Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Rehmannioside A Inhibits TRAF6/MAPK Pathway and Improves Psoriasis by Interfering with the Interaction of HaCaT Cells with IL-17A
Received 14 July 2023
Accepted for publication 12 September 2023
Published 21 September 2023 Volume 2023:16 Pages 2585—2596
DOI https://doi.org/10.2147/CCID.S430621
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Li-li Yuan, Chun-yu Cao
Department of Dermatology, Taizhou People’s Hospital, Taizhou, Jiangsu, 225300, People’s Republic of China
Correspondence: Chun-yu Cao, Email [email protected]
Objective: As a common chronic inflammatory skin disease, psoriasis seriously affects the physical health and psychological well-being of patients. Various clinical treatments for psoriasis have their own drawbacks, so it is important to find effective and safe drugs. Rehmannioside A (ReA) has anti-inflammatory properties and is the main active ingredient in Fuzhengzhiyanghefuzhiyang decoction (FZHFZY), an herbal compound for the treatment of psoriasis. But no studies have been conducted to determine whether ReA alone can treat psoriasis. Therefore, this study was designed to investigate the effect of ReA in the treatment of psoriasis and its potential mechanism of action.
Methods: HaCaT cells were treated with ReA and IL-17A alone for 24 h and 48 h, and the most effective concentrations of ReA and interleukin (IL)-17A were found at 25 μg/mL and 100 ng/mL, respectively. A psoriasis cell model was constructed by stimulating HaCaT cells with IL-17A, followed by intervention with ReA. Cell viability and cell cycle distribution were measured by MTT assay and flow cytometry. The expression levels of keratin family members and chemokines were detected by real-time quantitative PCR (RT-qPCR), the levels of pro-inflammatory cytokines by enzyme-linked immunosorbent assay (ELISA), and key proteins of TRAF6/MAPK signaling pathway by Western blot.
Results: ReA weaken cell viability, down-regulate the expression of keratin family members (KRT6 and KRT17), restore cell cycle distribution to normal distribution, inhibit the release of pro-inflammatory cytokines (IL-6, IL-8 and IL-1β) and lower the expression of chemokines (S100A7, S100A9 and CXCL2) by interfering with the interaction between HaCaT cells and IL-17A. Thus, it exerts an anti-psoriatic effect by reducing the inflammatory response and inhibiting abnormal proliferation of HaCaT cells. Mechanistically, ReA inhibited the TRAF6/MAPK signaling pathway activated by IL-17A stimulation in HaCaT cells.
Conclusion: ReA has in vitro anti-psoriatic effects and may be a new therapeutic agent for psoriasis.
Keywords: psoriasis, rehmannioside A, ReA, interleukin-17A, IL-17A, inflammation, proliferation
Introduction
Psoriasis is a common skin disease with a worldwide prevalence of approximately 2–4%.1 In the prevailing view, local inflammatory immune responses caused by abnormal interactions between keratinocytes and immune cells in the epidermis and uncontrolled overproliferation of keratinocytes are the two main pathological features of psoriasis.2,3 Although psoriasis is not usually life-threatening, it can have a negative impact on the mental and physical health of patients. For one thing, psoriasis patients develop red patches or silvery-white scales on the skin, which are usually located on the scalp, face, arms and legs where they can be easily noticed by others. In this condition, patients could endure a significant psychological load as well as social awkwardness and embarrassment.4 For another, psoriasis is often accompanied by pronounced itching and pain in the skin lesions, and the long-term discomfort can generate unhealthy emotions like anxiety, irritability and depression. Besides, patients with psoriasis will experience reduced sleep quality and poor physical health.5
Although Megna et al systematically evaluated the efficacy and safety of drugs for psoriasis in children and elderly people, they found that, compared with traditional systemic drugs, biologics and small molecule drugs are ideal treatment options for pediatric patients and have excellent performance in terms of efficacy and safety.6,7 There are several clinical treatments for psoriasis, but most of them have their own drawbacks. Corticosteroids, the most commonly used topical medication, can induce skin atrophy and interfere with the normal function of the adrenal axis after long-term use; vitamin D3 can easily cause irritant dermatitis; immunosuppressant drugs can increase the risk of infection and result in liver dysfunction and adverse cardiovascular outcomes; phototherapy requires frequent visits to medical institutions, which takes up a lot of time and energy for patients, and it may have side effects such as sunburn and hyperpigmentation.8,9 Therefore, the search for more simple, economical, safe and effective drugs for psoriasis will be of great significance in alleviating patients’ subjective discomfort and improving their quality of life.
Rehmannioside A (ReA, molecular formula: C21H32O15) is a natural small molecule isolated from the traditional Chinese herb Rehmanniae radix.10 There is evidence that ReA has positive neuroprotective benefits. Some animal experiments have shown that ReA can be used for the treatment of spinal cord injury, acute cerebral infarction and post-ischemic cognitive impairment. Its powerful effectiveness can be attributed to its potent efficacy of inhibiting oxidative stress and attenuating inflammatory immune responses.11–13 In addition, Chen et al demonstrated that ReA is one of the main active ingredients in Fuzhengzhiyanghefuzhiyang decoction (FZHFZY), the Chinese herbal compound used for the treatment of psoriasis.14 However, no study has specifically investigated the possibility that ReA used alone can treat psoriasis.
Therefore, this study constructed a psoriasis cell model by stimulating HaCaT cells with IL-17A to mimic abnormal cell proliferation. It attempted to further confirm the pharmacological effects and potential mechanism of action of ReA against psoriasis in this cell model. The findings of this study will contribute to the identification of novel clinical applications of ReA as well as new approaches to the management of psoriasis.
Materials and Methods
Cell Culture
The immortalized human epidermal keratinocyte cell line, HaCaT, purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), was used as a cell model in this study. HaCaT cells were cultured in high-sugar DMEM medium (Sigma-Aldrich, USA) containing 10% fetal bovine serum (FBS) (Beyotime, China) and 1% penicillin/streptomycin solution (Beyotime, China) under standard conditions (5% CO2, 37 °C, 95% humidity).
Cell Treatment and Grouping
The ReA powder used in this study was purchased from Sichuan Cuiyirun Biotechnology Co., Ltd (China). The ReA structure is shown in Figure 1. The procedure of this study was slightly adjusted based on the previous animal experiments11–13 that explored the pharmacological effects of ReA. At first, ReA powder was added to different volumes of double-distilled water at 37 °C for the preparation of the solutions at concentrations of 0 μg/mL (without ReA powder), 5 μg/mL, 10 μg/mL, 25 μg/mL and 50 μg/mL. The solutions were then shaken well in a bench-top shaker to dissolve the ReA powder. After passing through a filter and sterilization, all insoluble components were removed, and the remaining solutions were the ReA solutions for treating HaCaT cells in subsequent experiments. The above five concentration gradients of ReA solutions were added to HaCaT cell culture medium at 60–70% confluence for 24 hours to determine the optimal concentration of ReA solution.
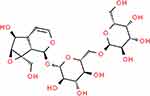 |
Figure 1 Diagram of ReA structure. |
The interleukin (IL)-17A powder was purchased from MedChemExpress (USA). Similarly, referring to previous related studies using IL-17A intervention on HaCaT cells,15,16 IL-17A powder was added to double-distilled water at 37 °C to prepare solutions at concentrations of 0 ng/mL (without IL-17A powder), 10 ng/mL, 50 ng/mL and 100 ng/mL. After shaking well to dissolve and filtering the insoluble components, the obtained solutions became the IL-17A solutions for stimulating HaCaT cells in subsequent experiments. IL-17A solutions of the above four concentration gradients were added to HaCaT cell culture medium at 60–70% confluence to stimulate cells for 48 hours to determine the most effective concentration for IL-17A solution.
Through applying ReA alone and IL-17A alone to intervene HaCaT cells, we found that 25 μg/mL was the optimal concentration of ReA solution and 100 ng/mL was the most effective concentration of IL-17A solution. HaCaT cells were divided into the following four groups and given different interventions: (1) Control group, HaCaT cells were conventionally cultured without the addition of ReA solution and IL-17A solution; (2) ReA group, HaCaT cells were treated with 25 μg/mL ReA solution for 24 h; (3) IL-17A group, HaCaT cells were stimulated with 100 ng/mL IL-17A solution for 48 h; (4) IL-17A+ReA group: HaCaT cells were stimulated with 100 ng/mL IL-17A solution for 48 h and then treated with 25 μg/mL ReA solution for 24 h. After 72 h of treatment, as described above, cells from each group were collected for subsequent experiments.
MTT Assay
HaCaT cells were resuspended in high-sugar DMEM medium and inoculated into 96-well plates at a concentration of 1 × 104/well, with a volume of 200 µL per well. Following that, the plates were incubated at room temperature for 72 hours. Next, 10 µL of MTT solution (Shanghai Regal Biological Technology Development Co., Ltd., China) were added to each well. The incubation was continued for 3 hours to allow HaCaT cells to fully absorb the MTT solution and reduce it to the purple-colored formazan. Afterwards, the supernatant was removed and 150 µL of DMSO (Beyotime, China) was introduced to each well and shaken for 10 min to dissolve the formazan. The cell viability was reflected by the OD value at 490 nm in each well measured using an enzyme marker (Thermo Fisher Scientific, USA). Three replicates were set up for each group, and the average value was taken as the final result.
Real-Time Quantitative PCR (RT-qPCR)
After mixing well with Trizol reagent (Servicebio, China), HaCaT cells were centrifuged (12,000 × g, 4 °C, 10 min). Then total RNA was collected from the supernatant. The total RNA was reverse-transcribed into cDNA using the PrimeScript RT Reagent Kit (Takara Biotechnology Ltd., Japan). Later, qRT-PCR was performed on a Quant Studio 6 Flex system (Applied Biosystems, USA) using the SYBR Premix Ex Taq kit (Takara Biotechnology Ltd., Japan). The expression levels of KRT6, KRT17, S100A7, S100A9 and CXCL2 were calibrated according to the internal reference GAPDH expression level, quantified and analyzed using the 2−ΔΔct method. The relative expression levels of all target RNAs were normalized based on the mean value of those in 0 ng/mL group (Control group). The qRT-PCR was repeated three times for each group of cells and the mean value was taken as the final result. The primers used in the qRT-PCR are shown in Table 1.
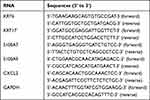 |
Table 1 RT-qPCR Primers |
Flow Cytometry
Flow cytometry was used to analyze the cell cycle distribution of HaCaT cells in this study. HaCaT cells were digested using trypsin and fixed with pre-cooled 75% ethanol overnight at 4 °C to inhibit cell cycle progression. Upon washing with PBS, the cells were incubated with PI/RNase Staining Buffer (#abx090619, Abbexa, UK) for 30 min at 37 °C protected from light. Following that, HaCaT cells were filtered using a 70 μm nylon cell strainer (FALCON, USA) to remove cell clumps and later assayed on a FACScan™ flow cytometer (BD Biosciences, USA). The results obtained were quantified by FlowJo 10.0 software (FlowJo, Canada).
ELISA
HaCaT cells were centrifuged (12,000 × g, 4 °C, 10 min) to collect supernatants. Commercial IL-6 (Invitrogen, #BMS213-2, USA), IL-8 (Invitrogen, #BMS204-3, USA) and IL-1β (Invitrogen, #BMS224-2 USA) ELISA assay kits were used to measure the levels of these three pro-inflammatory cytokines in the supernatants. The measurements were repeated three times for each group of cells and the average value was taken as the final result.
Western Blot
HaCaT cells were lysed using RIPA lysis buffer (Thermo Fisher Scientific, USA) containing 1% protease/phosphatase inhibitor (Servicebio, China), and then total protein was extracted. After centrifugation (12,000 × g, 4 °C, 10 min), the concentration of the total protein was quantified using a bicinchoninic acid (BCA) protein assay kit (Abcam, UK). The obtained protein samples were boiled in SDS sample loading buffer (Elabscience, China) for 15 min at 95 °C, separated by 10% SDS-PAGE (Servicebio, China), and transferred to PVDF membranes (Abcam, UK). Next, the PVDF membranes were blocked at room temperature for 1 h using 5% skim milk and TBST buffer. Subsequently, the membranes were then incubated overnight at 4 °C with the following primary antibodies: tumor necrosis factor receptor associated factor 6 (TRAF6) (1:1000, #8028, Cell Signaling Technology, USA); extracellular regulated protein kinases (ERK) (1:10,000, #ab184699, Abcam, UK); p-ERK (1:10,000, #3510, Cell Signaling Technology, USA); c-Jun N-terminal kinase (JNK) (1:1000, #ab179461, Abcam, UK); p-JNK (1:5000, #ab76572, Abcam, UK); P38 (1:1000, #ab32142, Abcam, UK); p-P38 (1:1000, #ab178867, Abcam, UK); β-actin (1:5000, #ab115777, Abcam, UK). The next day, after washing three times with TBST buffer, the protein samples were incubated with HRP-labeled Goat Anti-Rabbit IgG H&L secondary antibody (1: 10000, #ab6721, Abcam, UK) for 1 hour at room temperature. Finally, the membranes were visualized in a GEL imaging system (Bio-Rad, USA) and protein bands were quantified using Image J software (NIH, USA). The relative expression levels of all target proteins were corrected by the expression level of the internal reference protein β-actin.
Statistical Analysis
The measured data were expressed as mean ± standard deviation. The Shapiro–Wilk method was used to test the normality of all data. The continuous variables conforming to normal distribution were expressed as mean ± standard deviation (SD), and comparisons between multiple groups were analyzed by one-way ANOVA with post hoc test. The continuous data that were not normally distributed were expressed as median (interquartile range, IQR) and analyzed using the Kruskal waila H-test. Statistical analysis was carried out using SPSS 20.0 software (IBM Corp. Armonk, NY, USA). P < 0.05 was used as the criterion for statistically significant differences.
Results
Rehmannioside A Inhibits the Proliferation of HaCaT Cells
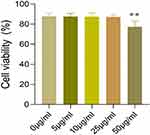
Keratinocytes are the main cell type of the skin, and they proliferate and differentiate moderately to ensure regular cell renewal under normal conditions. However, in the pathological process of psoriasis, the proliferation of keratinocytes is significantly accelerated. As a result, the excessive accumulation of keratinocytes within the epidermis contributes to the formation of scaly erythema.17 Therefore, the ability to inhibit excessive proliferation of keratinocytes is an important criterion for assessing the effectiveness of drug therapy in psoriasis. In this study, we treated HaCaT cells with different concentrations of ReA solution (0 μg/mL, 5 μg/mL, 10 μg/mL, 25 μg/mL and 50 μg/mL) for 24 h and then evaluated the number of viable cells using MTT assay. The results of MTT assay showed that there was no significant difference in the viability of HaCaT cells in the 0 μg/mL, 5 μg/mL, 10 μg/mL and 25 μg/mL groups (P>0.05). But the viability of HaCaT cells was significantly lower in the 50 μg/mL group compared with the 0 μg/mL group (P<0.05) (Figure 2). This suggested that 25 μg/mL of ReA solution significantly inhibited the effect of HaCaT cell proliferation. Therefore, we adopted a concentration of 25 μg/mL of ReA solution to intervene HaCaT cells to further explore the potential mechanism of action of ReA.
Interleukin-17A Induces Overproliferation of HaCaT Cells
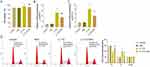
The role of IL-17A in the pathological process of psoriasis has been reported. For instance, IL-17A stimulates the proliferation of keratinocytes, which leads to an excessive increase in the number of keratinocytes under the epidermis. This pathogenic process results in characteristic histopathological features of psoriasis.18 We evaluated cell proliferation after stimulating HaCaT cells with different concentrations of IL-17A solutions (0 ng/mL, 10 ng/mL, 50 ng/mL and 100 ng/mL) for 48 h. The results of MTT assay showed that, compared to the 0 ng/mL group, HaCaT cell viability was significantly increased in the 10 ng/mL, 50 ng/mL and 100 ng/mL groups (P<0.05) in a concentration-dependent manner, with higher IL-17A concentration associated with higher cell viability (Figure 3A). As for the RT-qPCR results, the expression levels of KRT6 and KRT17 in HaCaT cells in the 10 ng/mL, 50 ng/mL and 100 ng/mL groups were also obviously increased compared with the 0 ng/mL group (P<0.05) in a concentration-dependent manner; the higher the concentration of IL-17A, the higher the expression levels of KRT6 and KRT17 (Figure 3B and C). Both KRT6 and KRT17, which are mainly expressed in epidermal cells, are members of the keratin family. They are involved in the composition of the cytoskeleton and maintenance of cell structure. When cell proliferation is active, the expression of KRT6 and KRT17 is up-regulated to meet the cytoskeleton reassembly requirements.19 Therefore, the expression levels of KRT6 and KRT17 in this study could reflect the proliferation level of IL-17A-induced HaCaT cells. The above results imply that IL-17A solution at 100 ng/mL may have the strongest effect on promoting HaCaT cell proliferation, so this concentration of IL-17A solution was used to stimulate HaCaT cells in subsequent experiments.
Rehmannioside A Inhibits Interleukin-17A-Induced Overproliferation of HaCaT Cells
Given ReA inhibited HaCaT cell proliferation while IL-17A inducing it, we further explored whether the anti-pyretic effect of ReA was achieved by interfering with the interaction between HaCaT cells and IL-17A. The results of MTT assay demonstrated that the IL-17A group displayed significantly increased cell viability than the Control group (P<0.05); however, after ReA treatment, HaCaT cells showed observably reduced viability (P<0.05); the differences in cell viability between the Control and ReA groups were not statistically significant (P>0.05) (Figure 4A). With regard to the results of RT-qPCR, the expression levels of KRT6 and KRT17 were much higher in HaCaT cells of the IL-17A group than the Control group (P<0.05), which were much lower in HaCaT cells of the IL-17A+ReA group than the IL-17A group (P<0.05). Notably, there was no significant difference in the expression levels of KRT6 and KRT17 in the Control and ReA groups (P> 0.05) (Figure 4B and C).
The results of flow cytometry are as follows. In comparison with the Control group, the proportion of G1 phase was considerably reduced (P<0.05) and that of S phase was markedly elevated (P<0.05) in the cells of the IL-17A group. But there was no significant difference in the G2/M phase in this group (P>0.05). In contrast to the IL-17A group, the number of HaCaT cells in G1 phase was significantly higher (P< 0.05) while in S-phase observably reduced (P<0.05) in the IL-17A+ReA group, and there was no significant difference in G2/M-phase between the two groups (P>0.05). Besides, the difference in the proportion of each cell cycle between the Control and ReA groups was not statistically significant (P>0.05) (Figure 4D). It is known that a high percentage of cells in the G1 phase indicates a low cell proliferation rate. On the contrary, a high percentage of cells in the S phase (during DNA replication) indicates active proliferation. In terms of DNA replication, a large number of cells are undergoing DNA synthesis and preparing to enter the mitotic phase, suggesting active proliferation.20 Overall, ReA effectively inhibits the IL-17A-induced proliferation of HaCaT cells, and the interference with the interaction between HaCaT cells and IL-17A may be the main mechanism of ReA in the treatment of psoriasis.
Rehmannioside A Attenuates Interleukin-17A-Induced Up-Regulation of Pro-Inflammatory Cytokine Release and Chemokine Expression in HaCaT Cells
Psoriasis is an inflammatory skin disease, and it can be induced by IL-17A. With IL-17A treatment, keratinocytes may release large amounts of pro-inflammatory cytokines, which directly aggravate local inflammation in the epidermis. IL-17A can also up-regulate the expression level of chemokines in keratinocytes. The recruitment of inflammatory cells induced by the chemokines indirectly aggravates local inflammation in the epidermis.21,22 With the superposition of these two effects, IL-17A can promote the pathological process of psoriasis by exacerbating the inflammatory immune response in the epidermis. According to the ELISA results, a notable rise was observed in the levels of IL-6, IL-8, and IL-1β in the supernatant of cell culture in the IL-17A group compared with the Control group (P<0.05); compared with the IL-17A group, the levels of IL-6, IL-8, and IL-1β in the supernatant of the 17A+ReA group were much lower (P<0.05); however, the differences in IL-6, IL-8, and IL-1β levels in the Control and ReA groups were not statistically significant (P>0.05) (Figure 5A–C). IL-6, IL-8, and IL-1β are common pro-inflammatory cytokines in psoriasis. The interaction between these pro-inflammatory cytokines leads to the persistence of a local inflammatory response in the epidermis, ultimately causing lesion formation and development of psoriasis.23
In addition, RT-qPCR results revealed that the expression levels of S100A7, S100A9, and CXCL2 were significantly raised in cells of the IL-17A group relative to the Control group (P<0.05); as opposed to the IL-17A group, the IL-17A+ReA group exhibited much lower expression levels of S100A7, S100A9, and CXCL2 in HaCaT cells (P<0.05); and there was no statistically significant difference in the expression levels of S100A7, S100A9 and CXCL2 in the Control and ReA groups (P>0.05) (Figure 5D–F). With the properties of chemokines, S100A7, S100A9 and CXCL2 can attract the migration of inflammatory cells to the skin lesion, thus triggering local inflammatory response and lesion formation in the epidermis.24 Combining the above results, we suggest that ReA exerts a therapeutic effect on psoriasis via effectively inhibiting pro-inflammatory cytokine release and upregulation of chemokine expression in IL-17A-induced HaCaT cells.
Rehmannioside A Inhibits Interleukin-17A-Induced Activation of TRAF6/MAPK Signaling Pathway in HaCaT Cells
The TRAF6/MAPK signaling pathway is over-activated in psoriasis. Not only does this signaling pathway worsen the inflammatory response, but it also accelerates the proliferation of keratinocytes. The two effects of TRAF6/MAPK signaling pathway exacerbate psoriasis.25 Accordingly, we explored the effect of ReA on the TRAF6/MAPK signaling pathway in HaCaT cells. Western blot assay results showed that the protein levels of TRAF6, p-ERK, p-JNK, and p-P38 were significantly up-regulated in cells of the IL-17A group compared to those of the Control group (P<0.05), and the protein levels of ERK, JNK, and P38 were not significantly different between the two groups (P>0.05). Clearly, the ratios of p-ERK/ERK, p-JNK/JNK, and p-P38/P38 were increased (P<0.05). As opposed to the IL-17A group, the protein levels of TRAF6, p-ERK, p-JNK, and p-P38 were significantly lowered in cells of the IL-17A+ReA group (P<0.05). Likewise, there was no significant difference in the protein levels of ERK, JNK, and P38 (P>0.05), and the ratios of p-ERK/ERK, p-JNK/JNK, and p-P38/P38 were noticeably reduced (P<0.05). In addition, the differences in the ratios of p-ERK/ERK, p-JNK/JNK, and p-P38/P38 in the Control and ReA groups were not statistically significant (P>0.05) (Figure 6A and B). In the TRAF6/MAPK signaling pathway, TRAF6 is located at the initiation of signaling. ERK, JNK and p38 are the main members of MAPK subfamilies activated in psoriasis.26,27 It is evident that ReA significantly inhibits the TRAF6/MAPK signaling pathway activated by IL-17A stimulation in HaCaT cells to exert therapeutic effects in psoriasis.
Discussion
In this study, the therapeutic effect of ReA on psoriasis was investigated in a psoriasis cell model induced by IL-17A. The results suggested that ReA attenuated the inflammatory response and abnormal proliferation of keratinocytes by inhibiting the TRAF6/MAPK signaling pathway that was activated by the interaction of keratinocytes with IL-17A, thereby delaying the progression of psoriasis. To the best of our knowledge, this study is the first to elucidate the potential mechanism of action of ReA in the treatment of psoriasis, which provides a new theoretical basis for the selection of clinical treatments for psoriasis.
FZHFZY, a herbal compound with ReA as one of the main active ingredients, has been applied clinically for decades to treat psoriasis, and its excellent efficacy has been generally recognized by patients.14 It is clear that there is sufficient evidence to support the effectiveness of ReA for the treatment of psoriasis. Furthermore, previous animal studies conducted to explore ReA in the treatment of central nervous system diseases disclosed that ReA did not significantly alter body weight and organ index values of animals.11,12,28 As such, ReA has high safety in clinical treatment. Therefore, we drew the conclusion that ReA may be a new drug candidate for the treatment of psoriasis, based on a comprehensive analysis of its efficacy and safety.
Unlike prior research, this study constructed a psoriasis cell model by stimulating HaCaT cells with IL-17A alone. The M5 cytokines, which include IL-1, IL-17A, IL-22, oncostatin M (OSM), and tumor necrosis factor (TNF)-α, have been employed in many recent in vitro psoriasis research29–31 to intervene cells and simulate psoriasis. However, this modeling approach has the disadvantage that it does not allow exploring the effect of candidate drugs on the interaction between keratinocytes and one cytokine alone. Previous studies have shown that dynamic interactions between keratinocytes and T-cell-derived cytokines are crucial in both the pathogenesis and progression of psoriasis, and such interactions result in a vicious cycle of persistent inflammatory responses locally in the epidermis.32 Among the various cytokines, IL-17A is involved in the pathological process of psoriasis through multiple pathways such as activation of immune cells, disruption of the skin barrier, acceleration of peripheral angiogenesis, and triggering of various inflammatory loops.18 For this reason, in our investigation, we only employed IL-17A to activate HaCaT cells. By comparing the Control and IL-17A groups, we found that IL-17A significantly enhanced the proliferation level of HaCaT cells, induced the release of a large number of pro-inflammatory cytokines, and up-regulated the expression level of chemokines in HaCaT cells. It confirms that the application of IL-17A alone is sufficient to drive HaCaT cells to exhibit (even if not fully exhibit) the typical pathological features of psoriasis. This finding is generally consistent with that of Zhang et al.33
The two fundamental characteristics of psoriasis, as previously established, are the inflammatory immune response and the abnormal proliferation of keratinocytes, and they work in tandem to encourage one another. The inflammatory response to the skin lesion attracts a large number of immune cells to accumulate locally. The various cytokines secreted by these accumulating immune cells will stimulate keratinocytes and ultimately cause them to overproliferate and differentiate abnormally. When keratinocytes overproliferate, they produce and release a large number of pro-inflammatory cytokines in response to their surrounding cytokines, thereby worsening the local inflammatory response.34,35 Therefore, the ability to inhibit overproliferation of keratinocytes and reduce excessive local inflammation are two important indicators for assessing the effectiveness of drug therapy in psoriasis. It is by these two indicators that we assessed the effect of ReA on psoriasis.
The effect of ReA on cell proliferation level in the psoriasis cell model was explored at first in our study. We discovered that ReA reduced the proliferation of IL-17A-stimulated HaCaT cells to normal levels as well as restored their cell cycle distribution to normal proportions. It is logical to speculate that ReA exerts its effect by interfering with the interaction between HaCaT cells and IL-17A.
Subsequently, we examined the effect of ReA on the inflammatory response in the psoriasis cell model. Upon stimulation by IL-17A, the levels of S100A7, S100A9 and CXCL2, proteins with properties of chemokines, were significantly up-regulated.36,37 Previous clinical studies have confirmed that S100A7, S100A9 and CXCL2 genes are overexpressed in patients with psoriasis and that changes in the protein concentrations of S100A7, S100A9 and CXCL2 in the serum can reflect whether psoriasis is moving into an active phase.38 In addition, IL-6, IL-8 and IL-1β are common pro-inflammatory cytokines in psoriasis.39 Therefore, the changes in the expression levels of S100A7, S100A9 and CXCL2 (as representatives of chemokines) as well as the concentrations of IL-6, IL-8 and IL-1β (as representatives of pro-inflammatory cytokines) in the pathological process of psoriasis were focused on in this study. Through the pairwise comparisons between the Control group, IL-17A group and IL-17A+ReA group, ReA was revealed to lower the levels of the pro-inflammatory cytokines released by IL-17A-stimulated HaCaT cells and the chemokines to normal ones. Collectively, ReA exerts its efficacy in suppressing excessive inflammation of HaCaT cells by interfering with the interaction between HaCaT cells and IL-17A.
Although we uncovered that ReA inhibited abnormal proliferation and reduced excessive inflammation, the specific molecular mechanisms involved remained a mystery. In this section, we concentrated on the effect of ReA on the TRAF6/MAPK signaling pathway. The TRAF6/MAPK signaling pathway is an important pathway implicated in various physiological and pathological processes. It mainly regulates inflammatory responses, cell proliferation, cell differentiation and cell survival. Upon receiving foreign signals, TRAF6 activates and phosphorylates multiple MAPKs in the downstream of the pathway, including ERK, JNK, and p38, resulting in MAPK cascade responses that produce multiple effects.40 Over-activated TRAF6/MAPK signaling pathway has been demonstrated to accelerate the progression of psoriasis. The acceleration is discussed in two aspects. For one thing, keratinocyte proliferation is encouraged through the release of pro-inflammatory cytokines and chemokines and the regulation of keratin synthesis by this pathway. For another, this pathway can activate immune cells such as T cells, dendritic cells and macrophages to exacerbate the local inflammatory response.25 In the present study, it was discovered that ReA reduced the ratios of p-ERK/ERK, p-JNK/JNK and p-P38/P38 in HaCaT cells stimulated by IL-17A to normal levels. Taken together, ReA strongly inhibits the TRAF6/MAPK signaling pathway activated by IL-17A intervention.
However, there are some shortcomings in our study. First, this study lacks in vivo experiments using animals as models to further validate the effect of ReA in treating psoriasis. Second, this study only elucidated the effect of ReA in interfering with IL-17 signaling in only one keratinocytes. Future studies may need to further explore whether there is a similar interfering effect of ReA on other M5 cytokines and cells.
Conclusion
In summary, this is the first study to reveal that ReA, a natural active molecule of traditional Chinese medicine, has in vitro therapeutic efficacy in the treatment of psoriasis. Specifically, ReA interferes with the interaction between HaCaT cells and IL-17A to inhibit the abnormal proliferation of HaCaT cells and reduce their inflammatory response, which is most likely achieved by down-regulating the IL-17A-activated TRAF6/MAPK signaling pathway. The results of this study not only identify a new direction for the clinical application of ReA, but also provide a new idea for the treatment of psoriasis.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The authors declare no conflicts of interest.
References
1. Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. 2021;8:743180. doi:10.3389/fmed.2021.743180
2. Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. doi:10.3390/ijms20061475
3. Tashiro T, Sawada Y. Psoriasis and systemic inflammatory disorders. Int J Mol Sci. 2022;23(8):4457. doi:10.3390/ijms23084457
4. Rousset L, Halioua B. Stress and psoriasis. Int J Dermatol. 2018;57(10):1165–1172. doi:10.1111/ijd.14032
5. Bolotna L, Sarian O. Psychopathological disorders as comorbidity in patients with psoriasis (review). Georgian Med News. 2020;2020 (301):143–147.
6. Megna M, Camela E, Battista T, et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part I: focus on pediatric patients. Expert Opin Drug Saf. 2023;22(1):25–41. doi:10.1080/14740338.2023.2173170
7. Megna M, Camela E, Battista T, et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part II: focus on elderly patients. Expert Opin Drug Saf. 2023;22(1):43–58. doi:10.1080/14740338.2023.2173171
8. Bakshi H, Nagpal M, Singh M, Dhingra GA, Aggarwal G. Treatment of psoriasis: a comprehensive review of entire therapies. Curr Drug Saf. 2020;15(2):82–104. doi:10.2174/1574886315666200128095958
9. Zhang P, Wu MX. A clinical review of phototherapy for psoriasis. Lasers Med Sci. 2018;33(1):173–180. doi:10.1007/s10103-017-2360-1
10. Liu C, Ma R, Wang L, et al. Rehmanniae Radix in osteoporosis: a review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J Ethnopharmacol. 2017;198:351–362. doi:10.1016/j.jep.2017.01.021
11. Xiao S, Wang C, Yang Q, Xu H, Lu J, Xu K. Rea regulates microglial polarization and attenuates neuronal apoptosis via inhibition of the NF-kappaB and MAPK signalings for spinal cord injury repair. J Cell Mol Med. 2021;25(3):1371–1382. doi:10.1111/jcmm.16220
12. Sun M, Shen X, Ma Y. Rehmannioside A attenuates cognitive deficits in rats with vascular dementia (VD) through suppressing oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2019;120:109492. doi:10.1016/j.biopha.2019.109492
13. Fu C, Wu Y, Liu S, et al. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J Ethnopharmacol. 2022;289:115021. doi:10.1016/j.jep.2022.115021
14. Chen L, Chen H, Lu Y, et al. Decoding active components in a formulation of multiple herbs for treatment of psoriasis based on three cell lines fishing and liquid chromatography-mass spectrometry analysis. J Pharm Biomed Anal. 2020;186:113331. doi:10.1016/j.jpba.2020.113331
15. Ma WY, Jia K, Zhang Y. IL-17 promotes keratinocyte proliferation via the downregulation of C/EBPalpha. Exp Ther Med. 2016;11(2):631–636. doi:10.3892/etm.2015.2939
16. Xu D, Wang J. Downregulation of cathepsin B reduces proliferation and inflammatory response and facilitates differentiation in human HaCaT keratinocytes, ameliorating IL-17A and SAA-induced psoriasis-like lesion. Inflammation. 2021;44(5):2006–2017. doi:10.1007/s10753-021-01477-0
17. Ortiz-Lopez LI, Choudhary V, Bollag WB. Updated perspectives on Keratinocytes and psoriasis: keratinocytes are more than innocent bystanders. Psoriasis. 2022;12:73–87. doi:10.2147/PTT.S327310
18. Furue M, Furue K, Tsuji G, Nakahara T. Interleukin-17A and keratinocytes in psoriasis. Int J Mol Sci. 2020;21(4):1275. doi:10.3390/ijms21041275
19. Zhang X, Yin M, Zhang LJ. Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells. 2019;8(8):807. doi:10.3390/cells8080807
20. Matson JP, Cook JG. Cell cycle proliferation decisions: the impact of single cell analyses. FEBS J. 2017;284(3):362–375. doi:10.1111/febs.13898
21. Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159(5):1092–1102. doi:10.1111/j.1365-2133.2008.08769.x
22. Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129(9):2175–2183. doi:10.1038/jid.2009.65
23. Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol. 2012;51(4):
24. Zdanowska N, Kasprowicz-Furmanczyk M, Placek W, Owczarczyk-Saczonek A. The role of chemokines in psoriasis-an overview. Medicina. 2021;57(8):754. doi:10.3390/medicina57080754
25. Dainichi T, Matsumoto R, Mostafa A, Kabashima K. Immune control by TRAF6-mediated pathways of epithelial cells in the EIME (Epithelial Immune Microenvironment). Front Immunol. 2019;10:1107. doi:10.3389/fimmu.2019.01107
26. Hammouda MB, Ford AE, Liu Y, Zhang JY. The JNK signaling pathway in inflammatory skin disorders and cancer. Cells. 2020;9(4):857. doi:10.3390/cells9040857
27. Mavropoulos A, Rigopoulou EI, Liaskos C, Bogdanos DP, Sakkas LI. The role of p38 MAPK in the aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev Immunol. 2013;2013:569751. doi:10.1155/2013/569751
28. Yuan M, Yuan B. Antidepressant-like effects of Rehmannioside A on rats induced by chronic unpredictable mild stress through inhibition of endoplasmic reticulum stress and apoptosis of hippocampus. J Chem Neuroanat. 2022;125:102157. doi:10.1016/j.jchemneu.2022.102157
29. Ding Z, Liu J, Qian H, Wu L, Lv M. Cinnamaldehyde inhibits psoriasis‑like inflammation by suppressing proliferation and inflammatory response of keratinocytes via inhibition of NF‑kappaB and JNK signaling pathways. Mol Med Rep. 2021;24(3). doi:10.3892/mmr.2021.12277
30. Gao J, Chen F, Fang H, Mi J, Qi Q, Yang M. Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice. Biol Res. 2020;53(1):48. doi:10.1186/s40659-020-00316-0
31. Che D, Hang B, Li Y, Li K, Wang K, Wang H. Livin upregulation in keratinocytes of psoriasis patients to promote adhesion molecule expression. Int J Dermatol. 2023;62(7):900–909. doi:10.1111/ijd.16621
32. Jiang Y, Tsoi LC, Billi AC, et al. Cytokinocytes: the diverse contribution of keratinocytes to immune responses in skin. JCI Insight. 2020;5(20). doi:10.1172/jci.insight.142067
33. Zhang L, Ma X, Shi R, et al. Allicin ameliorates imiquimod-induced psoriasis-like skin inflammation via disturbing the interaction of keratinocytes with IL-17A. Br J Pharmacol. 2023;180(5):628–646. doi:10.1111/bph.15983
34. Albanesi C, Pastore S. Pathobiology of chronic inflammatory skin diseases: interplay between keratinocytes and immune cells as a target for anti-inflammatory drugs. Curr Drug Metab. 2010;11(3):210–227. doi:10.2174/138920010791196328
35. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–653. doi:10.1016/j.jaci.2017.07.004
36. Schonthaler HB, Guinea-Viniegra J, Wculek SK, et al. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity. 2013;39(6):1171–1181. doi:10.1016/j.immuni.2013.11.011
37. Freise N, Burghard A, Ortkras T, et al. Signaling mechanisms inducing hyporesponsiveness of phagocytes during systemic inflammation. Blood. 2019;134(2):134–146. doi:10.1182/blood.2019000320
38. Benoit S, Toksoy A, Ahlmann M, et al. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol. 2006;155(1):62–66. doi:10.1111/j.1365-2133.2006.07198.x
39. Bai F, Zheng W, Dong Y, et al. Serum levels of adipokines and cytokines in psoriasis patients: a systematic review and meta-analysis. Oncotarget. 2018;9(1):1266–1278. doi:10.18632/oncotarget.22260
40. Mu Y, Gudey SK, Landstrom M. Non-smad signaling pathways. Cell Tissue Res. 2012;347(1):11–20. doi:10.1007/s00441-011-1201-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.