Back to Journals » OncoTargets and Therapy » Volume 14
ET-1 Promotes Epithelial–Mesenchymal Transition in Oral Squamous Cell Carcinoma Cells via the microRNA-489-3p /TWIST Axis
Authors Tzeng HE, Tang CH , Tsai CH, Chiu CH, Wu MH, Yen Y
Received 6 January 2021
Accepted for publication 23 August 2021
Published 6 October 2021 Volume 2021:14 Pages 5005—5018
DOI https://doi.org/10.2147/OTT.S294312
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Federico Perche
Huey-En Tzeng,1– 3 Chih-Hsin Tang,4– 7 Chun-Hao Tsai,8 Chih-Hui Chiu,9 Min-Huan Wu,10,11 Yun Yen12,13
1Taipei Cancer Center, Taipei Medical University, Taipei, Taiwan; 2PhD Program & Graduate Institute of Cancer Biology and Drug Discovery, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan; 3Division of Hematology/Oncology, Department of Medicine, Taipei Medical University Hospital, Taipei, Taiwan; 4Graduate Institute of Biomedical Sciences, China Medical University, Taichung, Taiwan; 5School of Medicine, China Medical University, Taichung, Taiwan; 6Chinese Medicine Research Center, China Medical University, Taichung, Taiwan; 7Department of Biotechnology, College of Health Science, Asia University, Taichung, Taiwan; 8Department of Orthopedic Surgery, China Medical University Hospital, Taichung, Taiwan; 9Graduate Program in Department of Exercise Health Science, National Taiwan University of Sport, Taichung, Taiwan; 10Sports Recreation and Health Management Continuing Studies, Tunghai University, Taichung, Taiwan; 11Bachelor of Science in Senior Wellness and Sport Science, Tunghai University, Taichung, Taiwan; 12TMU Research Center of Cancer Translational Medicine, Taipei Medical University, Taipei, Taiwan; 13Graduate Institute of Medical Informatics, Taipei Medical University, Taipei, Taiwan
Correspondence: Min-Huan Wu Tel +886 4-23590121 Ext. 30703
Email [email protected]
Yun Yen Email [email protected]
Objective: Oral squamous cell carcinoma (OSCC) constitutes almost 90% of head and neck malignancies and has a poor prognosis. To improve the efficacy of OSCC therapy, it is of great significance to explore other therapy for OSCC. Endothelin-1 (ET-1), a potent vasoconstrictor peptide, is implicated in cancer pathogenesis. Moreover, ET-1 promotes epithelial-mesenchymal transition (EMT) during the development of human cancers. We further to found that ET-1 exposure induced EMT in human squamous cell carcinoma cell lines SCC4 and SAS, by enhancing the expression of EMT biomarkers N-cadherin and vimentin and reducing E-cadherin expression via upregulation of the transcription factor TWIST.
Materials and Methods: Cell motility was examined by migration, invasion and wound-healing assays. Quantitative real time polymerase chain reaction (q-PCR), and promoter assays confirmed the inhibitory effects of ET-1 on miRNAs expression in oral cancer cells. We demonstrate an intravenous injection model of lung metastasis followed by an advanced method for quantifying metastatic tumor using image analysis software.
Results: In addition, ET-1/ETAR reduced levels of microRNA-489-3p (miR-489-3p), a transcriptional repressor of TWIST. We have identified a novel bypass mechanism through which ET-1/ETAR are involved in TWIST signaling and downregulate miR-489-3p expression, enabling OSCC cells to acquire the EMT phenotype. Notably, ET-1 knockdown dramatically decreased levels of EMT markers and cell migration potential.
Conclusion: The role of ET-1 in OSCC progression is supported by our findings from an in vivo murine model of OSCC. ET-1 may therefore represent a novel molecular therapeutic target in OSCC metastasis.
Keywords: oral squamous cell carcinoma, endothelin-1, TWIST, microRNA-489-3p, epithelial–mesenchymal transition
Introduction
Oral squamous cell carcinoma (OSCC) is a common cause of mortality worldwide, with oral squamous cell carcinoma (OSCC) being the predominant form, accounting for over 90% of all oral malignancies.1 However, despite treatment advances including multi-agent chemotherapy, radiotherapy, and targeted therapy, the overall 5-year survival rate for OSCC remains disappointingly low (< 50%), because of its aggressively invasive and metastatic nature, such that most patients with metastatic disease die within 1 year. Thus, metastasis is a major obstacle that must be overcome for the successful treatment of OSCC. Exploring the molecular basis of metastasis may help to improve the early detection, prevention, intervention, and prognostic evaluation of OSCC patients.2,3
EMT plays a major role in local recurrence and lymph node metastasis and is associated with a low survival rate in patients with oral squamous cell carcinoma (OSCC).4 During EMT, epithelial cells gradually lose epithelial structural molecules, polarity, and adhesion capacity, and acquire mesenchymal traits, motility, and invasiveness.1,15 Activation of the EMT results in decreased expression of epithelial markers (E-cadherin and β-catenin) and increased expression of adhesion and mesenchymal proteins (including vimentin, N-cadherin and fibronectin). Twist1, a basic helix-loop-helix transcription factor that induces tumor initiation, cell proliferation, development of resistance to anti-cancer drugs, and inhibition of apoptosis. However, the most well characterized role of Twist1 is in cell invasion, epithelial-to-mesenchymal transition (EMT), and tumor metastasis.
Previously studies, shown Serum big ET-1 or salivary ET-1 both as a biomarker of OSCC, by correlating it with the clinical staging and the histopathological grading.5,6 The secreted peptide endothelin-1 (ET-1) is a widely expressed member of the endothelin family of proteins. Overexpression of the ET axis (ie, the combination of ET-1 and its receptors ET-A and ET-B) is implicated in the pathobiology of numerous different types of tumors.7 Evidence supports the importance of an association between ET-1 expression and tumor progression and metastasis8 and it is recognized that the ET-1/ET-A receptor (ETAR) autocrine pathway induces an invasive phenotype that drives EMT in ovarian cancer cells.9,10 Thus, it may be worth targeting ET-1-induced EMT-dependent phenotypes as a way of preventing or treating tumor angiogenesis and metastasis.
Endogenous microRNAs (miRNAs) are small, evolutionarily conserved non-coding ribonucleotide acids that are crucial players in many biological processes involving normal cell development and cellular homeostasis, as well as cancer-relevant processes.11 By binding to complementary sequences in the 3′-untranslated regions (3′-UTRs) of their target mRNAs, miRNAs interfere with mRNA degradation and translation.12,13 Moreover, miRNAs modulate the metastatic process in many tumors.14,15 Recently, using miRNA microarray analysis, we identified a group of miRNAs differentially expressed in mesenchymal-like versus epithelial-like cancer cells.16,17 Remarkably, miR-489-3p was downregulated in cancer cells that had undergone EMT compared with those that had a typical epithelial phenotype, suggesting that miR-489-3p may regulate the EMT process.18
Among patients with oral squamous cell carcinoma (OSCC), high ET-1 or Twist1 expression has been reported in OSCC patients with a poorly differentiated tumor accompanied by metastasis. Twist1, in turn, activates Bmi1, both of which are essential for promoting EMT and tumor-initiating capacity.19–21 However, there have been no studies on the importance of the expression of the ET-1/Twist1 axis, and this axis signalling pathways in OSCC is still poorly understood. Therefore, we examined whether miR-489-3p/Twist1 plays a role in ET-1-mediated metastasis in human OSCC. We found that ET-1 promotes tumor metastasis and EMT markers expression by down regulating miR-489-3p expression via the TWIST signaling pathway.
Materials and Methods
Cell Culture
Two kinds of human tongue squamous carcinoma derived cells were used, including SAS cell line (a gift from Ph.D. Chih-Hsin Tang, 2018 from China Medical University, Taichung, Taiwan), SCC-4 cell line (CRL-1624; American Type Culture Collection (ATCC)).22 The SAS cell line has been authenticated by short tandem repeat (STR) DNA typing (Mission Biotech, Taipei, Taiwan). Cells were maintained in F12/Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin, 15 mM HEPES, 1 mM sodium pyruvate and 400 mg/mL hydrocortisone. Cells were maintained at 37°C in an incubator with 5% CO2. The medium was changed every 3 days. Subcultivation was carried out at 70–80% of cell confluence.
Migration and Invastion Assay
The migration assay was performed using Transwell inserts (Costar, NY, USA; 8-mm pore size) in 24-well dishes. For invasion assay, filters were precoated with 30 μL Matrigel basement membrane matrix (BD Biosciences, Bedford, MA, USA) for 30 min. The following procedures were the same for both migration and invasion assays. After the treatment with ET-1 (0, 10, 30 and 100 nM) for 24 h, cells were harvested and seeded to Transwell at 1×104 cells/well in serum-free medium and then incubated for 24 h at 37 °C in 5% CO2. Cells were then fixed in 3.7% formaldehyde for 5 min and stained with 0.05% crystal violet in PBS for 15 min. Cells on the upper side of the filters were removed with cotton-tipped swabs, and the filters were washed with PBS. Cells on the underside of the filters were examined and counted under a microscope. Each experiment was performed in triplicate and repeated at least three times.23
Quantitative Real-Time PCR
Total cDNA (100 ng) was mixed with TaqMan® primers and probes as well as PCR Master Mix. The StepOnePlus™ system was used in the quantitative RT-PCR assays. For the detection of miRNAs, reverse transcription was performed using Mir-X™ miRNA First-Strand Synthesis and the SYBR® RT-PCR kit. qPCR analysis was carried out according to an established protocol. All mRNA sequences have been provided in the supplementary information (Supplementary data Table S1 and S2).
Plasmid Construction and Luciferase Activity Assay
Wild-type (wt) Twist-3ʹ-UTR was constructed into the pGL2-Control vector. The predicted Twist binding site for miRNA was identified by the Targetscan (http://www.targetscan.org/). Mutant plasmids that attenuate the interaction between Twist‐3 3′UTR and miRNA were generated using a QuikChange Site‐Directed Mutagenesis kit (Stratagene, Cedar Creek, TX, USA). These plasmids were transfected into cells using Lipofectamine 2000. Following transfection, these cells were incubated with the indicated agents. Cell extracts were prepared and used for measuring the luciferase and β‐galactosidase activities.24
In vivo Tumor Xenograft Study
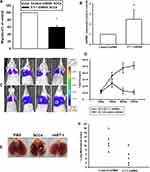
SCC4 cells that constitutively expressed pLenti CMV V5‐Luc were co‐transfected with pCMV plasmids alone or harboring human SCC-4/Luc control shRNA, or SCC4/Luc‐ET-1 shRNA, respectively. These cells (2 × 106) that were resuspended in serum‐free DMEN/α‐MEM were intravenously injected into the lateral tail vein of severe combined immunodeficiency (SCID) mice. Lung metastasis was monitored using an in vivo imaging system (Xenogen IVIS imaging system). After six weeks, the mice were humanely sacrificed and the tumor tissues were removed and photographed. The mice study was handled in accordance with the Animal Care and Use Guidelines of the Tunghai University (Taichung, Taiwan) under a protocol approved by the Institutional Animal Care and Use.
Immunohistochemistry (IHC)
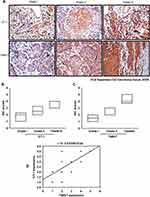
In this study, most human OSCC tissue array was purchased from Biomax (Rockville, MD; 6 cases for normal cartilage, 24 cases for grade I OSCC, 9 cases for grade II OSCC, and 15 cases for grade III OSCC). And some human tissues were purchased from Taipei medical hospital and the Ethics Committee of Taipei medical Hospital approved the study protocol, which followed institutional guidelines (N201804035). OSCC sample was treated with 3% hydrogen peroxide, then cover with 3% BSA. The tissue samples were incubated with primary antibodies against ET-1 and Twist. IHC analysis was carried out to determine the expression of lymphangiogenic marker according to the standard protocol.25
Statistics
All quantified results were calculated using GraphPad Prism 5.0 (GraphPad Software. Inc; San Diego, CA, USA) or SigmaPlot 10.0 software (Systat Software, Inc; San Jose, CA, USA) and are presented as the mean ± standard error of the mean (SEM). Pearson’s correlation analyses are presented in clinical data analysis. The Student’s t-test was used to compare the means of two experimental groups. We performed a one-way ANOVA followed by Bonferroni’s post hoc comparison tests on results from statistical comparisons involving more than 2 groups. In all cases, p < 0.05 was considered statistically significant.
Results
ET-1 Induced OSCC Cell Migration and Invasion via the ET-A Receptor
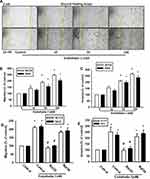
Cancer cells gain migratory and invasive properties through active EMT functioning, which occurs during wound healing and in the initiation of cancer metastasis.26,27 To investigate whether ET-1 was associated with migratory activity in OSCC cells, we examined the migratory ability of the human laryngeal squamous cell carcinoma SCC4 cell line. As shown in Figure 1A, ET-1 enhanced cell migration as demonstrated by the wound healing assay in a concentration-dependent manner. Under the same conditions, ET-1 also enhanced cell migration and invasion of the human SCC4 & SAS OSCC cell lines (Figure 1B and C). As ET-1 acts via two distinct subtypes of G protein-coupled receptors, subtype A (ETAR) and subtype B (ETBR), we hypothesized that ET receptors may be involved in ET-1-induced EMT in OSCC. Following pretreatment of OSCC cells with the ETAR antagonist BQ123 and the ETBR antagonist BQ788, only BQ123 abolished ET-1-induced cell migration and invasion, indicating that ET-1 acts through the ETAR to enhance the EMT process (Figure 1D and E).
The ETR Signaling Pathway is Involved in ET-1-Induced Expression of EMT Markers
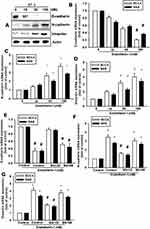
We hypothesized that ET-1 may promote cell migration by increasing the expression of specific EMT marker proteins. Using quantitative polymerase chain reaction (qPCR) analysis, we found that ET-1 increased levels of N-cadherin and vimentin expression, but suppressed E-cadherin expression in SCC4 and SAS cells (Figure 2B-D). Similar results were obtained with proteins levels by Western blot assay of SCC4 cells (Figure 2A). Pretreatment of SCC4 and SAS cells with the ETR antagonist BQ123 abolished ET-1-induced cell migration, invasion and levels of N-cadherin and vimentin expression, but increased E-cadherin levels (Figure 2E–G).
Transcription Factor TWIST is Required for ET-1-Mediated Expression of EMT Markers in OSCC Cells and Subsequently Elicits Cell Migration
Previous studies have indicated that TWIST is a key transcriptional factor EMT.28,29 We therefore hypothesized that TWIST may be involved in ET-1-mediated expression of EMT markers in human OSCC cells. Stimulation of SCC4 cells with ET-1 promoted TWIST mRNA and proteins expression (Figure 3A and F). Pretreatment of OSCC cells with the ETR antagonist BQ123 abolished ET-1-induced increases in TWIST mRNA expression (Figure 3B). After they were transfected with TWIST siRNA to suppress Twist1 protein levels (Supplementary data Figure S1). Stimulated with ET-1 the Twist1 proteins levels (Figure 3F), Transwell assay results revealed that ET-1 significantly increased cell migration and invasion, which were dramatically attenuated in the presence of TWIST siRNA (Figure 3C and D). Transfection of cells with specific siRNAs attenuated ET-1-induced expression of N-cadherin and vimentin, but suppressed E-cadherin mRNA levels (Figure 3E). These findings indicate that TWIST transactivation plays a critical role in ET-1-induced EMT marker expression and migration.
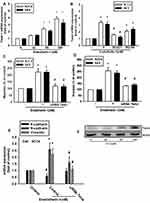
MiR-489-3p is an Important Factor in ET-1-Induced Cell Migration and TWIST Expression
MiRNAs are important regulators of cancer progression and metastasis. Our results indicate that ET-1 promotes cell migration by upregulating TWIST expression. We therefore undertook a bioinformatics analysis using open-source database software (miRDB and TargetScan) to screen for miRNAs that regulate TWIST expression. We found that the 3ʹ-UTR region of TWIST mRNA harbors potential binding sites for candidate miRNAs and that the most significant downregulation of miR-489-3p occurs after ET-1 stimulation (Figure 4A). Using RT-qPCR, we observed that ET-1 directly reduced miR-489-3p expression in a concentration-dependent manner of SCC4 cells (Figure 4B). To confirm the involvement of miR-489-3p in ET-1-mediated cell migration and invasion, a miR-489-3p mimic was transfected into OSCC cells. Following ET-1 treatment, we found that the miR-489-3p mimic abolished ET-1-induced cell migration and invasion (Figure 4C and D). To further examine whether EMT-related proteins are mediated by ET-1 via the reduction of miR-489-3p expression, we pre-treated cells with miR-489-3p mimic. This abolished ET-1-induced expression of E-cadherin, N-cadherin, vimentin and TWIST EMT-related proteins (Figure 4E and F).
To demonstrate whether miR-489-3p regulates the 3′-UTRs of TWIST, we constructed luciferase reporter vectors harboring the wild-type 3′-UTR of TWIST mRNA (WT-TWIST-2-3′-UTR) and a vector containing mismatches in the predicted miR-489-3p binding site (MUT-TWIST-3′-UTR; Figure 4H). In addition, treatment with ET-1 induced luciferase activity with the WT-TWIST-3′-UTR plasmid, but not with the MUT-TWIST-3′-UTR, indicating that miR-489-3p directly suppresses by ET-1. Furthermore, ET-1 can inhibit the expression of miR489-3p binding WT-TWIST 3'UTR further increasing TWIST mRNA expression. On the contrary, this phenomenon has not been seen in MT-TWIST 3'UTR. This shows that miR489-3p is specific to TWSIT 3'UTR and will be regulated by ET-1 (Figure 4I). These data indicate that ET-1 promotes cell migration via suppresses miR-489-3p and up-regulated Twist-1mRNA level.
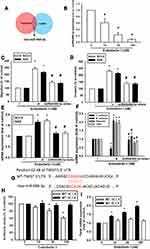
Knockdown of ET-1 Inhibits Metastasis of OSCC Cells in the Lungs of an Animal Model
To verify the involvement of ET-1 expression in the migration of OSCC cells, ET-1 expression was knocked down via lentivirus-mediated delivery of ET-1 shRNA. Knockdown efficiency of ET-1 was determined by Western blot and qPCR (Supplementary data Figure S2 and S3). As shown in Figure 5A, silencing of ET-1 expression reduced ET-1-shRNA SCC4 cell migratory activity and enhanced miRNA489-3p expression (Figure 5B). As shown in Figure 5C and D, ET-1 shRNA repressed OSCC cells growth via a remarkable reduction of bioluminescence activity. Furthermore, we development of lung metastases in mouse models of OSCC. According to recent studies, whether it is orthotopic or tail vein metastasis, is suitable for the construction of animal models of oral cancer metastasis. The orthotopic model presented spontaneous lung metastases in 50% of the animals and the tail vein model, a lung metastasis rate of 60% was observed.30,31 ET-1 shRNA decreased the number of lung metastatic nodules of OSCC xenografts (Figure 5E and F). Therefore, our results suggest that ET-1 is involved in OSCC metastasis.
ET-1 and TWIST Expression Levels Correlate Positively with the Degree of Malignancy in OSCC
We observed strong positive correlations between increased concentrations of ET-1 and TWIST expression with tumor progression in clinical samples of OSCC lung metastases (Figure 6A–C). Pearson’s correlation analysis confirmed a significant positive correlation between ET-1 and TWIST expression (Figure 6D). These findings suggest that ET-1 is associated with TWIST expression and tumor metastasis in OSCC.
Discussion
Despite the introduction of targeted treatment protocols combining EGFR monoclonal antibodies32 and radiotherapy combined with anticancer agents,33 in the hopes of improving therapeutic outcomes for OSCC, 5-year survival rates are poor and marked by toxicity.34 Moreover, the rapid division of cells in the mucosal lining makes them highly susceptible to damage from radiotherapy, resulting in serious oral cavity problems and deleterious quality of life outcomes.35,36
ET has previously been reported to be directly related to cell metastasis. Recently studies reported that the endothelin receptors ETA and ETB are highly expressed in head and neck cancer. And they were overexpressed in tumor cells of tongue cancer samples by immunohistochemistry. Previously studies mentioned the survival rates were significantly lower among the patients who were strongly positive for ET-1 and the ETAR-positive patients compared to negative patients. There was also a significant difference between ET/ETAR expression and the degree of histological differentiation and mode of invasion, and the survival rate of the positive cases was significantly lower than that of the negative cases.37 These reports suggested that ET/ETAR assessments are important for assessing the malignancy of cancer cells and predicting the prognoses of OSCC patients. These results are consisting with our founding. Moreover, our research further indicated that endothelin signalling may, in part, play important roles in miRNA 489–3p/Twist1 in SCCs through the ET-1/ETAR pathway.
ET-1 plays an important role in several cancers, promoting tumor cell migration and metastasis.38 Previous study indicated that ET-1 facilitates oncogenesis in human chondrosarcoma by induced angiogenesis or metastasis via increasing VEGF-A, matrix-metalloproteinase (MMP) family, and cyclooxygenase (COX)-2.39–42 Clinical studies have indicated that the presence of ET-1 immunoreactivity in OSCC specimens enhances the aggressive behavior of poorly-differentiated OSCC, especially metastasis.43–45 In contrast, while salivary ET-1 levels have been detected in patients with OSCC, the levels do not appear to reflect tumor progression.46–48 Thus, the biological function and significance of ET-1 expression in malignancy has been controversial. In this study, we hypothesized that ET-1 may drive metastasis of OSCC. Direct administration of exogenous ET-1 promoted cell migration and invasion activity in OSCC cells. Conversely, ET-1-regulated TWIST expression and cell motility were abolished by ET-1 shRNA. These data suggest that ET-1 increases TWIST expression and subsequently promotes cell migration in human OSCC.
Indeed, most research about EMT are related with Snail & Slug pathways but the major purpose of our research is previously reported mentioned that twist1 is a molecular marker for a poor prognosis in oral cancer and represents a potential therapeutic target.49 This result was corroborated by the clinical observation that Twist1 up‐regulation predicted the occurrence of lymph node and lung metastases as well as poor patient survival.50 In support of Twist1 as a driver of OSCC progression, the up‐regulation of Twist1 was observed in cells isolated from patients with metastatic OSCC. Seemly, TWIST1 is a critical role in clinical metastasis; therefore, we further verify whether ET-1 is the main cause of TWSIT1 activation.
As shown in previous studies, ET receptors are present on the surface of tumor cells and are responsible for ET-1-mediated cell motility.51 We found in this study that pretreatment of OSCC with an ETAR inhibitor blocked ET-1-induced cell migration and reduced levels of TWIST, N-cadherin and vimentin, but increased E-cadherin expression. The ET-1/ETAR axis interaction therefore mediated EMT activity and promoted cell migration in OSCC. MiRNAs control gene expression by binding to complementary sequences in the 3′-UTRs of target mRNAs. Deregulated miRNA expression has been noted in human cancers and may affect multiple steps during metastasis. In particular, the following miRNAs can regulate metastatic ability in OSCC: miR-146a, miR-191, RNA-1297, miR-155, miR-221 and miR-31-5p.52–55 Our study indicates that miR-489-3p is downregulated in response to ET-1; miR-489-3p reportedly suppresses tumor formation in prostate cancer, making it an attractive candidate biomarker for predicting responses to cancer treatment.56–58
In this study, transfection of cells with a miR-489-3p mimic reduced ET-1-induced cell migration, suggesting that miR-489-3p can function as a tumor suppressor. Our tumor metastasis animal model provides strong support that ET-1 is associated with higher incidence of metastasis. Here, we found that decreased ET-1 levels significantly downregulated OSCC cell migration and upregulated miRNA-489-3p expression. These changes affect metastatic ability in OSCC. In the present study, staining of clinical tissue specimens showed that levels of ET-1 and TWIST expression correlate with tumor development, which is consistent with previous clinical studies. These in vivo findings suggest that ET-1 regulates TWIST and OSCC metastasis through ET-1/ETAR/ miRNA-489-3p mechanisms.
Conclusions
In conclusion, existing therapeutic options for OSCC are characterized by poor survival outcomes and significant toxicity. Novel therapeutic targets are needed. Our study findings provide novel insights into the role of ET-1 in OSCC metastasis through ETRs/miR489-3p/Twist pathways (Supplementary data Figure S4) and indicate that ET-1 may be a novel therapeutic target.
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology of Taiwan (MOST110-2320-B-039 -022 -MY3; MOST109-2622-E-029 -005 -CC3; MOST106-2632- B-029-001-MY3; MOST 106-2320-B-029-002; MOST 106-2420-H-029-003-MY2), Ministry of Health and Welfare (PG10603-112) and Taipei Medical University Hospital (106TMU-TMUH-02).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Huey-En Tzeng and Chih-Hsin Tang contributed equally to this work and are are co-first authors. Min-Huan Wu and Yun Yen contributed equally to this work and are co-corresponding authors.
Funding
This research received no external funding.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Moro J, Maroneze MC, Ardenghi TM, Barin LM, Danesi CCJE. Oral and oropharyngeal cancer: epidemiology and survival analysis. Einstein. 2018;16:2.
2. Seyedmajidi M, Seifi S, Moslemi D, Mozaffari SF, Gholinia H, Zolfaghari Z. Immunohistochemical expression of TWIST in oral squamous cell carcinoma and its correlation with clinicopathologic factors. J Cancer Res Ther. 2018;14(5):964–969. doi:10.4103/0973-1482.224350
3. Chandavarkar V, Uma K, Sangeetha R, Mishra M. Immunomorphological patterns of cervical lymph nodes in oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2014;18(3):349–355. doi:10.4103/0973-029X.151311
4. Joseph JP, Harishankar MK, Pillai AA, Devi A. Hypoxia induced EMT: a review on the mechanism of tumor progression and metastasis in OSCC. Oral Oncol. 2018;80:23–32. doi:10.1016/j.oraloncology.2018.03.004
5. Tang L, Li H, Gou R, et al. Endothelin-1 mediated high glucose-induced epithelial-mesenchymal transition in renal tubular cells. Diabetes Res Clin Pract. 2014;104(1):176–182. doi:10.1016/j.diabres.2013.12.021
6. Kang Y, Zhang Y, Sun Y, Wen Y, Sun F. MicroRNA-300 suppresses metastasis of oral squamous cell carcinoma by inhibiting epithelial-to-mesenchymal transition. Onco Targets Ther. 2018;11:5657–5666. doi:10.2147/OTT.S173236
7. Herrmann E, Bogemann M, Bierer S, Eltze E, Hertle L, Wulfing C. The endothelin axis in urologic tumors: mechanisms of tumor biology and therapeutic implications. Expert Rev Anticancer Ther. 2006;6(1):73–81. doi:10.1586/14737140.6.1.73
8. Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80(4):705–713. doi:10.1189/jlb.1105656
9. Anggorowati NP, Ghozali AM, Widodo IP, Sari P, Mansyur RMMS, Arfian NP. Upregulation of Endothelin-1/Endothelin A receptor expression correlates with heparanase expression in ovarian carcinoma. Iran J Med Sci. 2018;43(3):286–295.
10. Rosano L, Cianfrocca R, Sestito R, Tocci P, Di Castro V, Bagnato A. Targeting endothelin-1 receptor/beta-arrestin1 network for the treatment of ovarian cancer. Expert Opin Ther Targets. 2017;21(10):925–932. doi:10.1080/14728222.2017.1361930
11. Moradi Marjaneh R, Khazaei M, Ferns GA, Avan A, Aghaee-Bakhtiari SH. MicroRNAs as potential therapeutic targets to predict responses to oxaliplatin in colorectal cancer: from basic evidence to therapeutic implication. IUBMB Life. 2019;71(10):1428–1441. doi:10.1002/iub.2108
12. Singla N, Lafin JT, Bagrodia A. MicroRNAs: turning the tide in testicular cancer. Eur Urol. 2019;76(5):541–542. doi:10.1016/j.eururo.2019.06.010
13. Shirjang S, Mansoori B, Asghari S, et al. MicroRNAs in cancer cell death pathways: apoptosis and necroptosis. Free Radic Biol Med. 2019;139:1–15.
14. Asadzadeh Z, Mansoori B, Mohammadi A, et al. microRNAs in cancer stem cells: biology, pathways, and therapeutic opportunities. J Cell Physiol. 2019;234(7):10002–10017. doi:10.1002/jcp.27885
15. Uhr K, Prager-van der Smissen WJC, Heine AAJ, et al. MicroRNAs as possible indicators of drug sensitivity in breast cancer cell lines. PLoS One. 2019;14(5):e0216400.
16. Afshar E, Hashemi-Arabi M, Salami S, Peirouvi T, Pouriran R. Screening of acetaminophen-induced alterations in epithelial-to-mesenchymal transition-related expression of microRNAs in a model of stem-like triple-negative breast cancer cells: the possible functional impacts. Gene. 2019;702:46–55. doi:10.1016/j.gene.2019.02.106
17. Nair D, Singhvi H, Mair M, et al. Outcomes of surgically treated oral cancer patients at a tertiary cancer center in India. Indian J Cancer. 2017;54(4):616–620. doi:10.4103/ijc.IJC_445_17
18. Liu Q, Yang G, Qian Y. Loss of MicroRNA-489-3p promotes osteosarcoma metastasis by activating PAX3-MET pathway. Mol Carcinog. 2017;56(4):1312–1321. doi:10.1002/mc.22593
19. Kim MS, Lee HS, Kim YJ, Lee DY, Kang SG, Jin W. MEST induces Twist-1-mediated EMT through STAT3 activation in breast cancers. Cell Death Differ. 2019;26(12):2594–2606. doi:10.1038/s41418-019-0322-9
20. Meng J, Chen S, Han JX, et al. Twist1 regulates vimentin through Cul2 circular RNA to promote EMT in Hepatocellular carcinoma. Cancer Res. 2018;78(15):4150–4162. doi:10.1158/0008-5472.CAN-17-3009
21. Georgakopoulos-Soares I, Chartoumpekis DV, Kyriazopoulou V, Zaravinos A, Factors EMT. Metabolic pathways in cancer. Front Oncol. 2020;10:499.
22. Chuang JY, Yang WH, Chen HT, et al. CCL5/CCR5 axis promotes the motility of human oral cancer cells. J Cell Physiol. 2009;220(2):418–426. doi:10.1002/jcp.21783
23. Chen PC, Liu JF, Fong YC, Huang YL, Chao CC, Tang CH. CCN3 facilitates Runx2 and osterix expression by inhibiting miR-608 through PI3K/Akt signaling in osteoblasts. Int J Mol Sci. 2019;20:13.
24. Chao CC, Chen PC, Chiou PC, et al. Melatonin suppresses lung cancer metastasis by inhibition of epithelial-mesenchymal transition through targeting to Twist. Clin Sci (Lond). 2019;133(5):709–722. doi:10.1042/CS20180945
25. Su CM, Tang CH, Chi MJ, et al. Resistin facilitates VEGF-C-associated lymphangiogenesis by inhibiting miR-186 in human chondrosarcoma cells. Biochem Pharmacol. 2018;154:234–242. doi:10.1016/j.bcp.2018.05.001
26. Roche J. The epithelial-to-mesenchymal transition in cancer. Cancers. 2018;10:2.
27. Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12(4):361–373. doi:10.1007/s11684-018-0656-6
28. Krisanaprakornkit S, Iamaroon A. Epithelial-mesenchymal transition in oral squamous cell carcinoma. ISRN Oncol. 2012;2012:681469.
29. Li R, Wu C, Liang H, et al. Knockdown of TWIST enhances the cytotoxicity of chemotherapeutic drugs in doxorubicin-resistant HepG2 cells by suppressing MDR1 and EMT. Int J Oncol. 2018;53(4):1763–1773.
30. Marcazzan S, Dadbin A, Brachi G, et al. Development of lung metastases in mouse models of tongue squamous cell carcinoma. Oral Dis. 2021;27(3):494–505. doi:10.1111/odi.13592
31. Peng QS, Cheng YN, Zhang WB, Fan H, Mao QH, Xu P. circRNA_0000140 suppresses oral squamous cell carcinoma growth and metastasis by targeting miR-31 to inhibit Hippo signaling pathway. Cell Death Dis. 2020;11(2):112. doi:10.1038/s41419-020-2273-y
32. Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666–2672. doi:10.1200/JCO.2005.04.8306
33. Seyedmahmoud R, Wang Y, Thiagarajan G, et al. Oral cancer radiotherapy affects enamel microhardness and associated indentation pattern morphology. Clin Oral Investig. 2018;22(4):1795–1803. doi:10.1007/s00784-017-2275-z
34. Lin CC, Lin HC. Effects of surgeon and hospital volume on 5-year survival rates following oral cancer resections: the experience of an Asian country. Surgery. 2008;143(3):343–351. doi:10.1016/j.surg.2007.09.033
35. Pu YM, Yang Y, Wang YJ, et al. Postoperative radiotherapy is dispensable for OSCC patients with micrometastases in lymph nodes. Virchows Arch. 2018;472(5):797–805. doi:10.1007/s00428-018-2351-z
36. Sharan Singh S, Kumar R, Singh Kushwaha V, et al. Expression of radioresistant gene PEG10 in OSCC patients and its prognostic significance. Asian Pac J Cancer Prev. 2017;18(6):1513–1518.
37. Miyazawa H, Kato K, Kobayashi Y, et al. Clinicopathological significance of the ET axis in human oral squamous cell carcinoma. Pathol Oncol Res. 2019;25(3):1083–1089. doi:10.1007/s12253-018-0514-5
38. Rosano L, Bagnato A. Endothelin therapeutics in cancer: where are we? Am J Physiol Regul Integr Comp Physiol. 2016;310(6):R469–75. doi:10.1152/ajpregu.00532.2015
39. Wu MH, Huang CY, Lin JA, et al. Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells. Oncogene. 2014;33(13):1725–1735. doi:10.1038/onc.2013.109
40. Wu MH, Chen LM, Hsu HH, et al. Endothelin-1 enhances cell migration through COX-2 up-regulation in human chondrosarcoma. Biochim Biophys Acta. 2013;1830(6):3355–3364. doi:10.1016/j.bbagen.2013.03.014
41. Wu MH, Lo JF, Kuo CH, et al. Endothelin-1 promotes MMP-13 production and migration in human chondrosarcoma cells through FAK/PI3K/Akt/mTOR pathways. J Cell Physiol. 2012;227(8):3016–3026. doi:10.1002/jcp.23043
42. Wu MH, Huang PH, Hsieh M, Tsai CH, Chen HT, Tang CH. Endothelin-1 promotes epithelial-mesenchymal transition in human chondrosarcoma cells by repressing miR-300. Oncotarget. 2016;7(43):70232–70246. doi:10.18632/oncotarget.11835
43. Mankapure PK, Barpande SR, Bhavthankar JD, Mandale M. Serum big endothelin-1 as a biomarker in oral squamous cell carcinoma patients: an analytical study. J Appl Oral Sci. 2015;23(5):491–496. doi:10.1590/1678-775720150125
44. Satomura K, Tokuyama R, Yamasaki Y, et al. Possible involvement of stem cell factor and endothelin-1 in the emergence of pigmented squamous cell carcinoma in oral mucosa. J Oral Pathol Med. 2007;36(10):621–624. doi:10.1111/j.1600-0714.2007.00587.x
45. Hinsley EE, Kumar S, Hunter KD, Whawell SA, Lambert DW. Endothelin-1 stimulates oral fibroblasts to promote oral cancer invasion. Life Sci. 2012;91(13–14):557–561. doi:10.1016/j.lfs.2012.04.001
46. Hoffmann RR, Yurgel LS, Campos MM. Evaluation of salivary endothelin-1 levels in oral squamous cell carcinoma and oral leukoplakia. Regul Pept. 2011;166(1–3):55–58. doi:10.1016/j.regpep.2010.08.006
47. Kar A, Shwetha V, Sukesh V, Reddy Sujatha S, Rakesh N, Tupakula Pavan K. Assessment of salivary endothelin-1 in patients with leukoplakia, submucous fibrosis, oral cancer and healthy individuals- a comparative study. J Stomatol Oral Maxillofac Surg. 2019;120(4):326–331.
48. Cheng Y-SL, Rees T, Jordan L, et al. Salivary endothelin-1 potential for detecting oral cancer in patients with oral lichen planus or oral cancer in remission. Oral Oncol. 2011;47(12):1122–1126. doi:10.1016/j.oraloncology.2011.07.032
49. Jayanthi P, Varun BR, Selvaraj J. Epithelial-mesenchymal transition in oral squamous cell carcinoma: an insight into molecular mechanisms and clinical implications. J Oral Maxillofac Pathol. 2020;24(1):189. doi:10.4103/jomfp.JOMFP_334_19
50. Zhuo X, Luo H, Chang A, Li D, Zhao H, Zhou Q. Is overexpression of TWIST, a transcriptional factor, a prognostic biomarker of head and neck carcinoma? Evidence from fifteen studies. Sci Rep. 2015;5:18073. doi:10.1038/srep18073
51. Pattani KM, Zhang Z, Demokan S, et al. Endothelin receptor type B gene promoter hypermethylation in salivary rinses is independently associated with risk of oral cavity cancer and premalignancy. Cancer Prev Res. 2010;3(9):1093–1103. doi:10.1158/1940-6207.CAPR-10-0115
52. Zhang H, Li T, Zheng L, Huang X. Biomarker MicroRNAs for diagnosis of oral squamous cell carcinoma identified based on gene expression data and MicroRNA-mRNA network analysis. Comput Math Methods Med. 2017;2017:9803018.
53. Gissi DB, Morandi L, Gabusi A, et al. A noninvasive test for MicroRNA expression in oral squamous cell carcinoma. Int J Mol Sci. 2018;19:6. doi:10.3390/ijms19061789
54. Liang L, Feng L, Wei B. microRNA-1297 involves in the progression of oral squamous cell carcinoma through PTEN. Saudi J Biol Sci. 2018;25(5):923–927. doi:10.1016/j.sjbs.2018.01.013
55. Gombos K, Horvath R, Szele E, et al. miRNA expression profiles of oral squamous cell carcinomas. Anticancer Res. 2013;33(4):1511–1517.
56. Lemecha M, Morino K, Imamura T, et al. MiR-494-3p regulates mitochondrial biogenesis and thermogenesis through PGC1-alpha signalling in beige adipocytes. Sci Rep. 2018;8(1):15096. doi:10.1038/s41598-018-33438-3
57. Faversani A, Amatori S, Augello C, et al. miR-494-3p is a novel tumor driver of lung carcinogenesis. Oncotarget. 2017;8(5):7231–7247. doi:10.18632/oncotarget.13933
58. Weng JH, Yu CC, Lee YC, Lin CW, Chang WW, Kuo YL. miR-494-3p induces cellular senescence and enhances radiosensitivity in human oral squamous carcinoma cells. Int J Mol Sci. 2016;17(7):7. doi:10.3390/ijms17071092
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.