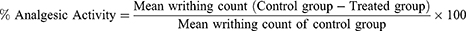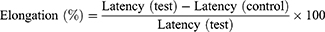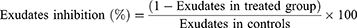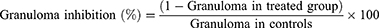Back to Journals » Journal of Experimental Pharmacology » Volume 15
Analgesic and Anti-Inflammatory Activities of 80% Methanol Extract and Solvent Fractions of Ehretia cymosa Thonn (Boraginaceae) Leaves in Rodents
Authors Ashagrie G , Abebe A, Umer S
Received 6 December 2022
Accepted for publication 15 February 2023
Published 23 February 2023 Volume 2023:15 Pages 63—79
DOI https://doi.org/10.2147/JEP.S396769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Getachew Ashagrie,1 Abiy Abebe,2 Shemsu Umer3
1Department of Pharmacy, College of Health Sciences, Woldia University, Woldia, Ethiopia; 2Biomedical Research Team, Traditional and Modern Medicine Research Directorate, Ethiopian Public Health Institute, Addis Ababa, Ethiopia; 3Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Getachew Ashagrie, Tel +251927368467, Email [email protected]
Background: Ethnobotanical studies in various districts of Ethiopia reported that Ehretia cymosa (E. cymosa) is used for the management of headache, abdominal pain, arthritis and rheumatism. However, there is no scientific investigation done so far to confirm these traditional claims. Thus, the aim of this study was to assess the analgesic and anti-inflammatory effects of the 80% methanol extract and fractions of E. cymosa leaves.
Methods: The dried and pulverized leaves of E. cymosa were soaked with 80% methanol to obtain a crude extract. Fractionation was done using chloroform, ethyl acetate and water by a soxhlet apparatus. The analgesic effects of the crude extract and solvent fractions were assessed using acetic acid-induced writhing and hot plate tests whereas anti-inflammatory activities were investigated using carrageenan-induced paw edema and cotton-pellet-induced granuloma models.
Results: In all the tested doses, the 80% methanol extract and solvent fractions revealed substantial (p < 0.001) analgesic activities in acetic acid induced writhing test. In the hot plate method, all the tested doses of E. cymosa crude extract and the solvent fractions produced significant analgesic activities (p < 0.05). In the carrageenan-induced acute inflammation model, all tested doses of the crude extract and solvent fractions resulted in a significant decline in paw edema. The 80% methanol extract and solvent fractions of E. cymosa at all the tested doses significantly reduced inflammatory exudates and granuloma mass formations (p < 0.001).
Conclusion: From the results of this investigation, it can be stated that 80% methanol extract, aqueous, ethyl acetate and chloroform fractions of E. cymosa exhibited considerable analgesic and anti-inflammatory activities, supporting the plant’s traditional use as a remedy for a variety of painful and inflammatory conditions.
Keywords: analgesic, hot plate, anti-inflammatory, Ehretia cymosa, carrageenan-induced paw edema, cotton pellet granuloma
Background
Pain is an unpleasant sensory and emotional sensation connected to or similar to actual or potential tissue injury.1 Although it is an unpleasant sensation, it has protective benefits through providing warning signal about the existence of a problem or threat.2 Pain is a dynamic and intricate phenomenon involving the interaction of numerous receptors, neurotransmitters, neural fibers, neural pathways and both distinct and dispersed anatomical sites. It begins physiologically with the stimulation of high-threshold primary sensory neurons (the nociceptor that detect noxious stimuli) and by particular transmission mechanism in their peripheral terminals and transmitted to the CNS via specialized neuronal networks.3 Drugs used to manage both chronic and acute pain are opiates, non-opioid analgesics (primarily non-steroidal anti-inflammatories), antidepressants, anticonvulsants, cannabinoids, and topical agents.4 Pain could be due to inflammatory or non-inflammatory responses to tissue damage.
Inflammation is one of the immune system’s initial defense responses to an infection or cellular/tissue damage. This reaction may also be directed against irritants, invading infections as well as autoimmune or neurodegenerative disorders. The changes that occur during an acute inflammatory event are crucial in the survival of the host, despite the fact that it can also cause numerous illnesses like cancer, rheumatoid arthritis, and cardiovascular dysfunctions.5,6 Varieties of cells are involved in the process of inflammation, and the four primary cell types that trigger inflammation are macrophages, neutrophils, lymphocytes, and mast cells. Once they have been attracted to the damaged area, they emit a variety of inflammatory mediators, including cytokines, histamine, nitric oxide (NO), prostaglandins, leukotrienes, and other substances.7 As a result, plasma proteins can enter into the tissue due to modification of the endothelial cell junctions in the blood vessel wall.8 These vascular actions are the cause of the three typical signs of inflammation; the increased blood perfusion provides the redness and heat, whereas the leakage of fluid into the tissue promotes swelling. Non-steroidal anti-inflammatory drugs (NSAIDs) are useful to diminish the detrimental effects of inflammation.9 Corticosteroids also prevent several mechanisms involved in inflammation. Glucocorticoids are useful drugs to reduce inflammation and immune activation in a variety of disorders including asthma, allergy, rheumatoid, collagen, vascular, dermatological, inflammatory bowel, and other systemic disorders.10–12 Currently available conventional medications for pain relief and management of inflammation-related disorders are challenging, especially as they cause numerous adverse effects including gastrointestinal irritation, cardiovascular toxicities, tolerance and dependency.13 In addition, most patients fail to achieve sufficient pain relief, even with the use of various analgesics and anti-inflammatory agents.14 Thus, there is a requirement for steeping up research on medicinal plants that are useful for the treatment of painful and inflammatory conditions.
In many parts of the world, medicinal plants have been utilized for thousands of years as traditional therapies for a wide range of human ailments.15 They are crucial for maintaining the wellbeing of the societies by possessing a variety of compounds with promising biological activities that alter biological process in the body.16,17 Secondary metabolites of medicinal plants, including polyphenols, flavonoids, terpenoids and alkaloids, are indispensable bases for developing analgesic and anti-inflammatory drugs.18 Ethiopia has a diversified heritage of traditional medicinal practices, known for using plants to prepare more than 90% of the remedies.19 According to reports, up to 80% of the people rely on traditional medicines as a main source of health care.20
E. cymosa is a deciduous shrub or small tree that grows 2–9 m tall and is mainly present in the Savanna and secondary jungle of Africa. The leaves are oval shaped, while the fruits are black, ovoid to globose drupe of 2–6 mm long.21 It is an indigenous plant widely distributed in various parts of Ethiopia where it is locally called “Game” in Amharic and “Hulaga, Ulaga, Garmi” in Afan Oromo. Experimental studies revealed that different extracts and solvent fractions of E. cymosa have a number of activities, including antioxidant, antihyperglycemic, antidiarrhea, for skin wounds, for paralysis, antiepileptic and antimicrobial.21,22 Ethnobotanical survey in different parts of Ethiopia indicated that E. cymosa is used in rheumatism,23 headache,24 and febrile illness (mich).25 Even though the leaves of E. cymosa were claimed for its analgesic and anti-inflammatory activities by traditional practitioners, as far as our knowledge no research has been done on the antinociceptive and anti-inflammatory activities of the plant. Therefore, it is crucial to conduct scientific research to the analgesic and anti-inflammatory effects of this plant.
Materials and Methods
Drugs and Chemicals
Carrageenan (Sigma Aldrich, Germany), Ketamine (India), Acetyl Salicylic Acid (Bayer Germany), Morphine, Indomethacin (Cadila, Ethiopia), Methanol (Carlo Erba, Italy), Chloroform & Ethyl Acetate (Bulex Laboratory, India), Sodium Phosphate, Sodium Hydroxide, Sodium Carbonate, Distilled Water (DW), Normal Saline (Addis Pharmaceutical Factory, Ethiopia), Tween 80 (Sigma Aldrich, Germany), and Glacial Acetic Acid (Basell, India) were used in the experiment.
Materials and Instruments
Lyophilizer (OPERON, OPR-FDU-5012, Korea), UV Spectrophotometer (Jenway Model 6500, England), Digital Plethysmometer (Ugo Basile-Cat no 7140, Italy), Hot plate (Ugo Basile 720), Rotary Evaporator (Heidolph, Germany), Soxhlet Apparatus, Electronic Balance (KERN-ALJ 220-4, Germany), tissue drying oven (Medite - Medizin technik, Germany).
Plant Material Collection and Authentication
The fresh leaves of E. cymosa were collected from Shashamane, Oromia, Ethiopia, in February 2021. The plant material was identified and authenticated by Mr. Melaku Wondaferash at the National Herbarium, Department of Plant Biology and Biodiversity Management, Addis Ababa University. For future use, a voucher specimen was placed with the voucher number GA 001.
Preparation of the Extract
Three-hundred grams (300g) of the dried and pulverized leaves of E. cymosa were extracted with 80% methanol by maceration for 72 hr (three times) and shaking sometimes using mini orbital shaker at 120 rpm. The resulting liquid was filtered first with muslin cloth and then with Whatman No. 1 filter paper. After extraction, methanol was vaporized under vacuum by a rotary evaporator at 40°C. The resultant solution was then frozen at −20°C until it solidified, and the residual solvent was dried by a lyophilizer. The dried powder residue/extract was weighed, placed into a glass vial, appropriately labeled and kept in a desiccator on silica gel until use.
Fractionation
The dried and powdered leaves of E. cymosa (400g) were packed into a thimble and sequentially extracted with chloroform, ethyl acetate and water using a soxhlet apparatus. The solvents were evaporated under reduced pressure and the fractions were then dried in a drying oven at a temperature not exceeding 40°C. The final semisolid mass of each fraction was kept in a desiccator until use under the same manner as the crude extract.
Experimental Animals
Healthy Swiss albino mice (6–8 weeks of age) weighing 25–35 g and Wistar albino rats (6–8 weeks of age) weighing 200–250 g were involved in the procedure. The animals were kept under standard environmental conditions in plastic cages at normal temperature and on a 12 hr light–dark cycle with unlimited access to standard pelleted diet and water ad libitum. To minimize stress, animals were acclimatized to laboratory settings before the start of the experiment in all procedures. The animals used in this study were treated according to internationally recognized standard guidelines for the use of laboratory animals.26 The School of Pharmacy, College of Health Sciences, Addis Abeba University’s ethical review committee granted its approval under protocol number ERB/SOP/180a/13/2020.
Acute Toxicity Study
Acute oral toxicity study was performed according to the internationally accepted protocol of OECD Guideline 425.26 For toxicity investigation fasted female albino mice, which were between 6 and 8 weeks old were used. A single female mouse was given 2000 mg/kg of the extract as a single dose in the initial screening test to determine the starting dose. Since no death was noticed within 24 hr, another four mice were given the same dose of extract. The animals were watched for the first four hours at intervals of 30 minutes, and then for the following 14 days at intervals of 24 hours. They were monitored for general toxic signs and symptoms like changes in skin and fur, somatomotor activities and behavioral patterns, eyes and mucous membranes, diarrhea and salivation, convulsions and tremor, food and water intake, weight loss, lethargy, paralysis and mortality.
Animal Grouping and Dosing
Swiss albino mice of both sex weighing 25–35 g (for analgesic activities) and Wistar albino rats weighing 200–250 g (for anti-inflammatory activities) were divided randomly into five groups of six animals each. Group I was allocated as negative control and received vehicles. Group II was assigned as a positive control and was given standard drugs such as morphine (20 mg/kg) for hot plate test, aspirin (150 mg/kg) for acetic acid induced writhing test, indomethacin (10 mg/kg) for carrageenan-induced paw edema and cotton-pellet-induced granuloma model of inflammation. The other three groups (test groups) received different doses (100, 200 and 400 mg/kg) of the 80% methanol extract orally.
For testing the solvent fractions, the animals were randomly assigned into eleven groups of six animals each. Group I was used as a negative control and received 2% tween 80 at a dose of 10 mL/kg. Group II served as a positive control and received morphine (10 mg/kg) for hot plate test, aspirin (150 mg/kg) for acetic acid induced writhing test, and indomethacin (10 mg/kg) for carrageenan-induced paw edema and cotton-pellet-induced granuloma model of inflammation. The next three test groups (group III–V) were given three different doses (100, 200 and 400 mg/kg) of the aqueous fraction. Another three test groups (Group VI–VIII) received ethyl acetate fraction at three dose levels, whereas the remaining three test groups (Group IX–XI) received the same three dose levels of the chloroform fraction.
Evaluation of Analgesic Activities of the Extract
Acetic Acid-Induced Writhing Test
The method reported by Yimer et al was used to demonstrate the peripheral analgesic effects of the extract (or solvent fractions).14 This procedure was carried out by randomly grouping mice that had been fasted overnight with free access to water. Mice of either sex were administered different doses of the crude extract (or solvent fractions), a vehicle (negative control) and a standard drug aspirin 150 mg/kg (positive control) 1 hr prior to acetic acid (0.6% v/v) (10 mL/kg, i.p) administration based on their particular groups. The analgesic effect of the extract (or solvent fractions) was determined five minutes after the acetic acid injection by counting the numbers of writhing, which involves contraction of the abdominal muscle along with stretching of the hind limbs for 30 minutes. The percentage protection of the number of writhes compared to the control group was served as an index of analgesia and was determined by the subsequent formula.14
Hot Plate Method
Hot plate test was performed to assess the anti-nociceptive activities of the extracts (or solvent fractions) by placing the mouse into an open-ended cylindrical chamber with a floor containing a metallic plate heated by a thermode. A plate was maintained at a constant temperature of 55°C ± 1°C and result in two behavioral responses that are described in terms of their response times that is paw licking and jumping which are supraspinally-integrated responses. Mice of either sex received different doses of the crude extract (or solvent fractions), a vehicle (negative control, P.O.) and standard drug morphine (20 mg/kg oral) based on their respective groups. They were placed individually on a hot plate with a 15-second cutoff period to prevent injuries to the animals’ paws. The latency to lick the paw or jump off the hot plate was measured at 0, 30, 60, 90, and 120 minutes to determine the reaction time. The percentage increase in reaction time or inhibition of pain threshold was calculated using the formula:27
Evaluation of Anti-Inflammatory Activity of the Extract
Carrageenan Induced Paw Edema
Acute inflammation was induced by injection of carrageenan (1% w/v carrageenan in normal saline, 100 µL) into the right hind paw of the rats. Before induction of inflammation, the paw was labeled with ink at the lateral malleolus. Carrageenan were injected into each group of rats 1 hr after oral administration of the crude extract (or solvent fractions), the vehicle and the standard drug. Inflammation was measured in mL by which displacement of water by edema with a digital plethysmometer at time 0, 1, 2, 3, 4, 5 and 6hrs after carrageenan injection. The percentage protection of edema was determined in comparison to the control rats using the formula.14
where PEC paw edema in control group, PET paw edema in test group.
Cotton Pellet Granuloma Method
This method is used to determine the transudative and proliferative (granulomatous) features of chronic inflammation.28 Male albino Wistar rats (200–250 g) were fasted for 12 h with free access to water prior to the beginning of the experiment. 2% tween 80, indomethacin (10 mg/kg P.O.) and crude extract (or solvent fractions) were given to the control, standard and test groups of rats, respectively. By rolling a piece of cotton weighing 10 mg and sterilizing it in an autoclave for 30 minutes at 120°C and 15 lbs of pressure, sterile cotton pellets weighing 10±1 mg were prepared. The rats were sedated with ketamine (50 mg/kg), and using forceps, a subcutaneous tunnel was created aseptically on both parts of the recently shaved groin region of each rat 20 minutes after administration of the standard drug and extracts (or solvent fractions). Then, a chromic catgut (0/4metric-1/2 Circle) was used to stitch two sterilized cotton pellets into a subcutaneous tunnel on either side. The standard drug (indomethacin 10 mg/kg) and extracts (or solvent fractions) were given for seven days (P.O., once a day). On the 8th day, the rats were sacrificed with anesthesia, subsequently; the pellets enclosed by granuloma tissue were carefully dissected and cleared from extraneous tissue. The wet weight of the cotton was measured and then dried at 60°C for 24 hrs and by subtracting the weight of the cotton pellets the net dry weight was recorded. The exudate amount (mg), granulation tissue formation (mg), and percent inhibition of exudate and granuloma tissue formation was calculated by the formula:
Where: Measure of exudates formation = immediate wet weight of pellet - Constant dry weight of pellet
Measure of granuloma tissue formation = Constant dry weight - Initial weight of cotton pellet.
Determination of Total Phenol, Flavonoid and Alkaloid Contents
Determination of Total Phenolic Content
The total phenolic content of leaf extract and solvent fractions of E. cymosa were estimated according to the Folin Ciocalteu colorimetric method.29 To construct the calibration curve different concentrations of the standard (gallic acid) solutions (100, 50, 25, 12.5, 6.75 μg/mL) were prepared in methanol. One mL of the standard was then moved into test tubes and 5 mL of methanol and 0.5 mL of Folin–Ciocalteu’s reagent were added into the test tubes. After 5 minutes, 1.5 mL of Na2CO3 (20%) was added and the volume was adjusted to 10 mL with methanol. Reactions were incubated for 90 min at room temperature and kept under the dark condition. Using a UV spectrophotometer, the solution’s absorbance was determined at 760 nm. The experiments were performed in triplicates to give more precise results. A similar procedure was performed for the extract or solvent fractions (100 µg/mL) and the blank solutions. The total phenolic content was estimated using a standard curve of gallic acid (y = 0.0055x - 0.195, R2 = 0.9767). Total phenolic contents were described as mg of gallic acid equivalent per g of extract or fractions.
Determination of Total Flavonoid Content
The total flavonoid content of the extract or solvent fractions of E. cymosa were determined using aluminum chloride colorimetric assay.30 Different concentrations of the standard (Quercetin) (1, 0.50, 0.25, 0.125 and 0.065 mg/mL) were prepared in methanol to obtain calibration curve. One mL of the standard was then transferred into test tubes. 0.3 mL 5% NaNO2 was added and left for 5 minutes. An additional 0.3 mL of 10% AlCl3 was mixed with the solution and left for 5 minutes. Then, 2 mL solution of 1M NaOH was added into the solution and filled with methanol to make final volume to 10 mL. Finally, the solutions were allowed to stand for 30 minutes in the dark at room temperature. The absorbance of the solution was measured at 510 nm using a UV spectrophotometer. A similar procedure was performed for the extract or solvent fractions (1 mg/mL) and the blank solutions. The total flavonoid content was calculated using a standard curve of quercetin (y=0.4321x +0.0656, R2 = 0.9783). The total flavonoid contents were described as mg of quercetin equivalent per 100 g of extracts. All of the steps were done in triplicate.
Determination of Total Alkaloid Contents
The total Alkaloid Content of the extract or solvent fractions of E. cymosa were determined by Bromocresol green (BCG) method.31 To construct a calibration curve, different concentrations of atropine (120, 60, 30, 15 and 7.5 g/mL) were made in methanol. One mL of the atropine solution was placed into separatory funnels. Then, 5 mL of phosphate buffer (PH, 4.7) and 5mL BCG solution were added to the filtrates and the mixture was shaken with 4 mL of chloroform two times. The chloroform extracts were then collected in a test tube and its final volume was adjusted to 10 mL with chloroform.
The plant extract or solvent fractions (1 mg/mL) prepared in methanol were dissolved in 2N HCl solution and then filtered. One mL of the filtrate was added into a separatory funnel and extracted with 5 mL of chloroform 2 times. The chloroform extract was removed and the PH of the remaining solution was adjusted to neutral with 0.1 M NaOH solution. To the neutralized solution 5 mL of BCG and 5 mL of buffer solution (PH, 4.7) were added and shaken. The complex was washed twice with 4 mL of chloroform using vigorous shaking. The final volume of the extract was then set to 10 mL and collected in a test tube. The absorbance of the chloroform extracts of the atropine and extract/or solvent fractions were determined at a wavelength of 470 using UV‑Spectrophotometer. The blank solution was treated in the same way. All procedures were carried out three times. The average absorbance of the blank solution was subtracted from the standard and sample solutions and the total alkaloid content was estimated using the standard curve of Atropine (y=0.00378x+0.0474, R2=0.9896) (Figure 1).
 |
Figure 1 Calibration curve of standards ((A) for gallic acid, (B) for quercetin, (C) for atropine). |
Statistical Analysis
The data were analyzed using statistical package for social science (SPSS) software version 25. The results were expressed as mean ± standard error of the mean (S.E.M.) and statistical significance was assessed by using One-way Analysis of Variance (ANOVA) followed by a Tukey post hoc test to compare variations among groups and the results were considered significant at p < 0.05. The analyzed data were then reported using tables and graph as necessary.
Results
Acute Toxicity Study
The results of acute oral toxicity test of 80% methanol extract of leaves of E. cymosa at a dose of 2000 mg/kg revealed that the extract did not produce any substantial alterations in behavior, respiration, cutaneous effects, sensory nervous system responses or gastrointestinal effects within 24 hr and in the next 14 days. There was no recorded mortality or hazardous reactions including convulsions, ataxia, diarrhea, or excessive urination. Based on the Limit Test at 2000 mg/kg of OECD guideline 425 (2008), it can be proposed that the oral LD50 of crude 80% methanol extract is higher than 2000 mg/kg in mice. Accordingly, the finding indicates that the extract has a low toxicity profile in mice.
Effect of 80% Methanol Leaves Extract and Solvent Fractions on Acetic Acid-Induced Writhing Test
The results of the acetic acid writhing test in mice are shown in Table 1. At all tested doses, the 80% methanol leaves extract of E. cymosa significantly (p < 0.001) reduced the writhing reactions of the mice produced by the intra-peritoneal injections of acetic acid in a dose-dependent manner. This was checked by performing a linear regression analysis (R2 = 0.878). The higher (400 mg/kg) dose of the extract displayed a significant analgesic effect (p < 0.001) compared to the lower and middle (100 and 200 mg/kg) doses of the extracts. Similarly, acetyl salicylic acid exhibited a significant effect (p < 0.001) compared to the 100 and 200 mg/kg dose of the extracts. However, the effect of the extract at 400 mg/kg did not differ significantly from that of aspirin (Table 1).
 |
Table 1 Effect of 80% Methanol Leaves Extract of E. cymosa Against Acetic Acid Induced Writhing Model in Mice |
Similarly, at all the tested doses, the solvent fractions exhibited significant analgesic activities (p < 0.001) compared to the control. Ethyl acetate and chloroform fraction demonstrated better reduction in the number of writhes in mice compared to the control. The ethyl acetate and chloroform fraction, in higher doses, decreased the number of abdominal constrictions significantly with 64% and 57.2% inhibition, respectively. Higher dose of ethyl acetate fraction demonstrated comparable analgesic activity with that of acetyl salicylic acid (65.5%) (Table 2).
 |
Table 2 Effects of the Solvent Fractions of E. cymosa Against Acetic Acid Induced Writhing Model in Mice |
Effects of the 80% Methanol Extract and Solvent Fractions on Hot Plate Test
In this model, all the tested doses of E. cymosa extract demonstrated substantial analgesic activity (p < 0.05) at 90 and 120 minutes of observation when compared with the negative control (Table 3). The standard drug morphine resulted in significant analgesic activity (p < 0.001) at these intervals of observations. The latency delayed by the three doses of the extract was considerably lower (p < 0.05) than that of the standard treatment at these intervals of observations. Throughout the observation, there was no significant difference between the middle and lower dose levels of the extract. The higher dose of the extract revealed a significantly different analgesic effect compared the lower and middle dose of the extract (p < 0.05) at 90 and 120 minutes of observations. The 80% methanol extract of E. cymosa at 100, 200 and 400 mg/kg doses exerted maximum analgesic effect at 120 minutes with respective values of 60.2 65 and 77.2%. The analgesic effect at this moment was shown to be dose-dependently increased. (R2 = 0.88) (Table 3).
 |
Table 3 Effects of 80% Methanol Extract of E. cymosa on Hot Plate Latency Time in Mice |
All the tested doses of the fractions (except 100 mg/kg of aqueous fraction at 30 minutes) resulted in significant (p < 0.001) delay in reaction times during 30, 60, 90 and 120 minutes of observation compared to the control group (Table 4). Higher dose of ethyl acetate fraction (400 mg/kg) and the standard drug morphine exhibited significant (p < 0.05) analgesic activities compared to the other fractions at these intervals of observations. Higher doses of aqueous, chloroform and ethyl acetate fractions showed higher increments in latency time at 120 minutes, with respective values of 63.8%, 64.6%, and 72.1% (Table 4).
 |
Table 4 Effects of Solvent Fractions of E. cymosa on Hot Plate Latency Time in Mice |
Effect of 80% Methanol Extract and Fractions on Carrageenan Induced Paw Edema
In the carrageenan-induced paw edema, the 80% methanol extract at all tested doses demonstrated a significant reduction in paw edema that starts from 1 hr and continued for 6 hr after induction (p < 0.001 from 1st −6thhr) as compared to the control group (Table 5). Intergroup comparison among doses of the E. cymosa showed a statistically significant effect between 100 and 400 mg/kg (p < 0.05). However, no significant differences were observed between 100 mg and 200 mg/kg and 200 and 400 mg/kg dose groups. The standard drug indomethacin (10 mg/kg) revealed a significant (p < 0.05) anti-inflammatory activity compared to the lower and middle doses of the extracts.
 |
Table 5 Effects of 80% Methanol Extract of E. cymosa Against Carrageenan Induced Paw Edema Model in Rats |
The maximum anti-inflammatory effect of 100, 200, and 400 mg/kg E. cymosa was recorded 6 hours after induction, with values of 28.6%, 30.4%, and 36%, respectively, and the effect at this hour increased dose-dependently (R2 = 0.92). Higher dose of the crude extract (400 mg/kg) exhibited a comparable anti-inflammatory activity with 10 mg/kg of indomethacin throughout the observation period (Table 5).
All tested doses of the solvent fractions as well as the standard drug (indomethacin 10 mg/kg) exhibited a significant reduction of paw edema starting from 1hr and the effect continued for 6 hrs after induction compared with negative control (p < 0.001). Indomethacin 10 mg/kg exhibited a statistically significant anti-inflammatory activity compared to all fractions (except higher dose of ethyl acetate fraction) at 6 hr post induction. Maximum anti-inflammatory effect was detected with higher doses of aqueous, chloroform and ethyl acetate fractions at 6 hr post induction with corresponding values of 35.3%, 35.9% and 40.8% (Table 6). Although all three fractions displayed significant reduction of paw edema as compared to the negative control, ethyl acetate fraction was the most active fraction in terms of anti-inflammatory effects on carrageenan-induced rat paw edema (Table 6).
 |
Table 6 Effects of Solvent Fractions of E. cymosa Against Carrageenan Induced Paw Edema Model in Rats |
Effect of 80% Methanol Extract and Solvent Fractions of E. cymosa on Cotton Pellet Induced Granuloma
Subcutaneous implantation of cotton pellets in the groin region of rats caused granulomatous inflammation with a highest granuloma weight and exudates recorded in the 2% tween 80 treated controls as shown in Table 7. The 80% methanol extract of E. cymosa at all tested doses significantly prevented inflammatory exudates and granuloma mass formation (p < 0.001) in contrast to the control. The anti-inflammatory activities of the E. cymosa increased in a dose-dependent manner (R2 = 0.99). The standard drug (indomethacin 10 mg/kg) significantly reduced both exudate and granuloma formation compared to the negative control group, lower and medium doses of the extract (p < 0.05). The percentage inhibition of formation of exudate by the crude extract was 14.6, 22 and 30.2% whereas the percentage inhibition of formation of granuloma was 38.5, 41.2 and 50.6% for granuloma at 100, 200 and 400 mg/kg, respectively (Table 7).
 |
Table 7 Effects of 80% Methanolic Extract of E. cymosa on Cotton Pellet Induced Granuloma in Rats |
All the tested concentrations of the three solvent fractions significantly reduced the formation of inflammatory exudate and granuloma mass compared with the control group (p < 0.001) (Table 8). Higher dose of the ethyl acetate fraction revealed a considerable anti-inflammatory activity in both exudate and granuloma inhibition (except 400 mg CF) compared with the other fractions. The highest percentage suppression of exudate and granuloma formation was exhibited by 400 mg/kg of EAF (36.4% and 48.7%), respectively, compared to all other doses of the EAF, CF, and AF (Table 8).
 |
Table 8 Effects of Solvent Fractions of E. cymosa on Cotton Pellet Induced Granuloma in Rats |
Determination of Total Phenol, Flavonoid and Alkaloid Contents
The total phenolic content is expressed as mg of gallic acid equivalent (GAE) per gram of sample using the formula derived from the calibration curve y=0.0055x+0.195. The highest total phenolic content was observed in the ethyl acetate fraction (281.8 mg GAE/g). This was followed by the aqueous fraction (245.5 mg GAE/g) and 80% methanolic extract (214.5 GAE/g).
The total flavonoid content is described as mg of quercetin per gram of sample (mg QE/g) using the equation from the calibration curve y=0.4321x+0.0656. The total flavonoid contents in 80% methanolic extract, chloroform and ethyl acetate fractions are 109.6 mg QE/g, 112 mg QE/g and 125.9 mg QE/g, respectively. The total alkaloid content of the extracts/or solvent fractions were derived from the regression formula of the calibration curve y=0.0037x+0.0474 and described as mg of atropine equivalents per gram of sample (mg AE/g). The ethyl acetate fraction revealed the highest total alkaloid content of 192.8 mg AE/g, followed by the chloroform fraction (147.6 mg AE/g) and the 80% methanolic extract (144.9 mg AE/g) (Table 9).
 |
Table 9 Total Phenolic, Flavonoid and Alkaloid Content of 80% Methanol Extract and Fractions of E. cymosa |
Discussion
The results of the acute oral toxicity study revealed that the extract did not cause any substantial alterations in the behavior and respiration, cutaneous effects, sensory nervous system reactions or gastrointestinal effects within 24 hr and the follow up period of 14 days. Moreover, no death or any noxious responses like convulsion, ataxia and diarrhea were recorded at the limit dose of 2000 mg/kg of crude extract of E. cymosa. Thus, the oral LD50 of the 80% methanolic extract of the plant was predicted to be higher than 2000 mg/kg and the experimental doses were calculated on the basis of this finding.
The 80% methanolic extract and solvent fractions of the plant were assessed for analgesic potential using hot plate and acetic acid induced writhing test in mice. The acetic acid-induced abdominal constriction method is the preferred assay for assessing the peripheral anti-nociceptive activities of drugs or medicinal plants.32 It is reported that administration of acetic acid enhances the level of PGE2, PGE2α and lipoxygenase products in the peritoneal fluid.32 Moreover, it triggers the production of a number of unpleasant endogenous mediators, including histamine, serotonin, and bradykinin, which are responsible for the hallmarks of inflammation.33,34 Acetic acid induced writhing test mimics visceral pain and the writhing that results from the test is typically characterized by the contraction of the abdominal muscles, the expansion of the forelimbs, and the lengthening of the body.34,35 This elongation is supposed to be produced by the activation of local peritoneal receptors and prostaglandin pathways in the experimental animal model. Peritoneal muscles may be partially responsible for abdominal writhing.36 The crude extract of E. cymosa produced significant analgesia (p < 0.001) at the given doses (100, 200, 400 mg/kg) in mice. The number of writhing was reduced by the extract dose-dependently as compared with the control (R2 = 0.878). When compared to the control, all tested doses of the three fractions demonstrated significant analgesic activities (p < 0.001). Another similar study revealed that, triterpenes isolated from Ehretia microphylla exhibited significant analgesic activity against acetic acid-induced abdominal constriction.37
The result of this study indicated that the extract and solvent fractions of the plant significantly inhibited abdominal contractions similar to that of aspirin. Thus, the potential mechanism for peripheral analgesic activities of the extract/solvent fractions might be related to suppression of the production and release of several endogenous inflammatory mediators (prostaglandin pathway in the pain perception cascade) and inhibition of sensitivity of peripheral nociceptors. Previous reports have proven that drugs which block the cyclooxygenase enzyme pathway reduce writhing, which is a marker of pain in experimental animal models.38 The analgesic action of the plant is probably attributed to its phytochemical constituents. Studies confirmed that phenols, flavonoids, terpenoids and steroids all acted as inhibitors of prostaglandin synthesis.39
Heat induced nociceptive pain in the paw of mice is particularly a sensitive model to assess the analgesic activities of the medicinal agents. Hot plate test is frequently used in illuminating the centrally mediated anti-nociceptive effects of the test substances due to its sensitivity to potent analgesics, limited tissue damage with a cutoff time of 15 sec that is normally carried out to limit the time by which the mouse exposed to the hot plate.14,32 Additionally, the model takes less time, and measurements are typically precise. In this test, jumping and paw licking are both regarded supraspinally integrated behavioral responses, and the time of the latency to the onset of this reaction following injection is an indicator of the analgesic activity.40 The plate was kept at 55±1°C, and only opioid-like agents are active at this temperature. All the test doses of E. cymosa extract displayed significant analgesic activity (p < 0.05) at 90 and 120 minutes of observation compared to control. All the test doses of the solvent fractions (except 100 mg/kg aqueous fraction at 30 minutes) resulted in significant (p < 0.001) delay in reaction times during 30, 60, 90 and 120 minutes of observation compared to the control. In consistence with this, morphine provides significant analgesia at all observations. In line with this study, methanol extract of the leaf, fruit, and stem bark of Ehretia serrata and Ehretia obtusifolia exhibit pronounced analgesic effect in mice by reducing pain.41 The probable analgesic activity of extract and solvent fractions of E. cymosa might be a central mechanism via stimulation of the opioid system. It has been demonstrated that phenols like ellagic acid have a central analgesic effect that may act through the opioid system.42
In the current study, carrageenan-induced paw edema for acute inflammation and cotton pellet-induced granuloma for chronic inflammation were used as models to assess the anti-inflammatory effects of plant extract and solvent fractions. Carrageenan is a phlogistic, non-antigenic substance and is without obvious systemic effect. Additionally, it is thought that this experimental model showed excellent reliability for acute phase inflammation.14 Carrageenan-induced paw edema is an extensively used primary assay for examining potential novel anti-inflammatory agents and is thought to be biphasic.43 The initial phase starts within 1–2 hr after the injection of carrageenan and is brought on by the release of serotonin, histamine, and bradykinin from mast cells into the nearby injured tissues. Meanwhile, the second phase starts 3–6 hr following carrageenan injection, which is associated with the production and release of prostaglandins, leukotrienes, and various cytokines such as IL-1β, IL-6, IL-10, and TNF-α.43 In the brain, cyclooxygenase (COX)-2 is in charge of mediating prostaglandin synthesis, which results in peripheral inflammation and, in turn, induces hyperalgesia and allodynia. In mast cells, PGD2, PGF2α and PGE2 are products of cyclooxygenase pathway that enhance vascular permeability and vasodilatation, resulting in edema.44 The 80% methanolic extract of E. cymosa significantly (p < 0.001) inhibits carrageenan-induced paw edema over a period of 1 to 6 hr compared to the control group. All tested doses of the fractions also significantly inhibited paw edema starting from 1hr and the effect lasted until 6hrs post-induction compared with the control group (p < 0.05). In agreement with this study, the chloroform, methanolic and aqueous extract of Ehretia laevis showed effective anti-inflammatory activity by decreasing paw volume at different doses and the plant has phenolic acids, flavonoids, fatty acids, steroids and alkaloids.45,46 The late phase of carrageenan-induced inflammation is an intensifying phase of swelling owing to increased vascular permeability and edema associated prostaglandins and can be prevented by NSAIDs.47 Similarly, the extracts and solvent fractions had reduced paw edema during the late phases, so the anti-inflammatory activity of the extract and solvent fractions may be a mechanism that entails blockage of COX related to the inflammatory cascade triggered by carrageenan. Natural compounds such as phenols, flavonoids, alkaloids, terpenoids, glycosides prevent the formation of prostaglandins in the late phase of inflammation.39,44 Thus, the presence of these phytochemical constituents in the extract and fractions might inhibit the production of prostaglandins and bradykinin which are involved in the anti-inflammatory activity.
Cotton pellet-induced granuloma is a common method to assess anti-inflammatory potential in transudative and proliferative constituents of chronic inflammation. In this model, inflammatory responses involve infiltration of monocytes, proliferation of fibroblasts, angiogenesis and exudation.48 In the cotton pellet-induced granuloma formation, the responses can be divided into three phases. The initial, transudative phase, which occurs 0–3 hr following cotton pellet implantation, characterized by the leakage of fluid from blood vessels produced by an increase in vascular permeability. The second, exudative phase, which occurs 3–72 hr after cotton pellet implantation, is associated with protein leakage from the bloodstream around the granuloma as a result of the thorough preservation in vascular permeability change. The formation of granulomatous tissues as a result of ongoing release of pro-inflammatory mediators is known as the last, proliferative phase, which lasts for three to six days.49,50 An increase in fibroblasts, the production of collagen and mucopolysaccharide, and the penetration of proliferating fibroblasts into exudate are the characteristics of granuloma tissue formation. This process finally results in the formation of a vascularized mass.50 The process of granuloma formation arises owing to the discharge of pro-inflammatory mediators and oxygen-derived free radicals, as well as lysosomal enzymes like activating protein, which results in tissue injury. The transudative phase of inflammation is represented by an increase in the cotton pellet’s wet weight, whereas the proliferative phases of inflammation is measured by an increase in the cotton pellet’s dry weight. The amount of fluid (exudate) absorbed by the pellet has a substantial effect on the granuloma’s wet weight, whereas the amount of granulomatous tissue generated correlates well with the granuloma’s dry weight.51 All the tested doses of 80% methanol extract and the three fractions of E. cymosa significantly reduced inflammatory exudates and granuloma mass formations (p < 0.001) as compared to the control. As a non-steroidal anti-inflammatory drug, indomethacin produces its inhibitory effect in cotton pellet-induced granuloma experiment by preventing granulocyte infiltration to the cotton pellet and inhibiting the generation of collagen fibers.52 Indomethacin also caused a reduction in weight gain by suppressing the inflammation, this mechanism was due to the inhibition of the prostaglandin synthesis in the site of inflammation that was induced by the cotton pellet.53 It can be assumed that the extract also acted similarly and the mechanism was assumed to be the same as compared to the indomethacin which is the inhibition of the synthesis of prostaglandin. The study also revealed a reduction in granuloma formation and these might be attributed to the ability of E. cymosa extract and solvent fractions to decrease the number of fibroblasts and production of collagen and mucopolysaccharide, which are natural proliferative indicators of granulation tissue formation.
The total phenolic content is described as mg GAE per gram of sample using the equation obtained from the calibration curve. It can be seen that the total phenolic content was highest with ethyl acetate fraction (281.8 mg GAE/g). This was followed by the aqueous fraction (245.5 mg GAE/g) and 80% methanolic extract (214.5 GAE/g), respectively. In previous study conducted elsewhere, the total phenolic content of ethyl acetate and methanol fractions of E. cymosa was 25.27 and 27.44 mg GAE/g, respectively, which is significantly lower than the present study.54 This difference might be due to the fact that phenolic content of plants depends on a number of intrinsic (genetic) and extrinsic factors (maturity at harvest and storage condition). The higher analgesic and anti-inflammatory effects of ethyl acetate fraction corresponded to its highest phenolic contents.
The total flavonoid content is expressed as mg QE/g using the equation from the calibration curve. The total flavonoid contents in 80% methanolic extract, chloroform and ethyl acetate fractions are 109.6 mg QE/g, 112 mg QE/g and 125.9 mg QE/g, respectively. The finding of this study is corroborated with other study in which the total flavonoid content of ethyl acetate and methanol fractions of E. cymosa was 221.44 and 235.31 mg QE/g, respectively.54 The high concentration of flavonoids in plant extract correlated with its analgesic and anti-inflammatory activities.
The total alkaloid content of the extracts/or solvent fractions were obtained from the regression formula of the calibration curve and described as mg of atropine equivalents per gram of sample (mg AE/g). The ethyl acetate fraction showed the maximum total alkaloid content of 192.8 mg AE/g, followed by the chloroform fraction (147.6 mg AE/g) and 80% methanolic extract (144.9 mg AE/g). This is the first report on the determination of the total alkaloid content of E. cymosa.
In general, the anti-inflammatory activities of E. cymosa extract and fractions in this study are consistent with previous reports that indicated plants which contain predominantly alkaloids, flavonoids, saponins, tannins, Phenols, glycosides, and triterpenoids showed powerful anti-inflammatory effects.7
Conclusion
From the finding of this study, it can be concluded that the 80% methanolic extract, aqueous, ethyl acetate and chloroform fractions of E. cymosa exhibited significant analgesic and anti-inflammatory activities which support the traditional use of the plant for the treatment of various painful and inflammatory conditions. The plant extract or solvent fractions attained peripheral analgesic activity and central pain inhibition potential. It also demonstrated an anti-inflammatory activity, in both acute and chronic phases of inflammation.
Abbreviations
NO, nitric oxide; NSAID, non-steroidal anti-inflammatory drugs; COX, cyclooxygenase; IL, interleukin; AE, atropine equivalent; GAE, gallic acid equivalent; QE, quercetin equivalent; OECD, Organization for Economic Cooperation and Development.
Data Sharing Statement
The data is available on the hand of the corresponding author and provided on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the School of Pharmacy, College of Health Sciences, Addis Ababa University, ethical review committee with protocol number ERB/SOP/180a/13/2020. OECD guideline 25 was used for the welfare of the laboratory animals.
Acknowledgments
The authors would like to thank Ethiopian Public Health Institute (EPHI) and Department of Pharmacology and Clinical Pharmacy, Addis Ababa University for providing laboratory animals and chemicals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published.
Funding
The study is supported by Addis Ababa University.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Raja S, Carr D, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976.
2. Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox J, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63(1):127–133. doi:10.1016/0304-3959(95)00110-E
3. Tracey I, Woolf CJ, Andrews NA. Composite pain biomarker signatures for objective assessment and effective treatment. Neuron. 2019;101(5):783–800. doi:10.1016/j.neuron.2019.02.019
4. Beal BR, Wallace MS. An overview of pharmacologic management of chronic pain. Med Clin. 2016;100(1):65–79. doi:10.1016/j.mcna.2015.08.006
5. Balkrishna A, Ranjan R, Sakat SS, et al. Evaluation of polyherbal ayurvedic formulation ‘Peedantak Vati’for anti-inflammatory and analgesic properties. J Ethnopharmacol. 2019;235:361–374. doi:10.1016/j.jep.2019.01.028
6. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
7. Wu X, Xie J, Qiu L, et al. The anti-inflammatory and analgesic activities of the ethyl acetate extract of Viburnum taitoense Hayata. J Ethnopharmacol. 2021;269:113742. doi:10.1016/j.jep.2020.113742
8. Munn LL. Cancer and inflammation. Wiley Interdiscip Rev Syst Biol Med. 2017;9(2):e1370.
9. Haley RM, von Recum HA. Localized and targeted delivery of NSAIDs for treatment of inflammation: a review. Exp Biol Med. 2019;244(6):433–444. doi:10.1177/1535370218787770
10. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–465. doi:10.1517/14740338.2016.1140743
11. Pahwa R, Goyal A, Bansal P, Jialal I. Chronic inflammation; 2018.
12. Ou Z, Zhao J, Zhu L, et al. Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomed Pharmacother. 2019;118:109347. doi:10.1016/j.biopha.2019.109347
13. Mathew E, Kim E, Zempsky W. Pharmacologic treatment of pain. In: Seminars in Pediatric Neurology. Elsevier; 2016.
14. Yimer T, Birru EM, Adugna M, Geta M, Emiru YK. Evaluation of analgesic and anti-inflammatory activities of 80% methanol root extract of Echinops kebericho M. (Asteraceae). J Inflamm Res. 2020;13:647. doi:10.2147/JIR.S267154
15. Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med. 2011;2011:1–15. doi:10.1093/ecam/nep067
16. Mohamed D, Mahmoud E, Abdel-Moniem S, Hassan M. Anti-inflammatory and anti-arthritic activity of some spices extracts on adjuvant induced arthritis in rats. J Appl Sci Res. 2013;9:5303–5312.
17. Yang R, Yuan B-C, Ma Y-S, Zhou S, Liu Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol. 2017;55(1):5–18. doi:10.1080/13880209.2016.1225775
18. Velu G, Palanichamy V, Rajan AP. Phytochemical and pharmacological importance of plant secondary metabolites in modern medicine. In: Bioorganic Phase in Natural Food: An Overview. Springer; 2018:135–156.
19. Tesfaye S, Belete A, Engidawork E, Gedif T, Asres K. Ethnobotanical study of medicinal plants used by traditional healers to treat cancer-like symptoms in eleven districts, Ethiopia. Evid Based Complement Alternat Med. 2020;2020:1–23. doi:10.1155/2020/7683450
20. Kassaye KD, Amberbir A, Getachew B, Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop J Health Dev. 2006;20(2):127–134.
21. Sarkodie J, Squire S, Oppong Bekoe E, et al. The antihyperglycemic, antioxidant and antimicrobial activities of Ehretia cymosa. J Pharmacognosy Phytother. 2015;4(3):105–111.
22. Ogundajo AL, Nnaemeka CO, Olawunmi RO, Ogunwande IA. Chemical constituents of essential oil of Ethretia cymosa Thonn. Br J Appl Sci Technol. 2016;14(4):1–6. doi:10.9734/BJAST/2016/24240
23. Etana B Ethnobotanical Study of Traditional Medicinal Plants of Goma Wereda, Jimma Zone of Oromia Region, Ethiopia. Ethiopia: Addis Ababa University Repository;2010.
24. Alemayehu G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants used by local communities of Minjar-Shenkora District, North Shewa Zone of Amhara Region, Ethiopia. J Med Plants Stud. 2015;3(6):1–11.
25. Fassil A, Gashaw G. An ethnobotanical study of medicinal plants in chiro district, West Hararghe, Ethiopia. Afr J Plant Sci. 2019;13(11):309–323. doi:10.5897/AJPS2019.1911
26. Couto M, Cates C. Laboratory guidelines for animal care. In: Vertebrate Embryogenesis. Springer; 2019:407–430.
27. Neto A, Costa J, Belati C, et al. Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen. J Ethnopharmacol. 2005;96(1–2):87–91. doi:10.1016/j.jep.2004.08.035
28. Afsar S, Kumar KR, Gopal JV, Raveesha P. Assessment of anti-inflammatory activity of Artemisia vulgaris leaves by cotton pellet granuloma method in Wistar albino rats. J Pharm Res. 2013;7(6):463–467. doi:10.1016/j.jopr.2013.04.056
29. Shi P, Du W, Wang Y, Teng X, Chen X, Ye L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci Nutr. 2019;7(1):148–154. doi:10.1002/fsn3.834
30. Nigatu H, Belay A, Ayalew H, et al. In vitro antileishmanial activity of some Ethiopian medicinal plants. J Exp Pharmacol. 2021;13:15. doi:10.2147/JEP.S285079
31. Ajanal M, Gundkalle MB, Nayak SU. Estimation of total alkaloid in Chitrakadivati by UV-spectrophotometer. Anc Sci Life. 2012;31(4):198. doi:10.4103/0257-7941.107361
32. Jan S, Khan MR. Antipyretic, analgesic and anti-inflammatory effects of Kickxia ramosissima. J Ethnopharmacol. 2016;182:90–100. doi:10.1016/j.jep.2016.02.020
33. Olela B, Mbaria J, Wachira T, Moriasi G. Acute oral toxicity and anti-inflammatory and analgesic effects of aqueous and methanolic stem bark extracts of Piliostigma thonningii (Schumach.). Evid Based Complement Alternat Med. 2020;2020:1–10. doi:10.1155/2020/5651390
34. Yasmen N, Aziz M, Tajmim A, Akter M, Hazra AK, Rahman S. Analgesic and anti-inflammatory activities of Diethyl Ether and n-Hexane extract of Polyalthia suberosa leaves. Evid Based Complement Alternat Med. 2018;2018:1–8. doi:10.1155/2018/5617234
35. Azab A, Nassar A, Azab AN. Anti-inflammatory activity of natural products. Molecules. 2016;21(10):1321. doi:10.3390/molecules21101321
36. Gawade S. Acetic acid induced painful endogenous infliction in writhing test on mice. J Pharmacol Pharmacother. 2012;3(4):348. doi:10.4103/0976-500X.103699
37. Villaseñor IM, Canlas AP, Faustino KM, Plana KG. Evaluation of the bioactivity of triterpene mixture isolated from Carmona retusa (Vahl.) Masam leaves. J Ethnopharmacol. 2004;92(1):53–56. doi:10.1016/j.jep.2004.01.017
38. Shojaii A, Motaghinejad M, Norouzi S, Motevalian M. Evaluation of anti-inflammatory and analgesic activity of the extract and fractions of Astragalus hamosus in animal models. Iran J Pharm Res. 2015;14(1):263.
39. Tamrat Y, Nedi T, Assefa S, Teklehaymanot T, Shibeshi W. Anti-inflammatory and analgesic activities of solvent fractions of the leaves of Moringa stenopetala Bak. (Moringaceae) in mice models. BMC Complement Altern Med. 2017;17(1):1–10. doi:10.1186/s12906-017-1982-y
40. Verdam MC, Guilhon-Simplicio F, de Andrade KC, et al. Analgesic, anti-inflammatory, and antioxidant activities of Byrsonima duckeana W. R. Anderson (Malpighiaceae). Sci World J. 2017;2017:8367042. doi:10.1155/2017/8367042
41. Khan MS, Maalik A. Evaluation of antinociceptive potential of methanolic extract of different parts of Ehretia serrata Roxb and Ehretia obtusifolia in vivo. Biomed Res. 2018;29(9):1792–1796.
42. Brito T, Silva A, Cunha RXD, et al. Anti-inflammatory, hypoglycemic, hypolipidemic, and analgesic activities of Plinia cauliflora (Mart.) Kausel (Brazilian grape) epicarp. J Ethnopharmacol. 2021;268:113611. doi:10.1016/j.jep.2020.113611
43. Karbab A, Mokhnache K, Ouhida S, et al. Anti-inflammatory, analgesic activity, and toxicity of Pituranthos scoparius stem extract: an ethnopharmacological study in rat and mouse models. J Ethnopharmacol. 2020;258:112936. doi:10.1016/j.jep.2020.112936
44. Kifayatullah M, Rahim H, Jan NU, Chishti KA, Ullah I, Abbas S. In vivo analgesic, antipyretic and anti-inflammatory activities of ethanol extract of pericampylus glaucus in experimental animals. Sains Malaysiana. 2019;48(3):629–635. doi:10.17576/jsm-2019-4803-16
45. Jyothirmai N, Nagaraju B, Kumar JS. Evaluation of anti-inflammatory and anti- bacterial activities of different solvent extracts of Ehretia laevis Roxb. J Pharm Sci Res. 2016;8(8):715.
46. Sharma P, Shri R, Ntie-Kang F, Kumar S. Phytochemical and ethnopharmacological perspectives of Ehretia laevis. Molecules. 2021;26(12):3489. doi:10.3390/molecules26123489
47. Sharma VC, Kaushik A, Dey YN, Srivastava B, Wanjari M, Jaiswal B. Analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of stem bark of Anogeissus latifolia Roxb. Clin Phytoscience. 2020;6(1):1–9. doi:10.1186/s40816-020-00171-2
48. Asif M, Saadullah M, Yaseen HS, et al. Evaluation of in vivo anti-inflammatory and anti-angiogenic attributes of methanolic extract of Launaea spinosa. Inflammopharmacology. 2020;28(4):993–1008. doi:10.1007/s10787-020-00687-6
49. Pingsusaen P, Kunanusorn P, Khonsung P, Chiranthanut N, Panthong A, Rujjanawate C. Investigation of anti-inflammatory, antinociceptive and antipyretic activities of Stahlianthus involucratus rhizome ethanol extract. J Ethnopharmacol. 2015;162:199–206. doi:10.1016/j.jep.2014.10.060
50. Patil KR, Patil CR. Anti-inflammatory activity of bartogenic acid containing fraction of fruits of Barringtonia racemosa Roxb. in acute and chronic animal models of inflammation. J Tradit Complement Med. 2017;7(1):86–93. doi:10.1016/j.jtcme.2016.02.001
51. Wilches I, Tobar V, Peñaherrera E, et al. Evaluation of anti-inflammatory activity of the methanolic extract from Jungia rugosa leaves in rodents. J Ethnopharmacol. 2015;173:166–171. doi:10.1016/j.jep.2015.07.004
52. Ma J, Guo C, Pan Y, Lin D, Qiu L, Wen L. Antioxidant and anti‑inflammatory activities of ethyl acetate extract of Gynura formosana (Kitam) leaves. Exp Ther Med. 2017;14(3):2303–2309. doi:10.3892/etm.2017.4757
53. Pranitha D, Ch MR. Inflammation lowering property of Pistacia atlantica in cotton pellet granuloma. Int J Rev Life Sci. 2019;9(2):14–17. doi:10.26452/ijrls.v9i2.1329
54. Ogundajo A, Ashafa AT. Phytochemical compositions and in vitro assessments of antioxidant and antidiabetic potentials of fractions from Ehretia cymosa Thonn. Pharmacogn Mag. 2017;13(Suppl 3):S470. doi:10.4103/pm.pm_118_17
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.