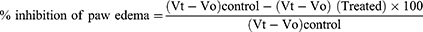Back to Journals » Journal of Inflammation Research » Volume 15
Anti-Inflammatory and Analgesic Activity of Methanolic Root Extract of Verbascum sinaiticum Benth
Authors Asefa M, Teshome N , Degu A
Received 10 September 2022
Accepted for publication 12 November 2022
Published 22 November 2022 Volume 2022:15 Pages 6381—6392
DOI https://doi.org/10.2147/JIR.S389430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Professor Ning Quan
Minda Asefa, Nathnael Teshome, Abel Degu
Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Abel Degu, Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, Addis Ababa University, P.O. Box 1176, Addis Ababa, Ethiopia, Email [email protected]
Introduction: Pain in its various forms is undoubtedly the most common ailment known to human beings. Non-steroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics are widely used to treat pain. However, long-term use of NSAIDs and opioids causes serious adverse effects on various organs. As a result, looking for drugs with better efficacy and lesser adverse effects appears crucial. For this purpose the obvious search begins from traditional medicines, particularly herbs. Therefore, this study investigated analgesic and anti- inflammatory activity of 80% methanol root extract of Verbasicum sinaiticum Benth (VS) in vivo.
Methods: The dried and crushed plant material was macerated with 80% methanol sequentially and dried with lyophilizer. As per the acute toxicity study conducted elsewhere, 100 mg/kg, 200 mg/kg and 400 mg/kg doses of extract were used in the acetic acid induced writhing, hot plate test, as well as carrageenan and formalin induced anti-inflammatory models. As a positive control, aspirin 150 mg/kg was used for anti-nociceptive and anti-inflammatory model and morphine 10 mg/kg was used for central analgesic models.
Results: VS200 and VS400 doses of the extract significantly (p< 0.05) reduced acetic acid induced writhing as compared with the control group. Similarly in hot plate test also, both VS200 and VS400 groups demonstrated significant (p< 0.05 at 30 min and p< 0.001 at 60 and 120 min) analgesic effect in comparison with the control and VS100 groups. Furthermore, in carrageenan and formalin induced anti-inflammatory test both VS200 and VS400 were shown to produce significant (p< 0.05) anti-inflammatory effect at the later hours and days.
Conclusion: The findings from this study suggest that 80% methanol root extract of V. sinaiticum possesses peripheral and central analgesic as well as anti-inflammatory activity, possibly emanating from the phytochemicals present in the hydroalcoholic crude extract.
Keywords: analgesic activity, anti-inflammatory, acetic acid induced writhing, hot plate, Verbascum sinaiticum
Introduction
Pain is the most commonly diagnosed symptom that people encounter starting from childhood. It is the body’s defense mechanism and unpleasant sensation, physical discomfort and emotional distress in response to harmful stimulus.1,2 It is a complex experience which has emotional affective, cognitive-evaluative, motivational and spiritual feelings beyond “sensory-discriminative” dimensions.
Pain in its various forms is undoubtedly the most common ailment familiar to humans. According to International Association for the Study of Pain (IASP), 1 in 5 persons globally have some form of chronic pain, 1 in 10 adults receive a yearly diagnosis of the condition,3 and 1 in 9 young adults live with chronic pain.4 While the prevalence of pain in childhood varies from 11% to 38% depending on pain type and the variability of studies.5 This epidemiology implies that pain is a major health concern around the world.
Pain is one of the cardinal features of inflammation which co-occurs with erythema, heat, swelling and loss of function where all this make up a typical inflammatory condition. Although, inflammation is one the physiological homeostasis mechanisms and sometimes its impact may go way beyond maintenance of balance. It will reduce the threshold of pain sensation and create a hyperalgesic state.6,7
The treatment of pain involves the use of various medications including NSAIDs and opioid analgesics. Despite the fact that such class of drugs provides huge relief in the management of pain, they are not free of debilitating adverse effects in some groups of individuals. Some of the most commonly associated adverse effects are hyperglycemia, gastrointestinal tract irritation, cardiovascular problems, hepatotoxicity and toxicity particularly in the elderly.8 Moreover, opioids are also not immune to major adverse effects, some of them are dependence, sedation, constipation, and respiratory problems.9
Thus, as experience shows, the ideal path to explore alternatives is plants. In this regard, in Ethiopia, around 80% of the population relies on traditional medicine for the treatment of various ailments.10 As a result, the plant V. sinaiticum was selected as experimental plant as it is widely used traditionally as a pain killer.
Verbascum L. is the largest genus of figwort family or Scrophulariaceae, which consists of about 360 species and are abundant in Africa around the Mediterranean region. Scrophulariaceae are grouped under the order of Lamiales, consisting of about 306 genera and over 5850 species.10,11
V. sinaiticum Benth is an erect herb up to 2 m tall which flowers perennially. Usually, it is noticeable around the roads and have a thick mat of soft white hairs which cover both leaves and stem.10 Locally, this plant is named Kutina, ye Ahya joro, Daba Keded in Amharic; Gurra harree, Abokena in Afan Oromo;12 Tirnake, Handega, Kunama Luta in Tigrigna.13
Ethiopians use V. sinaiticum root for tumor,14 rheumatic pain, wound healing, ophthalmic diseases, mental illness, amnesia, tapeworm, syphilis, gonorrhea, relapsing fever, elephantiasis, cold and chest diseases.15–17
The root is chewed for tooth ache, crushed, powdered and creamed on affected part with butter for wound healing, and taken with water for snake bite, leech infection and lymphadenitis through oral or nasal route.18–20 The phytochemical analysis of V. sinaiticum leaves show two flavonolignans, hydrocarpin, the novel sinaiticum, flavones, chrysoeriol, and luteolin. They have dose-dependent cytotoxicity against leukemia cancer cells.17
Thus, this study aimed to assess whether the experimental plant possesses anti-inflammatory and anti-nociceptive activities. Moreover, this investigation may provide a point of departure for further research into and identification of specific phytochemicals accountable for the reported activities.
Materials and Methods
Drugs, Chemicals and Equipment
Distilled water, methanol (Carlo-Erba, France), 0.6% acetic acid (Sigma-Aldrich, USA), aspirin (Aspirin Cardio, Germany), and morphine (Sandoz, Germany) were used for this experiment. The materials and equipment used to perform the experiments were hot plate, mortar and pestle, electronic balance, conical flask, measuring cylinder, test tubes, filtering funnel, filtering flask, filter paper, rotary evaporator (Buchi, Switzerland), lyophilizer (Operon, Korean), oral gavage, syringes, scissors, electronic balance, and permanent marker.
Collection and Authentication of Plant Specimen
Fresh roots of V. sinaiticum were collected from Menz mama woreda (Latitude, 10°14’60.00” N and longitude, 39°19’60.00” E) about 256 km from Addis Ababa on December, 2021 G.C. The plant was authenticated by Mr. Melaku Wondafrash, a taxonomist at the National Herbarium, Addis Ababa University, college of natural and computational sciences, department of biology, where a voucher specimen was kept for future reference with voucher number (MA 001).
Extraction
The roots (400 g) of the plant were thoroughly washed with tap water to remove dirt and soil. After that, they were cut into pieces manually and dried under shade in a well-ventilated area and then pulverized using a mortar and pestle to get a coarse powder for the extraction. The air-dried and powdered plant materials were macerated with 80% methanol and frequently shaken manually for 72 hrs. The mixture was filtered using gauze and Whatman filter paper number 1 with pore size of 150 mm in diameter. The methanol in the filtrate of the extract was removed under reduced pressure by rotary evaporator at 45 rpm and 40°C to obtain 80% methanol extract. The extract was further dried with lyophilizer at −50 °C and vacuum pressure (200 mBar). The net weight of dry root extract of V. sinaiticum was measured and yield percentage was 14.2%. Extract was stored in a refrigerator at −4°C until use.
Phytochemical Screening
Based on the qualitative phytochemical analysis procedure described elsewhere,21 the extract was examined for its potential composition of several natural constituents, including alkaloids, flavonoids, phenols, steroids, tannins, terpenes, and saponins.
Total Phenolic Content
The Folin-Ciocalteu technique was used to determine the amount of phenol present overall. Gallic acid was serially diluted in methanol at concentrations of 1, 0.5, 0.25, and 0.125 mg/mL to produce a calibration curve. Then one milliliter of gallic acid was added to test tubes. The test tubes were then filled with 0.5 milliliters of 2N Folin-reagent Ciocalteu’s and 5 milliliters of distilled water (1:20). After 8 minutes, 2 mL of Na2CO3 (7.5%) was added, and then, until the solution level reached 10 milliliters, pure water was added. After that, the solution was kept at room temperature for another 30 minutes. A UV-Vis spectrophotometer was used to test the solution’s absorbance at 765 nm (Jenway Model 6500, England). The experiment was run three times, with the average result being used. Similar procedures were followed in the preparation of the control solution and the extract (0.5mg/mL). The total phenolic content was calculated using the gallic acid equivalent milligrams per gram of extract.22
Total Flavonoid Content
A test for the production of aluminum chloride complexes was used to gauge the extracts’ overall flavonoid content. A calibration curve was created by sequentially diluting quercetin in methanol at concentrations of 0.5, 0.25, 0.125, and 0.065 mg/mL. Then, one milliliter of diluted quercetin was added to test tubes. The addition of 0.3 milliliters of 5% NaNO2 was followed by a 5-minute waiting period. An additional 0.3 milliliters of 10% AlCl3 were added to the solution, and it was left to stand for 5 minutes. After adding 2 milliliters of a 1M NaOH solution to the solution, 10 milliliters of distilled water were added. After that, the mixture was left to rest for 30 minutes at room temperature. The solution’s absorbance at 510 nm was measured by a UV-Vis spectrophotometer (Jenway Model 6500, England). The same steps were taken for the blank solution and the extract (0.5mg/mL). The total flavonoid concentration of the extract was reported as mg of quercetin equivalent per 1g of extract. The experiment’s average outcome was used after three tests.23
Experimental Animals
A total of sixty healthy Swiss albino mice with body weight ranging from 22 to 35 g, aged 4–6 weeks, either sex, were obtained from School of Pharmacy, Addis Ababa University. Sixty Wistar albino rats (Rattus norvegicus), weighing 250–350g and aged 4–6 months, were obtained from the Ethiopian Public Health Institute (EPHI) (animals used for anti-inflammatory activity test). Animals were left for fourteen days at standard housing conditions for acclimatization. They were housed in a stainless steel cage at room temperature with 12 h light-dark cycle and provided with a standard pellet diet and water ad libtum. All procedures and techniques used in this study were in accordance with the guide for care and use of laboratory animals.24 The Ethical Review Committee of Addis Ababa University, College of Health Sciences, School of Pharmacy (ERB/SOP/474/14/2022) granted its approval for the experimental procedure.
Grouping and Dosing
Mice were randomly divided into five groups with each group consisting of 6 mice. Group I served as negative control and mice were administered distilled water. Group II, Group III and Group IV were given 100mg/kg, 200mg/kg and 400mg/kg of the extract, respectively based on the acute toxicity study conducted elsewhere.25 Group V received standard drug morphine for hot plate 10mg/kg, while 150 mg/kg of aspirin (ASA) was used for writhing test, carrageenan induced paw edema and formaldehyde-induced paw edema models. Administration of all agents was performed via an oral route using entragastric gavage.
To assess the anti-inflammatory activity, five groups of six rats from each group were used in each model. Group I served as negative control and mice were administered distilled water. Three different doses of the extract (100 mg/kg, 200 mg/kg, and 400 mg/kg) were given to groups II through V, respectively, along with the conventional medication aspirin (150 mg/kg). The dose was calculated using the plant product’s safe dose, which was established as 2000 mg/kg via a limit test.25 A maximum of 10 mL/kg of the full dosage was taken orally. In order to create an oral solution, distilled water was used to dissolve both the extract and aspirin.
Experimental Models
Hot Plate Method
This test was used to measure the analgesic effect of herbal extract by applying thermal pain stimuli to animals and then the pain reaction time was measured as threshold for acute pain. Every mouse was placed on hot plate maintained at 55± 1◦C. The pain reaction time, latency per second, between placing the animal on hot plate and kicking, jumping, licking or holding hind limbs was measured for each tested mouse. Fifteen seconds was considered as cut-off time to avoid any thermal injury to the paw. Pain reaction times were recorded before and at 30, 60, 90, and 120 minutes after treatment in order to assess the analgesic effect of extract at different dose and the time effect response. The prolongation of latency time of treatment groups was compared with the value of control one. The percentage of antinociceptive maximal possible effect (MPE) was calculated from the formula:26
Acetic Acid Induced Writhing Method
This test was conducted to investigate the peripheral analgesic activity of extract. Five minutes after administration of acetic acid IP, the numbers of writhes were counted to determine analgesic activity of V. sinaiticum. Abdominal contractions together with stretching of the hind limbs were cumulatively counted over a period of 25 minutes. The percentage protection against writhing was taken as an index of analgesia and calculated using the following formula:27
Anti-Inflammatory Activity
Carrageenan Induced Paw Edema
Acute inflammation was induced via subplantar administration of carrageenan (0.03mL of 1% w/v in normal saline) to the rat’s right hind paw.28 The extract, the standard drug or the vehicles were administered 1hr prior to administration of the phlogistic agent, carrageenan. The inflammation was measured in mL, via quantifying the displaced water by edema using a digital plethysmometer (Ugo Basile Company, Cat No 7140, Italy) at 0, 1, 2, 3 and 4 hr after carrageenan injection.29 Acetylsalicylic acid (150 mg/kg) was used as a standard drug. The following formula was used to calculate the percent inhibition of edema in comparison to the control groups:30
Where, - Vt, is the right hind paw thickness volume (in mL) at time t,
Vo, is the right hind paw thickness volume (in mL) before carrageenan injection,
Vt –Vo, control and treated edema or paw size after carrageenan injection for control and drug treated groups respectively.
Formalin-Induced Paw Edema
Sub-acute inflammation was induced by sub-plantar administration of formalin (0.02 mL of 2% v/v, in distilled water) into the right hind paw of rats on the 1st and 3rd days of observation. Then a mark was placed at the level of lateral malleolus on the right hind paw before formalin induction. Thus, during the observation period, the injected paw would be immersed to the same extent in the measurement chamber of the Plethysmometer. After that, each test substance (extract, the standard drug and the vehicle) was administered 1hr before the paw volume was measured daily using Plethysmometer until the 7th day and the percentage of edema inhibition was calculated using the previously mentioned formula.31
Statistical Analysis
The analysis was conducted using statistical package for social science (SPSS) version 25 and Graph pad prism version 8.1. One way analysis of variance (ANOVA) was used to analyze the data followed by Tukey post hoc test to determine statistical significance. All the data were expressed as mean ± standard error of the mean (SEM). P values ≤ 0.05 were taken as statistically significant.
Result
Preliminary Phytochemical Screening
As indicated in Table 1, a qualitative phytochemical screening of an 80% methanol root extract of V. sinaiticum revealed the presence of alkaloids, tannins, flavonoids, phenols, steroids and glycoside; however, saponins and terpenoids were not detected in the extract.
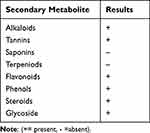 |
Table 1 Phytochemical Screening of Methanolic Root Extract of Verbascum Sinaiticum |
Total Phenol and Flavonoid Content
The total phenol and flavonoid content of V. sinaiticum root extract found from 80% methanol crude extract was determined to be 212mg GAE/g (Figure 1) and 87mg QE/g (Figure 2) respectively.
 |
Figure 1 Total phenol content of 80% methanol crude extract of root of Verbascum sinaiticum. |
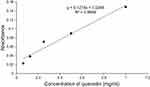 |
Figure 2 Total flavonoid content of 80% methanol crude extract of root of Verbascum sinaiticum. |
Hot Plate Assay
Both VS200 and VS400 groups demonstrated significant (p< 0.05 at 30 min and p< 0.001 at 60 and 120 min) analgesic effect in comparison with control and VS 100 groups (Table 2). The same pattern with delayed pain reaction time was also noticed in MO10 group. Likewise VS400 was found to exhibit similar significance as that of MO10 throughout the experiment period. In fact, maximum analgesic effect was found at 120 min and percentage inhibition of VS200, VS400 and MO10 was 72.7%, 81.8% and 88% respectively. From all extract groups, VS400 demonstrated higher percentage inhibition throughout experiment period than DW, VS100 and VS200. However, VS400 was found to exhibit lower percentage inhibition than MO10, until 90 min, where it exceeded the MO10 group in percentage inhibition of pain.
 |
Table 2 Effect of Methanolic Root Extract of Verbascum Sinaiticum on Hot Plate Test in Mice |
Acetic Acid Induced Writhing Assay
Unlike the VS100 group of the extract, VS200 and VS400 groups significantly reduced acetic acid induced writhing (p< 0.05) compared with the control group. Similarly, ASA150 also showed significant anti-nociceptive effect (p< 0.01) against acetic acid induced writhing (Table 3).
 |
Table 3 Effect of Methanolic Root Extract of Verbascum Sinaiticum on Acetic Acid Induced Writhing Test in Mice |
Anti-Inflammatory Activity
Carrageenan Induced Paw Edema
Figures 3 and 4 demonstrate how an anti-inflammatory test employing carrageenan-induced edema showed that 80% methanol extract of the V. sinaiticum plant extract had significant anti-inflammatory activities. Up until 3 hours after carrageenan administration, neither the test drug nor ASA150 showed particularly strong anti-inflammatory activity.
As shown in Figure 3, at the third and fourth hours, VS200, VS400, and ASA150 (P 0.001) significantly reduced paw edema. For VS200, VS400, and ASA150, respectively, the percentage of paw edema protection at 3 hours was 21.64%, 28.14%, and 28.57% (Figure 4). For VS200, VS400, and ASA150, respectively, the percentages of protection against paw edema at 4 hours were 19.05%, 19.52%, and 27.61% (Figure 4).
Formalin-Induced Paw Edema
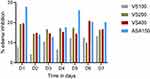
Starting on day 1, two of the treatment dose groups of 80% methanol extract of V. sinaticum (200 and 400mg/kg) resulted in a significant decrease in paw volume (Table 4). Despite the fact that there was a significant reduction in mean paw edema volume in VS200, VS400 and ASA150, the extract’s activity appears to fade away at the last days of measurement. Furthermore, the lower dose VS100 did not exhibit significant activity in all days of measurement. Besides, larger percentage inhibition of edema was detected for VS100, VS200, and VS400, respectively, as 12.9%, 19.04%, and 20.12% (Figure 5). However, all doses of the extract had a smaller effect than the positive control (ASA150), which resulted in a maximum percentage of edema inhibition of 27.61%.
 |
Table 4 Effect of Methanolic Root Extract of Verbascum Sinaiticum in Formalin-Induced Paw Edema in Mice |
Discussion
Long-term use of analgesics has various adverse effects. As a result, searching for effective drugs with minimal untoward effects from medicinal plants seems reasonable. V. sinaiticum is the most common traditionally utilized medicinal herb among Ethiopians.32 There are medicinal plants which have analgesic effect with good safety profile. This may be due to the various individual and/or synergistic pharmacological effects of phytochemical constituent present in the plant.33 Although this plant is used as pain killer traditionally, there are no studies which support the traditional claim. Therefore the aim of this study was to investigate the analgesic effect of 80% methanol root extract of V. sinaiticum Benth in vivo.
Hot plate assay was performed to investigate central analgesic activity of extract. In this model, it was investigated whether the test substance interfered in pain transduction, thereby inhibiting pain sensation. In this regard, the procedure follows placing mice on the hot plate and watching for jumping response and paw licking. The animals are more sensitive to thermal heat and thereby 15 seconds were considered as cut-off time to avoid injury. For this test, morphine was used as a positive control. It is a potent central acting analgesic drug which binds with opioid receptors namely mu, kappa and delta receptor. Morphine interferes with pain signaling from the periphery to the spinal cord and activates the periaqueductal gray matter to release endogenous peptides, which play a great role in descending inhibitory pathways.2,33
In the hot plate test, all experimental groups were found to exhibit maximum analgesia at 120 minutes. This may be due to lag time for the drug to enter into the central compartment and distribute to the target site. Apart from VS100, both VS200 and VS400 groups demonstrated significant (p< 0.05 at 30 min and p< 0.001 at 60 and 120 min) analgesic effect in comparison with control. The percentage inhibition of VS200, VS400 and MO10 was comparable (Table 2). In particular, VS400 and MO10 did not show kicking, jumping, licking or holding hind limbs responses at 15 minutes. This showed the significant analgesic activity of VS400 and MO10. The prolonged latency shown further corroborates the dose dependent pharmacologic effects of the extracts.
Acetic acid induced writhing method is a typical test to investigate the peripheral analgesic effect of the extract. Pain mediators; PGE2 and PGF are released after intraperitoneal administration of acetic acid at the peritoneal receptors. Those mediators induce irritation leading to stretching of limbs and elongation of the body together with constriction of abdominal muscles.34 Perhaps 80% methanol root extract of V. sinaiticum may elicit its effect via inhibition of pain mediators at the stage of synthesis, release or uptake.35,36
Apart from VS100, the remaining experimental groups VS200 and VS400 showed significant (p < 0.05) peripheral analgesic activities by reducing the amount of writhing in comparison with the control group (Table 3). The finding suggested that the peripheral analgesic activity of the extract increased from VS200 to VS400. This provides an impression that the extract seemed to follow a dose dependent analgesic effect.
Despite significant difference existing among the extracts, the lower average writhe recorded was for ASA150 and the extracts’ writhe level fell as the dose range increased. This also explains the dose dependent peripheral analgesia induced by the extract. However, the analgesic effect exhibited by the extract was found to be lower than that of standard drug aspirin.
Overall from the experimental groups, VS400 showed significant effect in reducing writhe in acetic acid induced test as well as in extending the pain reaction time throughout the whole experiment period compared to DW, VS100 and VS200. The individual and/or synergistic pharmacologic actions of the many phytochemicals found in the plant may be responsible for the reported extract’s peripheral and central analgesic effects.
The carrageenan-induced hind paw edema model has been widely used in the development and testing of anti-inflammatory medicines.37 The capacity of such medicines to inhibit the edema developed in the hind paw of mice after injection of a phlogistic agent is the basis for this model. The ability of anti-inflammatory drugs to reduce edema generated in mice paws by carrageenan is one of the most extensively used in vivo animal experiments.38
As evidenced by decreased paw edema and higher percent edema protection in carrageenan-induced paw edema model, VS200 and VS400 demonstrated strong anti-inflammatory properties. The extracts’ anti-inflammatory activity became more apparent over time, especially for VS200 and VS400; this may be a sign that a dose greater than 200mg/kg is required to produce an anti-inflammatory response. The extracts and standard drug exhibited their pharmacological activity after three hours, this might be explained by the lag-time required for extract and drug to reach the site of action. In terms of paw edema reduction and percentage edema protection, the extract’s effectiveness is comparable to that of aspirin (ASA150) (Figures 4 and 5).
Intraplantar injection of diluted formalin induces characteristic mechanistic changes in two distinct phases, the first phase is marked by pain, which is brought on by the direct activation of nociceptive neurons, and the second phase is marked by inflammation, which results from increased vascular permeability and tissue damage.39 According to numerous studies, the pain and inflammation brought on by the administration of formalin have been found to be reduced by bradykinin receptor antagonists that operate through the B2 receptor.40
In formalin induced paw edema, VS400 significantly reduced the edema compared to the other extract doses (p<0.05), whereas VS200 showed a better effect than VS100 (p<0.05). Furthermore, larger percentage inhibition of edema was detected for VS200, and VS400, respectively (Figure 5). However, all doses of the extract had a lesser effect than the positive control (ASA150), which resulted in a maximum percentage of edema inhibition.
As illustrated in the phytochemical screening and quantification result, V. sinaiticum extract is rich in numerous phytochemicals which are known to elicit several pharmacological activities including analgesia and anti-inflammatory effect. According to studies conducted on the biological activities of the various phytochemicals, the flavolignans41 and flavones,42,43 glycosides,44 saponins45 and tannins46 have been shown to induce analgesia and anti-inflammatory effects due to their ability to inhibit key enzymes involved in the inflammation process such as COX-1 (cyclooxygenase 1), COX-2 (cyclooxygenase 2), sPLA2 (secretory phospholipase A2) and 15-LOX-2 (15-lipoxygenase).
As illustrated elsewhere, different plant species within the genus verbascum namely V. chionophyllum Hub.-Mor., V. cilicicum Boiss., V. dudleyanum (Hub.-Mor.) Hub.-Mor., V. latisepalum Hub.-Mor., V. pycnostachyum Boiss. and Heldr., V. salviifolium Boiss., V. splendidum Boiss., and V. exuberans Hub.-Mor.47,48 have been shown to possess analgesic and anti-inflammatory activity. The observed analgesic and anti-inflammatory activity in V. sinaiticum is also in line with the activities observed in other species within the genus.
Conclusion
The findings from this study suggests that 80% methanol root extract of V. sinaiticum possesses significant peripheral anti-nociceptive and central pain inhibition effects as well as anti-inflammatory activity. The extract’s analgesic and anti-inflammatory activity possibly emanate from the phytochemicals present in the hydroalcoholic crude extract. Therefore, the results obtained justify the use of the roots of V. sinaiticum for analgesia, which supports the traditional use of the plant.
Abbreviations
ANOVA, analysis of variance; ASA, acetyl salicylic acid; COX;-Cyclooxygenase; DW, distilled water; IASP, international association for the study of pain; LOX;-15-lipoxygenase; MO, morphine; NSAIDs;- non-steroidal anti-inflammatory drugs; PG, prostaglandins; sPLA2;- secretory phospholipase A2; SEM, standard error of the mean; SPSS, statistical package for social science; VS, Verbascum sinaiticum.
Data Sharing Statement
The datasets used and/or analyzed during the current work are available from the corresponding author upon reasonable request.
Ethics Approval and Consent
The protocol was approved by institutional review board of the School of Pharmacy with Reference no. ERB/SOP/474/14/2022.
Acknowledgments
We would like to thank Addis Ababa University College of Health Sciences School of Pharmacy for allowing the study to be conducted with facility of the institution.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Swieboda P, Filip R, Prystupa A, Drozd M. Assessment of pain, types, mechanism and treatment. Ann Agric Environ Med. 2013;1:2–7.
2. Gordon DB, de Leon-Casasola OA, Wu CL, Sluka KA, Brennan TJ, Chou R. Research gaps in practice guidelines for acute postoperative pain management in adults, findings from a review of the evidence for an American pain society clinical practice guideline. J Pain. 2016;17:158–166. doi:10.1016/j.jpain.2015.10.023
3. International Association for the Study of Pain. Unrelieved pain is a major global healthcare problem; 2012.
4. Murray CB, de la Vega R, Murphy LK, Kashikar-Zuck S, Palermo TM. The prevalence of chronic pain in young adults, a systematic review and meta-analysis. Pain. 2022;163:972–984. doi:10.1097/j.pain.0000000000002541
5. King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited, a systematic review. Pain. 2011;152:2729–2738. doi:10.1016/j.pain.2011.07.016
6. Kidd BL, Urban LA. Mechanisms of inflammatory pain. BJA. 2001;87:3–11. doi:10.1093/bja/87.1.3
7. Leah E. An endogenous resolution for inflammatory pain? Nat Rev Drug Discov. 2010;9:433. doi:10.1038/nrd3192
8. Schofield P, Dunham M, Martin D, et al. National guidelines for the management of pain in older adults; 2019.
9. Baldini A, Von Korff M, Lin EH. A review of potential adverse effects of long-term opioid therapy, a practitioner’s guide. Prim Care Companion CNS Disord. 2012;14:27252.
10. Edwards. Some Wild Flowering Plants of Ethiopia. Addis Ababa University Press; 1976.
11. Mahmoud SM, Abdel-Azim NS, Shahat AA, Ismail SI, Hammouda FM. Phytochemical and biological studies on Verbascum sinaiticum growing in Egypt. Nat Prod Sci. 2007;13:186–189.
12. Yineger H, Kelbessa E, Bekele T, Lulekal E. Ethnoveterinary medicinal plants at Bale Mountains National Park, Ethiopia. J Ethnopharmacol. 2007;112:55–70. doi:10.1016/j.jep.2007.02.001
13. Chekole G. Ethnobotanical study of medicinal plants used against human ailments in gubalafto district, Northern Ethiopia. J Ethnobiol Ethnomed. 2017;13:1–29. doi:10.1186/s13002-017-0182-7
14. Abebe W. An overview of Ethiopian traditional medicinal plants used for cancer treatment. European Journal of Medicinal Plants. 2016;14(4):4. doi:10.9734/EJMP/2016/25670
15. Gondal HY, Zamir R, Nisar M, Choudhary MI. Verbascum sinaiticum, a rich source of antioxidant phenylethanoid glycosides. Nat Prod J. 2020;10:158–162.
16. Lalrinzuali K, Vabeiryureilai M, Jagetia GC. Investigation of the anti-inflammatory and analgesic activities of ethanol extract of stem bark of sonapatha oroxylum indicum in vivo. Int J Inflam. 2016;2016. doi:10.1155/2016/8247014
17. Yeabyo S, Zenebe Teka M, Gopalakrishnan VK, Hagos Z, Krishna Chaithanya K. Antibacterial activity of root extracts of Verbascum sinaiticum against multidrug-resistant Enterobacteriaceae family Gram-negative and two Gram-positive bacteria. Drug Invent Today. 2018;10:1387–1394.
18. Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in kilte awulaelo district, Tigray region of Ethiopia. J Ethnobiol Ethnomed. 2013;9:1–23.
19. Enyew A, Asfaw Z, Kelbessa E, Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche district. Int J Pharm Clin Res. 2014;6:154–167.
20. Megersa M, Jima TT, Goro KK. The use of medicinal plants for the treatment of toothache in Ethiopia. Evid Based Complement Alternat Med. 2019;2019. doi:10.1155/2019/2645174
21. Harborne AJ. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. springer science & business media; 1998.
22. Shi P, Du W, Wang Y, Teng X, Chen X, Ye L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.). Urb Int J Food Sci Nutr. 2019;7:148–154.
23. Nigatu H, Belay A, Ayalew H, et al. In vitro antileishmanial activity of some Ethiopian medicinal plants. J Exp Pharmacol. 2021;13:15–22. doi:10.2147/JEP.S285079
24. Care IoLARCo, Animals UoL. Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services. National Public Health Service; 1986.
25. Mergia E, Shibeshi W, Terefe G, Teklehaymanot T. Antitrypanosomal activity of Verbascum sinaiticum Benth. (Scrophulariaceae) against Trypanosoma congolense isolates. BMC Complement Altern Med. 2016;16:1–9. doi:10.1186/s12906-016-1346-z
26. Abbas DA. Analgesic, Anti-inflammatory and antidiarrhoeal effects of datura stramonium hydroalcoholic leaves extract in mice. Ijrras. 2013;14:193–199.
27. Alemu A, Tamiru W, Nedi T, Shibeshi W. Analgesic and anti-inflammatory effects of 80% methanol extract of Leonotis ocymifolia (Burm.f.) iwarsson leaves in rodent models. Evid Based Complement Alternat Med. 2018;2018:1–8. doi:10.1155/2018/1614793
28. Owoyele BV, Nafiu AB, Oyewole IA, Oyewole LA, Soladoye AO. Studies on the analgesic, anti-inflammatory and antipyretic effects of Parquetina nigrescens leaf extract. J Ethnopharmacol. 2009;122:86–90. doi:10.1016/j.jep.2008.11.027
29. Mahdi-Pour B, Jothy SL, Latha LY, Chen Y, Sasidharan S. Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac J Trop Biomed. 2012;2:960–965. doi:10.1016/S2221-1691(13)60007-6
30. María R, Shirley M, Xavier C, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci. 2018;30:500–505. doi:10.1016/j.jksus.2017.03.009
31. Masson‐Meyers DS, Andrade TA, Caetano GF, et al. Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol. 2020;101:21–37. doi:10.1111/iep.12346
32. Ayele TT. A review on traditionally used medicinal plants/herbs for cancer therapy in Ethiopia, current status, challenge and future perspectives. Org Chem Curr Res. 2018;7(2):8. doi:10.4172/2161-0401.1000192
33. Irem T, Zeliha S. Traditional uses and biological activities of Verbascum species. FABAD J Pharm Sci. 2006;31:85–96.
34. Yimer T, Birru EM, Adugna M, Geta M, Emiru YK. Evaluation of analgesic and anti-inflammatory activities of 80% methanol root extract of Echinops kebericho m. (Asteraceae). J Inflamm Res. 2020;13:647–658. doi:10.2147/JIR.S267154
35. Afsar T, Khan MR, Razak S, Ullah S, Mirza B. Antipyretic, anti-inflammatory and analgesic activity of Acacia hydaspica R. Parker and its phytochemical analysis. BMC Complement Altern Med. 2015;15:1–2.
36. Alam MM, Emon NU, Alam S, et al. Assessment of pharmacological activities of Lygodium microphyllum Cav. leaves in the management of pain, inflammation, pyrexia, diarrhea, and helminths, In vivo, in vitro and in silico approaches. Biomed Pharmacother. 2021;139:111644. doi:10.1016/j.biopha.2021.111644
37. Shahidi F, Yeo J. Bioactivities of phenolics by focusing on suppression of chronic diseases, A review. Int J Mol Sci. 2018;19:1573. doi:10.3390/ijms19061573
38. Ferraz CR, Carvalho TT, Manchope MF, et al. Therapeutic potential of flavonoids in pain and inflammation, mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules. 2020;25:762. doi:10.3390/molecules25030762
39. Damas J, Liégeois JF. The inflammatory reaction induced by formalin in the rat paw. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:220–227. doi:10.1007/PL00005345
40. Arzi A, Olapour S, Yaghooti H, Sistani Karampour N. Effect of royal jelly on formalin induced-inflammation in rat hind paw. Jundishapur J Nat Pharm Prod. 2015;10(1):e22466. doi:10.17795/jjnpp-22466
41. Shah M, Jan H, Drouet S, et al. Chitosan elicitation impacts flavonolignan biosynthesis in Silybum marianum (L.) Gaertn cell suspension and enhances antioxidant and anti-inflammatory activities of cell extracts. Molecules. 2021;26:791. doi:10.3390/molecules26040791
42. Aboulaghras S, Sahib N, Bakrim S, et al. Health benefits and pharmacological aspects of chrysoeriol. Pharmaceuticals. 2022;15:973. doi:10.3390/ph15080973
43. Taheri Y, Sharifi-Rad J, Antika G, et al. Paving luteolin therapeutic potentialities and agro-food-pharma applications, emphasis on in vivo pharmacological effects and bioavailability traits. Oxid Med Cell Longev. 2021;2021:1–20. doi:10.1155/2021/1987588
44. Khan H, Pervaiz A, Intagliata S, et al. The analgesic potential of glycosides derived from medicinal plants. Daru. 2020;28:387–401. doi:10.1007/s40199-019-00319-7
45. Passos FR, Araújo-Filho HG, Monteiro BS, et al. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines, A review of pre-clinical research. Phytomedicine. 2021;96:153842. doi:10.1016/j.phymed.2021.153842
46. Soyocak A, Kurt H, Cosan DT, et al. Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum Exp Toxicol. 2019;38:1296–1301. doi:10.1177/0960327119864154
47. Tatli II, Akkol EK, Yesilada E, Akdemir ZS. Antinociceptive and anti-inflammatory activities of seven endemic Verbascum species growing in Turkey. Pharm Biol. 2008;46:781–788. doi:10.1080/13880200802315758
48. Eyiiş E, Kaygısız B, Kılıç FS, Ayhancı A. The in vivo antinociceptive and antiinflammatory effects of verbascum exuberans. Hub -Mor Turk J Pharm Sci. 2020;17:586–592. doi:10.4274/tjps.galenos.2019.77992
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.