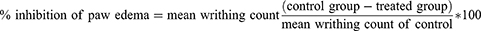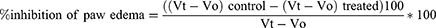Back to Journals » Journal of Experimental Pharmacology » Volume 15
Evaluation of Analgesic and Anti-inflammatory Activities of Methanolic Leaf and Root Extracts of Gomphocarpus purpurascens A. Rich (Asclepiadaceae) in Mice
Authors Ayanaw MA, Yesuf JS , Birru EM
Received 19 March 2022
Accepted for publication 24 December 2022
Published 9 January 2023 Volume 2023:15 Pages 1—11
DOI https://doi.org/10.2147/JEP.S361194
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Junmin Zhang
Meaza Adugna Ayanaw, Jibril Seid Yesuf, Eshetie Melese Birru
Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Meaza Adugna Ayanaw, Tel +251 962818585, Email [email protected]
Background: Regardless of the availability of drugs many people still experienced pain and inflammation because current medications often trigger potentially serious adverse effects. A range of medicinal plants with analgesic and anti-inflammatory properties have been widely used by traditional healers. Among them, Gomphocarpus purpurascens is one however there are no experimental studies that support this traditional use.
Objective: This study aimed to evaluate the analgesic and anti-inflammatory activities of 80% methanolic leaf and root extracts of G. purpurascens.
Methods: Air-dried leaves and roots of G. purpurascens were extracted with 80% methanol and an acute oral toxicity study was conducted for the 80% methanolic extract of G. purpurascens according to OECD guideline version eighteen. Preliminary phytochemical screening for the presence of different constituents was carried out. The hot plate method was used to evaluate centrally mediated analgesic activity while peripheral analgesic activity was tested by an acetic acid-induced writhing test. Carrageenan-induced paw edema test and formalin-induced pedal edema test were used to evaluate anti-inflammatory activity.
Results: Dose-dependent inhibition of acetic acid-induced writhing test was observed in mice by 100 mg/kg, 200 mg/kg, and 400 mg/kg of root extract with respective values of 16.6%, 68.9%, and 83%. In the hot plate method, the root extract at doses of 200mg/kg and 400 mg/kg showed a significant (p < 0.05) analgesic effect. Maximum anti-inflammatory effects by all doses of leaf extracts were observed from 2– 4hr post-induction in carrageenan-induced paw edema; and all tested doses of the extract inhibited the formalin-induced inflammation significantly (p < 0.001, p < 0.01). The presence of saponins, alkaloids, flavonoids, tannins, terpenoids, anthraquinone, steroids, and phenols might be responsible for these activities.
Conclusion: This study shows that the extract had potential analgesic and anti-inflammatory activity which supports the traditional claim.
Keywords: Gomphocar puspurpurascens, anti-inflammatory, analgesic, mice, carrageenan, formalin
Background
Pain is an increasing problem globally. Estimates suggest that 20% of adults suffer from pain globally and 10% are newly diagnosed with chronic pain each year.1,2 Pain is the reason for more than 80% of patients visiting physicians and for most of these patients, it is of short duration and quickly forgotten. Unfortunately, for some, the pain does not pass but becomes a continuous burden and interrupted suffering.3
When tissue injury occurs, whether caused by bacteria, trauma, chemicals, heat, or any other phenomenon, multiple substances are released by the injured tissues and cause dramatic secondary changes in the surrounding uninjured tissues. This entire complex of tissue changes is called inflammation.4 It is a most important mechanism to restore tissue homeostasis and initiate the healing process in the body.5 It is intrinsically unpleasant and is associated with hurting, soreness, avoidance of motor reflexes, and alterations in autonomic output and mostly results from a complex interaction of sensory, emotional, and behavioral factors which is expressed in terms of suffering. Pain is always subjective; accordingly, it differs from person to person and even in the same person from time to time.6
Acute inflammation is an immediate response to an injurious agent. It is characterized by fluid and plasma protein exudation and a predominantly neutrophilic leukocyte accumulation. It has a limited beneficial response, particularly during infectious challenges rather it is an aggravating component of infections.6,7 Chronic inflammation can be defined as a prolonged inflammatory process (weeks or months) where active inflammation, tissue destruction, and attempts to repair are proceeding simultaneously.
The use of medication can have the advantage of more profound relief of inflammation and pain than non-drug treatment (at least for a time being), but this is often offset by the disadvantage of side effects so it should be titrated individually according to the efficacy and possible contraindications or side effects.8
Those standard drugs are associated with many potentially significant adverse events mainly with prolonged use such as gastrointestinal (dyspepsia, bleeding, and peptic ulcer formation through inhibition of protective prostaglandin formation), hematologic (platelet inhibition due to inhibition of thromboxane synthesis), renal dysfunction, increased risk of myocardial infarction. The use of NSAIDs has been linked to coronary heart disease and heart failure.9,10 Acetaminophen is a mild-moderate painkiller that is strictly monitored by the FDA due to the risk of causing unpleasant skin responses, severe liver impairment from overdosing, and an increase in INR when combined with warfarin medication.11 Corticosteroids are one of the most commonly prescribed medications for a variety of inflammatory and immunological conditions, including asthma.12 Corticosteroids therapy affects endogenous corticosteroid production and has a suppressive effect on the HPA axis, which is mediated by the interaction of hormones with corticosteroid receptors, which regulates gene transcription, reducing inflammation and immune system activity inside the cell even after they are no longer in circulation, so their use requires close monitoring because their side effects are numerous.13
According to the World Health Organization, traditional medicine is used by 80% of the population in the developing world, and there has been a steady demand for and widespread use of traditional and complementary medicine around the world.14 Plants are a rich natural supply of chemicals that could be used as a starting point for the development of new medications. Ethiopia is thought to have around 6500 species of higher plants, with approximately 12% endemism. Traditional Ethiopian healers employ a variety of plants for the treatment of inflammation and pain. In Ethiopia, eighty to ninety percent of the population uses herbal treatments for primary healthcare due to a lack of access to modern health care.15 G. purpurascens is an annually grown endemic plant to Ethiopia and it is a shrubby perennial herb 0.6–2 m tall arising from a tap root; stems erect, much branched from base, woody below, densely spreading-pubescent or tomentose. Leaves paired; sessile or with petiole to 2 mm long; lamina 5–15×0.1–0.5 cm, flowering throughout the year in open rocky ground and disturbed areas; altitude of 1500–2500m and it has different Vernacular name Ari-Yuyo in Oromia, Tseba dimu in Tigray, Tefringe in Amharic and Mexxino in South Ethiopia. This plant has many reported uses in traditional medicine; the dried leaf part is applied topically for the treatment of eczema, evil eye, and itching skin; fresh leaf or stem latex is applied topically for warts and used as part of a formula for Rhesus Factor problems in pregnancy (shotelay); a pound of fresh or dry root bark is used for febrile illness, abdominal pain, hemorrhoids, and wounds (livestock). High curative evidence was noticed in wound management and wart treatment, where people used tifirigina (local name) as a medicament.16–19
As a result, there is a pressing need to expand research into medicinal plants that claim to be useful in the treatment of pain and inflammation. Traditional healers have long used a variety of medicinal herbs with anti-inflammatory and analgesic effects.20 However, the majority of these medicinal plants have yet to be properly tested.
G. purpurascens leaf and root extract have been used as an anti-inflammatory and analgesic drug in Tigray, Amhara, and the southern areas of Ethiopia,16–19 but no scientific investigations have been done to back up this traditional claim. The purpose of this research was to establish a baseline for the traditional use of G. Purpurascens for pain and/or inflammation. Furthermore, this research tried to identify the elements responsible for analgesic and anti-inflammatory effects to get insight into the nature of the phytochemical compounds involved. Furthermore, this research could serve as a starting point for additional research and identification of the precise compounds responsible for the plant’s analgesic and anti-inflammatory properties.
Materials and Methods
Materials and Instruments Used
Digital Plethysmometer (Orchid Scientific, India), Hot Plate (Orchid Scientific, India), Electronic Balance (Orchid Scientific, India), Tissue drying Oven (Yamato Scientific), and Lyophilizer (OPERON, OPR-FDU-5012, and Korea) were used.
Drugs and Chemicals Used
Aspirin, Morphine, and Diclofenac (Ethiopian Pharmaceuticals Manufacturing (EPHARM)), Indomethacin (Cadila pharmaceuticals Ethiopia), Normal saline (EPHARM), Carrageenan (Sigma Aldrich, Germany), Tween 80% (Lobe chemi, India), Formalin (Taflen industry, Ethiopia), Glacial Acetic Acid (BDH laboratory supplier, England), Methanol (Sigma Aldrich, Germany), Acetic Anhydride, Mayer’s reagent, Dragendroff’s reagent (Fisher Scientific, UK), Sulfuric Acid (Fisher Scientific, UK) and Distilled water (from Pharmaceutics Department, UoG) were used in the experiment.
Collection, Identification, and Preparation of Plant Materials
Fresh leaves and roots of G. purpurasence were collected from Gondar town, Amhara Regional State which is located 728 km to the northwest of Addis Ababa city on January 2019, and Authentication of the plant was done by a botanist.
Preparation of the Extract
The collected plant material was wrapped with plastic sheets during transportation then washed carefully under running tap water to remove dirt and soil then dried at room temperature under shade and reduced to the appropriate size by using mortar and pestles. A total of 2 kg of dried leaves and 900 g of dried roots were extracted by maceration (50 g of dried leaves in 300 mL of 80% methanol and 1 g of dried roots in 8–10mL of 80% methanol) for 72 h. The extraction process was facilitated manually by vigorous shaking and stirring. The mixture was first filtered using a muslin cloth and then with Whatman filters paper No.1. The residue has macerated a total of three times to obtain the maximum yield.
Filtration and collection of the extract were done three times with the whole extraction taking 9 days. After the extraction, methanol was evaporated under an oven at 40°C. The resulting solution was placed in a deep freezer operating at negative 20°C till it forms solid ice and then the remaining solvent (water) was removed using a lyophilizer. After water removal, a dark green gummy residue weighing 250.46g was obtained; giving rise to a percentage yield of 12.49% from the leaf, and a light yellow powder weighing 65g was obtained from the root part, giving rise to a percentage yield of 7.2%. The residue was then stored at 40°C (deep freezer) until use.
Experimental Animals
Healthy adult Swiss Albino mice of either sex (20–35g, and 6–8 weeks of age) were purchased from Ethiopian Public Health Institute (EPHI) and obtained from the Department of Pharmacology, School of Pharmacy. The animals were kept in cages at room temperature on a 12 h light/dark cycle with access to standard laboratory pellets and water ad libitum. They were allowed to acclimatize to the laboratory condition for a week before beginning the experiment. All animals used in this study were handled following the internationally accepted standard guidelines for use of animals.21
Acute Toxicity Test
An acute toxicity test was conducted for the crude extracts of leaf and root (single dose 2g/kg) using OECD guidelines. On the first day, one fasted mouse was given a limit dose of 2000 mg/kg and then four other mice were sequentially treated based on the outcome of the first animal. The animals were strictly observed for toxicities like weight loss, diarrhea, tremor, lethargy, and paralysis periodically for the first four hours during the 24 h period and later were followed per day for 14 days for any lethality. After the acute toxicity test, three dose levels were chosen. A middle dose, which was one-tenth of the maximum dose obtained during the acute toxicity study is 200mg; a low dose, which was half of the middle dose that is 100mg, and a high dose (400mg) was twice the middle dose.21
Preliminary Phytochemical Screening
The 80% methanol extracts of root and leaves of G. purpurasence were screened for the possible presence of secondary metabolites to relate to the anti-inflammatory and analgesic activity of the plant. Thus, the test for alkaloids, saponins, flavonoids, phenols, and tannins was performed according to standard tests as described in the Appendix.
Animal Grouping and Dosing
Mice were randomly divided into five groups with each group consisting of six mice. Group, I served as a negative control and was administered with a solvent used to dissolve the extract. Groups II, III, and IV were given 100mg, 200mg, and 400mg of the extract, respectively. Group V received standard drugs (ie, 10mg/kg of morphine for hot plate, 150 mg/kg of ASA for writhing test, 25 mg/kg of indomethacin for carrageenan-induced paw model, and 10 mg/kg diclofenac for formalin-induced pedal model). Administration of all agents was performed via an oral route using oral gavage.
Evaluation of Analgesic Activities of the Extract
Acetic Acid-Induced Writhing Test
This test was conducted to detect the peripheral analgesic activity of the extract. Mice of either sex were divided into five groups, each consisting of six animals. Three groups were given different doses of the root extract (determined based on acute toxicity), while one group was given a vehicle (a negative control), and the other group was given a standard drug like Aspirin, 150 mg/kg (a positive control) one hour before acetic acid administration. Sixty minutes later, the analgesic activity of G. purpurascens was assessed by counting the number of writhes induced by 0.6% acetic acid (10 mL/kg, i.p.).22 Five minutes after the acetic acid injection, the animals were placed in inverted flasks individually, and the contractions of abdominal muscles together with stretching of the hind limbs were cumulatively counted for 20 minutes.
Percentage protection against writhing was taken as an index
Hot Plate Method
This test was conducted to evaluate the central analgesic potential of G. purpurasence extract. In this test, each mouse was introduced into an open-ended cylindrical space with a floor consisting of a metallic plate that is maintained at 55 1°C. This plate produces two behavioral components that can be measured in terms of their reaction times, namely, paw licking and jumping, which are both considered to be supra-spinally integrated responses. The animals were placed on a hot plate with a cut-off time of 15 seconds to avoid lesions to the animals’ paws after 1 hour of administration of the standard drug, vehicle, and three different doses of the extract, and the reaction time of each animal was recorded. The latency to lick the paw or jump off the hot plate was noted as the reaction time. The reaction times were noted at 0, 30, 60, 90, and 120 min.23
Anti-Inflammatory Activity
Carrageenan-Induced Paw Edema
This experiment was conducted by producing acute inflammation in the paws of mice that had been starved overnight but had unrestricted access to water.
To induce acute inflammation, carrageenan (1% w/v carrageenan in normal saline) was injected into the mice’s left hind paw one hour after oral administration of the extract, the standard medication, and the vehicle. The displacement of water by edema was measured in milliliters (mL) at times 0, 1, 2, 3, and 4 hours following carrageenan injection (using a digital plethysmometer).
The percent inhibition of edema was calculated in comparison to the animals in the control group by using the following formula.24
Vo: is the left hind paw thickness volume before carrageenan injection,
Vt –Vo: control and treated is edema or paw size after carrageenan injection to control and drug-treated groups, respectively.
Formalin-Induced Pedal Edema
The induction of subacute inflammation was done with 2% v/v formalin in this technique. On the first and third days, all animals were given a sub-plantar injection of 20 μL of freshly made 2% formalin in the left hind paw to cause inflammation.22 The extract, the standard medication, and the vehicle were given orally one hour before the formalin injection and continued for seven days in the groups indicated earlier.17 The % inhibition was computed using the following formula after measuring the degree of inflammation with a plethysmometer daily for 7 days.25
Statistical Analysis
The raw data that was obtained from the experiment was expressed as mean ± SEM (standard error of the mean). The results were statistically analyzed using one-way ANOVA followed by the Tukey Post Hoc Test for multiple comparisons to compare results among groups and the results were considered significantly significant at p < 0.05. SPSS version 20 software was used for data processing.
Results
Acute Toxicity
Mice used in the acute toxicity study were observed for the first four hours continuously then for 24 hours and for the next 14 days to see if there is any toxicity. The LD50 of the root and leaf part of the plant was estimated to be above 2000 mg/kg as there were no visible signs of toxicity, gross behavioral changes, and mortality within 24 h as well as in the next 14 days.
Phytochemical Screening
Preliminary phytochemical screening of 80% methanol root and leaf extract of G. purpurasence was carried out to detect the possible presence or absence of different phytoconstituents. From the results, the possible presence of saponins, triterpenes, glycosides, phenols, steroids, tannins, anthraquinone, and alkaloids as well as the absence of flavonoids in the root part were confirmed through qualitative color changes of test reagents which will give a clue to the possible mechanisms of analgesic and anti-inflammatory effects of the extract as indicated in Table 1.
 |
Table 1 Preliminary Phytochemical Screening of 80% Methanol Extract of the Leaf and Roots of G. Purpurasence |
Analgesic Activity
Acetic Acid-Induced Writhing Method
In the acetic acid-induced writhing method, the extract at all doses significantly (P < 0.001) reduced the number of writhes in mice as compared to the negative control (Table 2). The higher (400 mg/kg) dose of the extract showed a significant (p < 0.001) difference compared to the lower and middle (100 mg/kg and 200 mg/kg) doses of the extract. Acetylsalicylic acid, on the other hand, showed a significant (p < 0.001) difference between the 100 mg/kg and 200mg/kg dose of the extracts; however, no significant difference was observed as compared with that of 400mg/kg doses of the extract.
 |
Table 2 Effect of 80% Methanolic Root Extracts of G. Purpurasence on Acetic Acid-Induced Writhing in Mice |
Intergroup comparison among doses of G. purpurascens also showed a statistically significant difference in 200 versus 100mg/kg (p < 0.001), 400 versus 100mg/kg (p < 0.001), and 200 versus 400mg/kg (p < 0.001) as indicated in Table 2.
Hot Plate Method
In the hot plate method, 200mg and 400mg doses of the extract produced a significant analgesic effect (p < 0.05) at 120 min when compared to the negative control, and Morphine 10mg/kg produced a significant analgesic effect at 60 and 90 min compared to the negative control. In the contrast, 100mg of the extract produce no significant analgesic effect at an all-time interval compared to the negative control. When compared to each other, there was no significant difference among the doses as described in Table 3.
 |
Table 3 Effect of 80% Methanolic Root Extracts of G. Purpurasence on Hot Plate Method |
Anti-Inflammatory Activity
Carrageenan-Induced Paw Edema
This test was conducted to evaluate the anti-inflammatory potential of the extract in the acute phase of inflammation. All doses of the plant extract and that of the standard drug showed a statistically significant inhibitory effect (p < 0.01 or p < 0.001) on the mean increase in paw volume starting from 2hr as compared to the negative control group. Minimum and maximum inhibition were recorded for all extract and standard drugs 1hr and 4hr after administration of carrageenan respectively, except G. purpurascens400mg at 2hr.
The extract at all doses shows a high percent of inhibition that was comparable to the standard drug indomethacin, especially in the case of 100mg and 400mg. The highest percent of inhibition was recorded by 400mg of the extract at 2hr which is 92.96%. No difference was observed in the onset of action between the extract and indomethacin which is 1hr after administration of the extract in all cases. However, indomethacin and G. purpurascens100mg shows a low percentage of inhibition at 1hr when compared to others and no difference in duration of action was observed between the different dose of extract and that of indomethacin as shown in Table 4.
 |
Table 4 Effect of 80% Methanolic Extract of Leaf of G. Purpurasence on Carrageenan-Induced Paw Edema in Mice |
Formalin-Induced Paw Edema
In the case of formalin-induced pedal edema, the extract at all doses showed statistically significant inhibition of edema on 5th, 6th and 7th day of measurement of paw volume when compared to the negative control (p < 0.001 for 100mg, 200mg, and Diclofenac, p < 0.01 at day 6 and p < 0.05 at day 7 for 400mg). The percentage inhibition of the extract was 76%, 68%, and 82% for 100 mg/kg, 200mg/kg, and 400 mg/kg doses respectively on 5th day; 94%, 92%, and 97% for 100 mg/kg, 200mg/kg and 400 mg/kg respectively on 6th day and 96.5%, 92% and 96.9% for 100 mg/kg, 200mg/kg and 400 mg/kg respectively on 7th day of measurement. When compared to diclofenac all dose shows a high percentage of inhibition as indicated in Table 5.
 |
Table 5 Effect of 80% Methanolic Extract of Leaf of G. Purpurasence on Formalin Induced Paw Edema |
Discussion
G. purpurascens leaf and root extracts have a long history of usage in Ethiopia as an anti-inflammatory and analgesic drug;16–19 however, no evidence of this plant’s analgesic and anti-inflammatory effectiveness in pain and inflammation models has been found. As a result, the purpose of this study was to scientifically evaluate the traditional claims for the above-mentioned uses of G. purpurascens. Because hydro alcoholic co-solvents have the best solubility qualities for initial extraction and As methanol 80 is a universal solvent for extraction, it was chosen as the solvent of choice in this study for extracting the root and leaves of G. purpurasence.26
The peripheral analgesic activity of the extracts was evaluated by the acetic acid-induced writhing test, which is used for a reliable and rapid evaluation of the peripheral analgesic action of plants. It is a very sensitive test that can detect anti-nociceptive effects of compounds at dose levels that may appear inactive in other methods like the hot plate test, but it is a non-selective pain test that gives false positive results with sedatives, muscle relaxants, and other pharmacological agents,27,28 because it involves different nociceptive mechanisms, such as biogenic amine release (eg histamine and serotonin, 5-HT), substance P (SP), bradykinins25–29 and pro-inflammatory cytokines (such as tumor necrosis factor, TNF-α, and interleukin, IL-1β).29,30
Methanol 80% crude extracts of the root of this plant showed significant peripheral analgesic action compared to the negative control. The inhibition of the writhing response is a sign of the plant’s peripheral analgesic properties. In an acetic acid-induced writhing model, the root extract inhibited the writhing response by 16.6%, 68.9%, and 83% for 100, 200, and 400 mg/kg, respectively. The aforesaid results indicate that the extract has analgesic action that is dose-dependent, ie, as the dose is increased, the analgesic activity increases as well, indicating an increase in the concentration of phytoconstituents with analgesic activity. The extract had an effect comparable to Acetylsalicylic acid, a well-known NSAID that blocks acetic acid action by eliminating inflammatory mediators of pain in peripheral tissues.31
The hot plate model, in which the pain threshold of mice towards heat is measured, is commonly used to examine medicines that display central mechanism analgesia. This test was chosen because it is sensitive to strong analgesics and has little tissue damage due to a 15-second cutoff duration that is commonly used to protect mice from harm.23 At dosages of 200 mg/kg and 400 mg/kg, an 80% methanolic extract of G. purpurasence roots displayed considerable central analgesic effectiveness. This could be due to the presence of alkaloids, which are known to have analgesic effects by interfering with pain-enhancing neurotransmitters in the CNS, or it could be due to their interaction with various receptors found in supra-spinal locations.33
The onsets of action of morphine and the extract were found to be distinct. The extract took 120 minutes to start functioning at doses of 200 mg and 400 mg/kg, whereas morphine required 60 minutes. This shows that the activity of the extract has been delayed. The time it takes for the drug to enter the central compartment and be delivered to the target site, or could be related to active analgesic metabolites.
Previous investigations have found similar results for peripheral analgesic activity. The effects of Swertia chirata and MoringastenopetalaBak were statistically significant when compared to the negative control.20 The extraction solvent, dose ranges, and models used in this study were similar to those used in a prior study that discovered a dose-dependent increase in analgesic activity when employing the writhing method. As previously indicated, secondary metabolites such as alkaloids may play a role in the extract’s anti-nociceptive activities. In the case of glycosides, the glycosidic metabolite morphine-6-glucuronide has been discovered to bind to 1 and 2 receptors in the mouse brain with affinities similar to morphine.31,32
Likewise, Carrageenan-induced paw edema was studied using a carrageenan-induced acute anti-inflammatory paradigm and a formalin-induced subacute anti-inflammatory model.
Carrageenan paw edema is a test often used to investigate anti-inflammatory medicines, both steroidal and nonsteroidal because it involves numerous mediators. Non-antigenic phlogistic agent carrageenan is the primary non-antigenic phlogistic agent for testing anti-inflammatory drugs with no clear systemic effects.24 Furthermore, it is a frequent method for testing natural substances’ anti-edematous effects.
A sub-plantar injection of 1% carrageenan in the hind paw causes inflammation in two phases: the first is triggered by histamine and serotonin, while the second, in which the edema reaches its peak, is triggered mostly by the production of prostaglandins. Nitric oxide is another essential mediator in acute inflammation (NO). Carrageenan has been linked to the production and release of NO at the damaged location. Moreover, the inflammatory response is also related to the local infiltration and activation of neutrophils and the production of oxygen-derived free radicals.
The formalin-induced paw edema model is a widely used model for subacute inflammation and one of the best ways to test anti-arthritic and anti-inflammatory drugs. Subcutaneous injection of formalin into the hind paw of mice causes localized inflammation and discomfort. Because it involves neutrophil infiltration, macrophage proliferation, and fibroblast proliferation, formalin-induced arthritis offers a model for evaluating an agent with possible anti-proliferative potential.25, on the 5th, 6th, and 7th days, the 80% methanolic leaf extract of G. purpurasence exhibits a high percentage of inhibition of edema, and 100mg of the extract shows a higher percent of inhibition than 200mg of the extract.
Because this model is utilized to evaluate medicines with potential anti-proliferative activity, the extract’s success in this model may have demonstrated its anti-proliferative activity. The existence of secondary metabolites may be responsible for the extract’s action.23 Secondary metabolites like saponins have been found to impede nitric oxide production. As a result, the extract’s efficacy in this model implies that it could be useful in the treatment of sub-chronic inflammation.28
Stronger effects of the extract compared to the standard drugs were observed in the Case of acetic acid-induced writhing, carrageenan-induced and formalin-induced paw edema models. This notion is in line with the proven concept that medicinal plants possess a combination of constituents, involving different mode(s) of action, offering synergistic effects. This idea is in keeping with the well-established theory that medicinal plants contain a variety of ingredients with various mechanisms of action, resulting in synergistic effects.14 The anti-inflammatory and analgesic effects observed in this study could indicate that inhibition of inflammatory mediator synthesis, release, and action could be a common mechanism of action for all activities and that the extract behaved like an NSAID because both analgesic and anti-inflammatory effects were present. NSAIDs, on the other hand, have been linked to stomach discomfort due to the suppression of PG production. Gastroprotective action has been documented for phytoconstituents such as terpenoids and saponins. These phytoconstituents could elicit anti-ulcer activity of the extract potentially reducing gastric irritation that may occur from inhibiting PG synthesis but this needs further study.8
Conclusion and Recommendations
We can conclude from the current findings that the G. purpurasence 80% methanol extract has analgesic and anti-inflammatory properties, which are likely mediated by both central and peripheral inhibitory mechanisms. The root and leaf components of this plant are similarly safe in high quantities, according to this study. The current findings support the traditional usage of this herb in the treatment of painful and inflammatory illnesses. However, the precise mechanisms through which G. purpurasence exerts its anti-inflammatory and analgesic effects are unknown. Further studies are recommended to identify the proper mechanisms of the anti-pain and anti-inflammatory activity of the plant extract.
Abbreviations
ASA, Acetylsalicylic acid, BK, Bradykinin CNS, Central Nervous System, COX, Cyclooxygenase, GP, GomphocarpusPurpurasence, IL, Interleukin, iNOS, Inducible Nitric Oxide Synthase, NO, nitric oxide, NSAID, Non-steroidal Anti-inflammatory Drug, NF-κ, nuclear factor Kappa, PGs, prostaglandins, TNF, Tumor necrosis factor.
Data Sharing Statement
All data are available in the manuscript; additional data may be acquired from the corresponding author as needed via email.
Ethics Approval and Consent
Ethical clearance was obtained from the Department of Pharmacology, School of Pharmacy College of Medicine and Health Science University of Gondar to conduct the study in animal models. Besides, all possible steps were taken to avoid animal suffering at each stage of the experiment based on the Eighth Edition of the Guide for the Care and Use of Laboratory Animals. However, no consent was needed for this study.
Acknowledgment
We would like to acknowledge the University of Gondar for allowing using the laboratory setup and for sponsoring the study (This is the MSc. thesis of the first author).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Scott A, Khan KM, Cook JL, Duronio V. What is ―inflammation‖? Are we ready to move beyond Celsus? BJSM. 2004;38(3):248–249. doi:10.1136/bjsm.2003.011221
2. Agli MD, Lorenzo C, Baeda M. Plant food supplements with anti-inflammatory properties, a systematic review (1). Crit Rev Food SciNutr. 2013;53(4):403. doi:10.1080/10408398.2012.682123
3. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil. 2013;21(9):1145–1153. doi:10.1016/j.joca.2013.03.018
4. Segall SK, Maixner W, Belfer I, Wiltshire T, Seltzer Z, Diatchenko L. Janus molecule I, Dichotomous effects of COMT in neuropathic vs. nociceptive pain modalities. CNS Neurology Disorders Drug Targets. 2012;11(3):1–26.
5. Khan FA, Khan MF. Inflammation and acute phase response. Ijabpt. 2010;1(2):312–321.
6. Sattar HA. Fundamentals of pathology, Medical course and step 1 review; 2011.
7. Debon D, Hoeksema LJ, Hobbs RD. Caring for patients with chronic pain, pearls and pitfalls. J Am Osteopath Assoc. 2013;113:620–627. doi:10.7556/jaoa.2013.023
8. Abebe D, Hagos E. Plants as a primary source of drugs in the traditional health practices of Ethiopia. In: Engles J, Hawkes G, editors. Plant Genetic Resources of Ethiopia. Cambridge: Cambridge University Press; 1991.
9. Yuan H, Ma Q, Ye L, Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules; 2016;21(5):559.
10. Chrubasik J, Roufogalis B, Chrubasik S. Evidence of effectiveness of herbal anti-inflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. PhytotherapyResearch. 2007;21:675–683.
11. Belayneh B, Bussa NF. Ethnomedicinal plants are used to treat human ailments in the pre historic place of Harla and Dengego valleys, in eastern Ethiopia. J Ethnobiol Ethnomed. 2014;10:18. doi:10.1186/1746-4269-10-18
12. Mesfin A, Demissew S, Teklehaymanot T. An ethnobotanical study of medicinal plants in WonagoWoreda, SNNPR, Ethiopia. J Ethnobiol Ethnomed. 2009;5:28. doi:10.1186/1746-4269-5-28
13. Teklay B, Abera B, Giday M. An ethnobotanical study of medicinal plants used in kilteawulaelo district, Tigray region of Ethiopia. J Ethnobiol Ethnomed. 2013;9:65. doi:10.1186/1746-4269-9-65
14. D’Avigdoret E, Wohlmuth H, Asfaw Z, Awas T. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J Ethnobiol Ethnomed. 2014;10:38. doi:10.1186/1746-4269-10-38
15. Attal N, Cruccu G, Haanpää M, Task Force EFNS. EFNS guidelines on the pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi:10.1111/j.1468-1331.2006.01511.x
16. Bieger D, Hein L, Lüllmann H, Mohr K. Color Atlas of Pharmacology. Barnes: Thieme; 2011.
17. Jèwiak-Benista M, Norwak JZ. Paracetamol, mechanism of action, applications, and safety concern. Acta Pol Pharm. 2014;71(1):11–23.
18. Gómez-Moreno G, Guardia J, Cutando A, Calvo-GuiradoJ L. Pharmacological interactions of anti-inflammatory-analgesics in odontology. Med Oral Patol Oral Cir Bucal. 2009;14(2):81–89.
19. Adcock IM, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905–1917. doi:10.1016/S0140-6736(09)60326-3
20. Awad A, Toczek J, Fink C. Phytosterols decrease prostaglandin release in cultured P388D1/MAB macrophages. ProstagLeukotEssent Fatty Acids. 2004;70(6):511–520. doi:10.1016/j.plefa.2003.11.005
21. Worlein JM, Baker K, Bloomsmith M, Coleman K, Koban TL, editors. The Eighth Edition of the Guide for the Care and Use of Laboratory Animals. Implications for Behavioral Management. Malden MA: Wiley-Blackwell; 2011.
22. Olukunle JO, Adenubi OT, Oladele GM. Studies on the anti-inflammatory and analgesic properties of Jatropha curcas leaf extract. ActaVeterinariaBrunesis. 2011;80:259–262.
23. Schaible HG, Ebersberger A, Natura G. Update on peripheral mechanisms of pain, beyond prostaglandins and cytokines. Arth Res Ther. 2011;13:210. doi:10.1186/ar3305
24. Liao CR, Kao CP, Peng W. Analgesic and anti-inflammatory activities of methanol extract of Ficus pumila L.in mice. Evid-based Complement Altern Med; 2012;2012. doi:10.1155/2012/340141
25. Collier HOJ, Dinneen LG, Johnson CA, Schnei-der C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Brit J Pharmacology. 1968;32:295–310.
26. Gupta A, Naraniwal M, Kothari V. Modern extraction methods for preparation of bioactive plant extracts. IJANS. 2012;1(1):8–26.
27. Akele B. In vivo anti-inflammatory and anti-nociceptive activities of aerial part extracts of Zehneria scabra. Int J Pharm Ind Res. 2012;2(4):479–484.
28. Das SC, Bhadra S, Roy S. Analgesic and anti-inflammatory activities of ethanolic root extract of Swertia chirata (Gentianaceae). Jordan J Biol Sci. 2012;5(1):31–36.
29. Yohannes T, Teshome N, Ephrem E. Analgesic and anti-inflammatory activities of 80% methanol root extract of Jasminum abyssinicum Hochst. ex. Dc. (Oleaceae) in mice. J Ethnopharmacol. 2017;8741(16):31812–31818.
30. Milind P, Monu Y. Laboratory models for screening analgesics. Int Res J Pharm. 2013;4:15–19.
31. Siddalingappa CM, Rajesh T, Kudagi BL. Evaluation of analgesic and anti-inflammatory activities of Tinospora cordifolia in rodents. Int J Medical Sci. 2012;2(6):305–311.
32. Posadas I, Bucci M, Roviezzo F, et al. Carrageenan-induced mouse paw edema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br J Pharmacol. 2004;142:331–338. doi:10.1038/sj.bjp.0705650
33. Çadirci E, Suleyman H, Gurbuz P, et al. Anti-inflammatory effects of different extracts from three Salvia species. Turk J Biol. 2012;36:59–64.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.