Back to Journals » Cancer Management and Research » Volume 12
ERα36 as a Potential Therapeutic Target for Tamoxifen-Resistant Breast Cancer Cell Line Through EGFR/ERK Signaling Pathway
Authors Li G, Zhang J, Xu Z, Li Z
Received 7 August 2019
Accepted for publication 16 December 2019
Published 14 January 2020 Volume 2020:12 Pages 265—275
DOI https://doi.org/10.2147/CMAR.S226410
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Guangliang Li, 1 Jing Zhang, 2 Zhenzhen Xu, 2 Zhongqi Li 2
1Institute of Cancer Research and Basic Medicine (ICBM), Chinese Academy of Sciences, Department of Medical Oncology (Breast), Cancer Hospital of University of Chinese Academy of Sciences, Zhejiang Cancer Hospital, Hangzhou, Zhejiang, 310022, People’s Republic of China; 2Department of Surgical Oncology, The 1st Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China
Correspondence: Guangliang Li
Institute of Cancer and Basic Medicine (ICBM), Chinese Academy of Sciences, Department of Medical Oncology (Breast), Cancer Hospital of University of Chinese Academy of Sciences, Zhejiang Cancer Hospital, No. 1, Banshan East Road, Hangzhou, Zhejiang 310022, People’s Republic of China
Email [email protected]
Zhongqi Li
Department of Surgical Oncology, The 1st Affiliated Hospital, School of Medicine, Zhejiang University, No. 79 Qingchun Road, Hangzhou 310003, People’s Republic of China
Email [email protected]
Background: Acquired tamoxifen resistance is one of the major barriers to the successful treatment of breast cancer. Recently, overexpression of ERα 36 was demonstrated to be a potential mechanism for the generation of acquired tamoxifen resistance. This study aims to evaluate the possibility of ERα 36 being a therapeutic target for tamoxifen-resistant breast cancer.
Methods: A tamoxifen-resistant cell subline (MCF-7/TAM) was established by culturing MCF-7 cells in medium plus 1 μM tamoxifen over 6 months. Colony-forming assay was used to determine the sensitivity of MCF-7/TAM cells to tamoxifen. Stable transfection was used to knockdown ERα 36 expression in MCF-7/TAM cells. MTT assay and Transwell migration assay were used to assess the in vitro proliferation and migration, respectively. Nude mouse tumorigenicity assay was used to evaluate in vivo tumorigenicity. Western blot analysis and quantitative real-time PCR (qRT-PCR) were used to examine the expression of ERα 36, ERα, EGFR and phosphorylated ERK1/2. The dual-luciferase reporter assay was used to determine the effect of ERα 36 on the activity of EGFR-promotor.
Results: MCF-7/TAM cells possessed greatly increased ERα 36 expression and EGFR expression and exhibited significantly increased in vitro proliferation and migration ability, as well as increased in vivo tumor growth ability, compared to parental MCF-7 cells. Knockdown of ERα 36 expression inhibited in vitro proliferation and migration, as well as in vivo tumor growth ability of MCF-7/TAM cells. ERα 36 regulated EGFR expression at the transcriptional level, and knockdown of ERα 36 in MCF-7/TAM cells downregulated EGFR expression and then blocked EGFR/ERK signaling pathway.
Conclusion: Knockdown of ERα 36 inhibits the growth of MCF-7/TAM cells in vitro and in vivo by blocking EGFR/ERK signaling pathway. ERα 36 may be a candidate therapeutic target for the treatment of tamoxifen-resistant breast cancer.
Keywords: breast cancer, acquired tamoxifen resistance, ERα 36, EGFR/ERK signaling
Introduction
Breast cancer is the major kind of malignant tumor and the second-leading cause of cancer-related death in women.1 Estrogen signaling through estrogen receptor (mainly ERα) plays an important role in breast cancer tumorigenesis and biology, and approximately 70% of breast cancer patients are ERα-positive in clinical.2–4 Tamoxifen, a selective estrogen receptor modulator (SERM), has been used to treat patients with ERα-positive breast cancer for over 40 years, both in the adjuvant and the recurrent setting.5 Although adjuvant tamoxifen therapy has been proven to reduce relapse, death rates and risk of contralateral breast cancer, a significant percentage (~30%) of ERα-positive tumors that initially respond to tamoxifen treatment will eventually develop acquired resistance.6 The development of acquired resistance limits the therapeutic efficacy of tamoxifen, which is a major challenge in the management of ERα-positive breast cancers.7,8 Despite the current deepening understanding of the mechanisms of tamoxifen resistance and intensive efforts to develop new therapeutic solutions, few effective regimens exist to treat tamoxifen-resistant breast cancer as ERα is still the only clinically used biomarker for ERα-positive breast cancer now.9 Significant efforts should be undertaken to explore new therapeutic target for tamoxifen-resistant breast cancer.
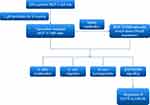 |
Figure 1 Schematic overview of the experimental design and workflow of experiment in this study. |
ERα36, a 36 kDa variant of ERα, was identified and cloned in 2005 by Wang et al10. ERα36 is a transcript from the ESR1 gene, with a different promotor to original 66 kDa ERα that located in the first intron of the ESR1 gene.10 ERα36 differs from the typical ERα, as it lacks both transcriptional activation domains (AF-1 and AF-2) of ERα. However, it retains the DNA-binding and dimerization domains, and partial ligand-binding domain, and is still capable of binding with its specific ligand.10 It is worth mentioning that ERα36 possesses a unique 27-amino acid sequence in the C-terminal region, instead of the 138-amino acid sequence in the C-terminal region of ERα.10 ERα36 is mainly localized in the plasma membrane and cytoplasm and mediates nongenomic estrogen signaling.10,11 Tamoxifen can bind to and stimulate the membrane-associated estrogen receptor ERα36.11,12 ERα36 is demonstrated to be able to mediate the agonist activity of tamoxifen via the MAPK/ERK and PI3K/Akt pathways in ERα-negative breast cancer cells and endometrial cancer cells that express high levels of endogenous ERα36.13,14 Furthermore, a large amount of studies has reported that an increased level of ERα36 expression in breast cancer cells is one of the underlying mechanisms for tamoxifen resistance. A retrospective study from Shi et al revealed that higher ERα36 expression was associated with poor prognosis and resistance to tamoxifen treatment in ERα-positive breast cancer patients.15 Zhang et al found that tamoxifen could induce ERα36 expression in tamoxifen sensitive breast cancer MCF-7 cells, which further led to the generation of acquired tamoxifen resistance.16 In addition, knockdown of ERα36 expression could restore tamoxifen sensitivity of tamoxifen-resistant breast cancer cells.17 In our previous study, we also demonstrated that ERα36 was involved in the development of acquired tamoxifen resistance via upregulating EGFR and downregulating ERα in breast cancer cells.33
In this study, as is shown in Figure 1, a tamoxifen-resistant cell subline MCF-7/TAM was established, which was found to possess increased level of ERα36 expression. To evaluate the possibility of ERα36 as a therapeutic target for tamoxifen-resistant breast cancer, the ERα36 expression in MCF-7/TAM cells was knocked down and a series of function assays were performed to prove that ERα36 was responsible for the tumorigenesis of tamoxifen-resistant breast cancer cells. Then, the main underlying mechanisms were examined in the tamoxifen-resistant MCF-7/TAM cells. In summary, the aim of this study is to ascertain the possibility of ERα36 as a potential therapeutic target for tamoxifen-resistant breast cancer.
Methods
Regents
Geneticin (G418), dimethyl sulfoxide (DMSO), tamoxifen, protease and phosphatase inhibitors were ordered from Sigma-Aldrich (St. Louis, MO, USA). 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was ordered from AMRESCO Inc (Solon, OH, USA). Polyvinylidene difluoride (PVDF) membranes were ordered from Millipore (Billerica, MA, USA). RPMI 1640 medium with phenol red indicator and fetal bovine serum (FBS) were purchased from Thermo Scientific HyClone (South Logan, UT, USA). The monoclonal ERα36 antibody was developed by Abmart, Inc. (Shanghai, China) as a custom service, which was raised against a synthetic peptide antigen corresponding to the C-terminal of ERα36. The polyclonal ERK1/2 antibody and phospho-ERK1/2 (Thr202/Tyr204) antibody were purchased from Cell Signaling Technology, Inc. (Boston, MA, USA). The antibody against EGFR and ERα was purchased from Epitomics, Inc. (Burlingame, CA, USA). The antibody against β-actin was purchased from Beyotime Biotech Inc. (Shanghai, China).
Cell Culture
Human breast cancer cell line MCF-7 (ATCC, No. HTB-22) was obtained from American Type Culture Collection (Manassas, VA, USA), and was routinely cultured at 37°C in the presence of 5% CO2 in routine RPMI 1640 medium with phenol red indicator supplemented with 10% FBS. MCF-7/TAM cells were established by culturing cell MCF-7 cells in the presence of 1 μM TAM for 6 months and were maintained in the culture medium supplemented with 1 μM tamoxifen consistently.
Colony-Forming Assay
Cells were seeded in a 6-well plate (500 cells per well) and incubated in medium containing 1 μM TAM or equivalent DMSO (vehicle) for 2 weeks. The colonies were fixed with 100% methanol for 15 mins, stained with 0.1% crystal violet and washed with phosphate buffer solution (PBS). Visible colonies (≥50 cells) were then counted for quantification. Three independent experiments were performed and the data were presented as the mean ± SEM.
Western Blot Analysis
Cellular proteins were prepared in lysis buffer (50 mM Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.5% sodium deoxycholate, 150 mM NaCl, 0.02% sodium azide, and 0.1% SDS) containing protease and phosphatase inhibitors and heated for 10 min at 100°C. Equal quantities of protein were fractionated by 10% SDS-PAGE and electro-transferred to PVDF membranes. The membranes were then blocked at room temperature with 5% nonfat milk in TBS-T (10 mmol/L Tris-HCl (pH 7.5), 0.5 mol/L NaCl, and 0.05% (w/v) Tween 20) buffer for 1 hour, incubated with primary antibodies overnight at 4°C, washed 3 times with TBS-T, and then incubated with suitable peroxidase-conjugated secondary antibodies for 1 hour at room temperature, and subjected to enhanced chemiluminescent staining using an ECL detection system (Millipore, Billerica, MA, USA). Molecular weights of the immunoreactive proteins were estimated based on PageRulerTM Prestained Protein ladder (MBI Fermentas, USA). Experiments were repeated at least 3 times.
Methyl-Thiazolyl-Tetrazolium (MTT) Assay
Cells were seeded in 96-well plates at 1000 cells per well and 5 wells were used for every experiment. Growth was measured daily by adding 20 μL 0.5 mg/mL MTT to each well, and the plate was incubated for another 4 hos at 37°C. Afterward, the supernatant was removed and the formazan crystals were dissolved in 200μL DMSO at room temperature for 15 mins, and absorbance was then measured at 570 nm wavelength using an ELx800 Absorbance Microplate Reader (Biotek, Winooski, VT, USA). Three independent experiments were performed and the data were presented as the mean ± SEM.
Plasmid Preparation and Transfection
An artificial microRNA-expressing vector pcDNA3.1/6mi36 was designed before.27 Transfection was performed using Fugene HD Transfection Reagent (Roche Applied Science, Mannheim, Germany) as recommended by the manufacturer. 48 hrs after transfection, the cells were replanted and selected via incubating cells with 600 ug/mL G418 for 2 weeks. Surviving colonies were then amplified and examined for ERα36 expression using Western blot analysis. The Coding sequence of ERα36 cDNA was successfully cloned, which was consistent with the NCBI database. On that basis, a eukaryotic expression vector of pcDNA3.1(+)-ERα36 was constructed and verified by sequencing. Transient transfection was performed using Fugene HD Transfection Reagent as recommended by the manufacturer. 48 hrs after transfection of pcDNA3.1(+)-ERα36, cells were harvested for further analysis.
Transwell Migration Assay
Cells were pre-starved in serum-free medium for 12 hrs, and the transwell plates were rehydration according to the protocol provided by the manufacturer (Millipore, Billerica, MA, USA). 5×104 cells, suspended in 200 μL serum-free medium, were seeded into the upper chamber of the transwell plate. The lower chamber was filled with 900 µL of medium with 10% FBS. After incubation for 24 hrs, cells adhering to the upper surface of the filter were removed using a cotton swab. After fixed with 95% ethanol, stained with 0.1% crystal violet, and washed with PBS, the cells that had migrated to the opposite side of the filter were counted at ×200 magnifications under a light microscope. Five separate fields per membrane were selected, and the number of stained cells was counted in each field.
Nude Mouse Tumorigenicity Assay
Tumor formation was assayed using female BALB/c nude mice (4- to 6-week-old, Slaccas Laboratory Animal, Shanghai, China), which were housed in a barrier facility and acclimated to 12-h light-dark cycles for at least 3 days before use. The use of experimental animals adhered to the “Principles of Laboratory Animal Care” (NIH publication #85-23, revised in 1985). All experiments were approved by the Institutional Animal Care and Use Committee of Zhejiang University (approval ID: SYXK(ZHE)2005-0072). Mice were randomly distributed into equal groups (5 mice per group) for each experiment. A total of 1 x 107 indicated cells were resuspended in 0.1 mL of PBS and inoculated subcutaneously into the left mammary fat pad of female nude mice. Tumor growth was monitored every 2 days by Vernier caliper measurement of the length (a) and width (b) of tumor, and tumor volume was calculated as (a × b2)/2.
RNA Extraction and Quantitative RT-PCR
Total RNA was extracted using TRIzol reagent according to the protocol provided by the manufacturer (Invitrogen, Carlsbad, Calif, USA). RNA concentrations were quantified by NanoDrop 1000 (Nanodrop, Wilmington, Del. USA). Reverse transcription reaction was performed using 2 μg of total RNA with Reverse Transcription System (Promega, Madison, WI, USA). The mRNA level of EGFR was analyzed using GoTaq® qPCR Master Mix Kit (Promega, Madison, WI, USA) in ABI PRISM 7500 Sequence Detection System (Applied Biosystems, CA, USA), and was carried out in triplicate for each sample. The β-actin gene was used as internal controls for mRNAs, respectively. Primers used were shown as follows: EGFR-forward: CGTCCGCAAGTGTAAGAA, reverse: AGCAAAAACCCTGTGATT; β-actin-Forward: TGAGCGCGGCTACAGCTT, reverse: TCCTTAATGTCACGCACGATTT.
Luciferase Assay
Luciferase assays were performed using a Dual-Glo luciferase assay system according to the manufacturer’s instructions (Promega, Madison, WI, USA). Briefly, MCF-7 cells were cultured in 6-well plates and co-transfected with pcDNA3.1(+)-NC or pcDNA3.1(+)-ERα36 and reporter plasmids (pGL3-promotor, pGL3-EGFR-promotor) with the pRL-CMV plasmid pRL/TK (Promega, Madison, WI, USA) to establish transfection efficiency. 48 hrs after transfection, the luciferase activity of cell lysate was determined with the Dual-Luciferase Reporter kit (Beyotime, China) according to the provided protocol. Luciferase signals were collected by Dual-Luciferase Assay System (Thermo Fisher Scientific, Waltham, MA, USA). Relative luciferase activity (RLA) was calculated by normalizing to the renilla luciferase activity. Each experiment was repeated 3 times, and the mean RLA was calculated for statistical analysis. The expression of ERα36 of MCF-7 cells transfected with pcDNA3.1(+)-NC or pcDNA3.1(+)-ERα36 was then determined by Western blot analysis using an anti-ERα36 antibody.
Statistical Analysis
The data were presented as the means ± standard error of the mean of three independent experiments. Statistical significance between various experimental and control groups was determined by two-tailed Student’s t-test (GraphPad Prism v.7.0, La Jolla, CA, USA). Differences were considered statistically significant at a level of P<0.05. * represents P<0.05; ** represents P<0.01.
Results
ERα36 Is Upregulated in Tamoxifen-Resistant MCF-7/TAM Cells
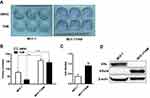
To investigate the molecular mechanisms underlying acquired tamoxifen resistance in breast cancer, a tamoxifen-resistant cell subline MCF-7/TAM was established by culturing ERα-positive breast cancer MCF-7 cells in medium plus 1 μM tamoxifen over 6 months, and MCF-7/TAM cells were finally maintained in the culture medium supplemented with 1 μM tamoxifen consistently. The tamoxifen sensitivity of MCF-7/TAM cells was then examined using colony-forming assay. Compared to parental MCF-7 cells, MCF-7/TAM cells exhibited dramatically decreased sensitivity to tamoxifen (8.63±5.12% vs 62.29±5.41%) (Figure 2A–C). Moreover, Western blot analysis revealed that the level of ERα36 expression in MCF-7/TAM cells was greatly increased than parental MCF-7 cells. Instead, the level of ERα expression in MCF-7/TAM cells was nearly undetectable (Figure 2D).
MCF-7/TAM Cells Exhibit Significantly Increased Proliferation and Migration in vitro
We further investigate the biological behaviors of MCF-7/TAM cells in vitro via MTT assay and Transwell migration assay in a routine medium containing estrogen. MTT assay revealed that MCF-7/TAM cells proliferated much faster than parental MCF-7 cells in vitro (Figure 3A). Transwell assay demonstrated that MCF-7/TAM cells exhibited a much stronger capability for migration in vitro than parental MCF-7 cells. Out of 5×104 cells seeded into the upper chamber of the transwell plate, 200.0 ± 9.55 MCF-7/TAM cells per field migrated through the membrane after 24-hr’ incubation versus 16.6 ± 0.9274 MCF-7 cells per field (Figure 3B and C).
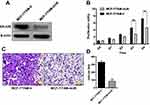
Knockdown of ERα36 Expression Inhibits in vitro Proliferation and Migration of MCF-7/TAM Cells
To evaluate the role of ERα36 in the regulation of proliferation and metastasis of MCF-7/TAM cells, we knocked-down ERα36 expression in MCF-7/TAM cells via stable transfection of an ERα36 multi-hairpin vector (pcDNA3.1/6mi36).27 Western blot analysis confirmed the significantly decreased expression of endogenous ERα36 in clones transfected with pcDNA3.1/6mi36 (Figure 4A). The in vitro proliferation and migration ability of MCF-7/TAM cells in a routine medium containing estrogen was then determined. MTT assay revealed that MCF-7/TAM-mi36 cells proliferated much more slowly than MCF-7/TAM-V cells (Figure 4B). In addition, Transwell migration assay demonstrated that knockdown of ERα36 expression significantly inhibited the in vitro migration ability of MCF-7/TAM cells (87.8 ± 11.01 MCF-7/TAM-mi36 cells vs 332.2 ± 15.08 MCF-7/TAM-V cells per field out of 5×104 cells seeded into the upper chamber of the transwell plate after 24-hr’ incubation, Figure 4C and D).
Knockdown of ERα36 Expression Inhibits in vivo Tumor Growth of MCF-7/TAM Cells in Nude Mice
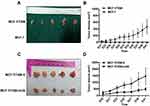
To assess the tumorigenicity of MCF-7/TAM cells in vivo, MCF-7/TAM cells, as well as parental MCF-7 cells, were implanted into the left mammary fat pad of female nude mice. In the absence of exogenous estrogen, parental MCF-7 cells failed to form tumors in nude mice, whereas MCF-7/TAM cells could form stable subcutaneous tumors (Figure 5A and B). To assess the effect of ERα36 on the tumorigenicity of MCF-7/TAM cells in vivo, the in vivo tumor growth of MCF-7/TAM-V cells and MCF-7/TAM-mi36 cells was evaluated using the same Nude mouse tumorigenicity assay (Figure 5C and D). Our results revealed that the tumor growth of MCF-7/TAM-mi36 cells in nude mice was significantly weaker than that of MCF-7/TAM-V cells, which indicated that knockdown of ERα36 expression could inhibit in vivo tumor growth of MCF-7/TAM cells.
Knockdown of ERα36 Expression Deactivates EGFR/ERK Signaling in MCF-7/TAM Cells
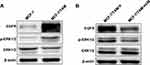
Similar to previous studies, our results also uncovered that EGFR expression in MCF-7/TAM cells was significantly upregulated, compared to parental MCF-7 cells. What’s more, the basal level of ERK1/2 phosphorylation in MCF-7/TAM cells was much higher than parental MCF-7 cells, indicating the activation of the EGFR/ERK signaling pathway, which was responsible for the enhanced capability of proliferation and migration gained by MCF-7/TAM cells (Figure 6A). Further investigation disclosed that knockdown of ERα36 expression downregulated EGFR expression, decreased ERK1/2 phosphorylation, and finally deactivated EGFR/ERK signaling pathway in MCF-7/TAM cells (Figure 6B). These results led to the suggestion that the EGFR/ERK signaling pathway may involve in the regulation of cell proliferation and metastasis driven by ERα36.
ERα36 Regulates EGFR Expression at Transcriptional Level
In order to explore the mechanism underlying the regulation of EGFR expression by ERα36, we detected the mRNA level of EGFR in MCF-7/TAM cells. We found that the mRNA level of EGFR in MCF-7/TAM cells was significantly higher than that of parental MCF-7 cells (Figure 7A). Further results indicated that knockdown of ERα36 expression could downregulate the mRNA level of EGFR in MCF-7/TAM cells (Figure 7B). In order to demonstrate if ERα36 positively regulates EGFR expression, we overexpressed ERα36 in MCF-7 parental cells via transmit transfection. We then found that EGFR expression in MCF-7/ERα36 cells was significantly increased both in mRNA level (Figure 7C) and protein level (Figure 7D), compared to MCF-7/V cells. These results meant that ERα36 may regulate EGFR expression at the level of transcription. To further illuminate the exact role of ERα36 in the transcription of the EGFR gene, a dual-luciferase reporter assay was performed to examine the effect of ERα36 on the activity of EGFR-promotor. Results revealed that luciferase activity of the pGL3-EGFR-promotor plasmid was dramatically increased when co-transfected with pcDNA3.1(+)-ER-α36 in MCF-7 cells, which indicated that overexpressed ERα36 could activate the promotor activity of EGFR gene, and then induce the transcription of EGFR gene (Figure 7E and F).
Discussion
Tamoxifen remains a cornerstone of endocrine therapy for ERα-positive breast cancer, which has effectively improved the survival rate and reduce the recurrence of ERα-positive breast cancer patients in the past decades.18–20 Despite the significant anti-tumor activities of tamoxifen, most initially responsive breast tumors are eventually resistant to tamoxifen therapy, which is a major obstacle in the management of patients with ERα-positive breast cancer.21 Actually, more women die from ERα-positive breast cancer than from any other molecular subtype in general.22 Previous studies have identified that upon the development of resistance to tamoxifen, breast cancer cells also acquired enhanced proliferation and metastasis potential.23 Multiple mechanisms involved in acquired tamoxifen resistance have been proposed by extensive researches, including nuclear hormone receptor coregulator differential expression, growth factor signaling crosstalk, acquired ER somatic mutations and alteration mutations in ERα, and changes in the tamoxifen absorption rate and metabolic pathways.24 Although the causes of tamoxifen resistance vary, the most predominant mechanisms responsible for this resistance remain largely unknown. And there is an urgency in exploring new therapeutic target for tamoxifen-resistant breast cancer.
Accumulating experimental and clinical evidence has demonstrated that ERα36, a truncated variant of ERα, is closely related to the generation of acquired tamoxifen resistance.15,16 The current study establishes a tamoxifen-resistant cell subline MCF-7/TAM, which susceptibility to tamoxifen is significantly decreased. We found that MCF-7/TAM cells possess increased ERα36 expression, but undetectable ERα expression. This finding is in good agreement with a previous study which revealed that tamoxifen-resistant MCF-7 cells possessed greatly overexpressed ERα36 but significantly downregulated ERα.25 However, the change of ERα expression level during the development of acquired tamoxifen resistance is still uncertain, as some groups show breast cancer cells with acquired tamoxifen resistance retain ERα expression.16,26 That may be because the biological reprogramming during the development of acquired tamoxifen resistance is so complicated that breast cancer cells usually undergo adaptive changes in response to the corresponding environment. The specific underlying mechanisms require further research to clarify.
In this study, we mainly evaluate the possibility of ERα36 as a potential therapeutic target for tamoxifen-resistant breast cancer rigorously. Through stable transfection, we knock down the expression of ERα36 in MCF-7/TAM cells and get MCF7/TAM-mi36 cells with lower ERα36 expression. A previous study has showed that downregulation of the ERα36 expression could reduce the in vitro migration and invasiveness of MDA-MB-231 breast cancer cells.27 Here, our results reveal that knockdown of ERα36 expression could inhibit in vitro proliferation and migration of MCF-7/TAM cells. Most importantly, consistent results are observed in vivo. Using nude mouse tumorigenicity assay, we further find that the tumor growth of MCF7/TAM-mi36 cells in nude mice is significantly weaker than that of control MCF-7/TAM-V cells, which indicated the pivotal role of ERα36 in maintaining aggressive biological behaviors of MCF-7/TAM cells. Unlike previous studies, the nude mouse tumorigenicity assay here is performed in the absence of exogenous estrogen. On that basis, we infer that the regulation of proliferation and metastasis of MCF-7/TAM cells by ERα36 may have nothing to do with the estrogen signaling pathway.
Acquired resistance to tamoxifen is clearly a complex phenomenon involving multiple pathways, and the exact molecular mechanism of ERα36 in regulating acquired tamoxifen resistance has not been elucidated until now. Many kinds of research have demonstrated that multiple signaling molecules and pathways such as EGFR and HER2 are involved in the induction of tamoxifen resistance.28,29 Previous studies have discovered the existence of the crossregulatory loops between ERα36 and the EGFR/HER2.30,31 Besides, ERα36 is demonstrated to promote the generation of acquired tamoxifen resistance via regulating EGFR/HER2 expression and their downstream signaling pathway, and disruption of the ERα36-EGFR/HER2 positive regulatory loops restores tamoxifen sensitivity in tamoxifen-resistant breast cancer cells.17 Not only that ERα36 is also reported to play critical roles in tamoxifen resistance and metastasis of breast cancer.32
In this study, our results uncover that MCF-7/TAM cells possessed much higher expression of EGFR than parental MCF-7 cells, as well as increased basal level of ERK1/2 phosphorylation that indicates the activation of EGFR/ERK signaling pathway, which leads to the suggestion that EGFR/ERK signaling pathway may involve in the regulation of cell proliferation and metastasis driven by ERα36. We further find that knockdown of ERα36 expression could downregulate EGFR expression, and then decrease ERK1/2 phosphorylation level in MCF-7/TAM cells, which supports a critical role of ERα36 in the regulation of EGFR/ERK signaling. Zhang et al once revealed that ERα36 regulated EGFR expression via stabilizing EGFR protein.30 In this study, our results reveal that MCF-7/TAM cells with high ERα36 expression possess a high mRNA level of EGFR, and knockdown of ERα36 could downregulate the mRNA level of EGFR. In order to demonstrate if ERα36 positively regulates EGFR expression, we overexpressed ERα36 in parental MCF-7 cells via transmit transfection. We then found that EGFR expression in MCF-7/ERα36 cells was significantly increased both in mRNA level and protein level, compared to MCF-7/V cells. Further results reveal that overexpressed ERα36 could activate the promotor activity of the EGFR gene and then induce the transcription of the EGFR gene, which indicates that ERα36 regulates EGFR expression at the transcriptional level.
Conclusions
In the present study, we have briefly discussed the pivotal role of ERα36 in the treatment of tamoxifen-resistant breast cancer. Our results suggest that knockdown of ERα36 inhibits the progress of tamoxifen-resistant MCF-7/TAM cells via blocking the EGFR/ERK signaling pathway. These findings present a potential therapeutic option to overcome tamoxifen resistance via targeting ERα36. However, further studies are warranted to verify these findings.
Ethical Statement
All animal experimental protocols conducted in the present study were approved by the Institutional Animal Care and Use Committee of Zhejiang University (approval ID: SYXK[ZHE]2005-0072).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81702976), Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ16H160015, LY15H160012), Science and Technology Major Projects of Ningbo (Grant No. 2015C0003), and Zhejiang Provincial Medicine & Health Science and Technology General Project (Grant No. 2016KYA045).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.v69.1
2. Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984;2(10):1102–1109. doi:10.1200/JCO.1984.2.10.1102
3. Tyson JJ, Baumann WT, Chen C, et al. Dynamic modelling of oestrogen signalling and cell fate in breast cancer cells. Nat Rev Cancer. 2011;11(7):523–532. doi:10.1038/nrc3081
4. Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9(6):1980–1989.
5. Jordan VC. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer. 2014;21(3):R235–R246. doi:10.1530/ERC-14-0092
6. Briest S, Stearns V. Tamoxifen metabolism and its effect on endocrine treatment of breast cancer. Clin Adv Hematol Oncol. 2009;7(3):185–192.
7. Jordan VC, O’Malley BW. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol. 2007;25(36):5815–5824. doi:10.1200/JCO.2007.11.3886
8. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi:10.1146/annurev-med-070909-182917
9. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134(7):e48–72.
10. Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336(4):1023–1027. doi:10.1016/j.bbrc.2005.08.226
11. Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-α, hER-α36: transduction of estrogen-and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci. 2006;103(24):9063–9068. doi:10.1073/pnas.0603339103
12. Deng H, Zhang XT, Wang ML, Zheng HY, Liu LJ, Wang ZY. ER-alpha36-mediated rapid estrogen signaling positively regulates ER-positive breast cancer stem/progenitor cells. PLoS One. 2014;9(2):e88034. doi:10.1371/journal.pone.0088034
13. Hong W, Zhang X, Ding L, Kang L, Wang Z-Y. Estrogen receptor-alpha 36 mediates mitogenic antiestrogen signaling in ER-negative breast cancer cells. PLoS One. 2012;7(1):e30174. doi:10.1371/journal.pone.0030174
14. Lin SL, Yan LY, Zhang XT, et al. ER-alpha36, a variant of ER-alpha, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PLoS One. 2010;5(2):e9013. doi:10.1371/journal.pone.0009013
15. Shi L, Dong B, Li Z, et al. Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol. 2009;27(21):3423–3429. doi:10.1200/JCO.2008.17.2254
16. Zhang X, Wang ZY. Estrogen receptor-alpha variant, ER-alpha36, is involved in tamoxifen resistance and estrogen hypersensitivity. Endocrinology. 2013;154(6):1990–1998. doi:10.1210/en.2013-1116
17. Yin L, Zhang XT, Bian XW, Guo YM, Wang ZY. Disruption of the ER-alpha36-EGFR/HER2 positive regulatory loops restores tamoxifen sensitivity in tamoxifen resistance breast cancer cells. PLoS One. 2014;9(9):e107369. doi:10.1371/journal.pone.0107369
18. Burstein HJ, Lacchetti C, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline update on ovarian suppression summary. J Oncol Pract. 2016;12(4):390–393. doi:10.1200/JOP.2016.011239
19. Turner NC, Neven P, Loibl S, Andre F. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet. 2017;389(10087):2403–2414. doi:10.1016/S0140-6736(16)32419-9
20. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi:10.1016/S0140-6736(12)61963-1
21. Fan P, Craig Jordan V. Acquired resistance to selective estrogen receptor modulators (SERMs) in clinical practice (tamoxifen & raloxifene) by selection pressure in breast cancer cell populations. Steroids. 2014;90:44–52. doi:10.1016/j.steroids.2014.06.002
22. Droog M, Beelen K, Linn S, Zwart W. Tamoxifen resistance: from bench to bedside. Eur J Pharmacol. 2013;717(1–3):47–57. doi:10.1016/j.ejphar.2012.11.071
23. Hiscox S, Jordan NJ, Morgan L, Green TP, Nicholson RI. Src kinase promotes adhesion-independent activation of FAK and enhances cellular migration in tamoxifen-resistant breast cancer cells. Clin Exp Metastasis. 2007;24(3):157–167. doi:10.1007/s10585-007-9065-y
24. Clarke R, Tyson JJ, Dixon JM. Endocrine resistance in breast cancer–an overview and update. Mol Cell Endocrinol. 2015;418(Pt 3):220–234. doi:10.1016/j.mce.2015.09.035
25. Zhao Y, Deng C, Lu W, et al. let-7 microRNAs induce tamoxifen sensitivity by downregulation of estrogen receptor alpha signaling in breast cancer. Mol Med. 2011;17(11–12):1233–1241. doi:10.2119/molmed.2010.00225
26. Clarke R, Liu MC, Bouker KB, et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22(47):7316–7339. doi:10.1038/sj.onc.1206937
27. Zhang J, Li G, Li Z, et al. Estrogen-independent effects of ER-alpha36 in ER-negative breast cancer. Steroids. 2012;77(6):666–673. doi:10.1016/j.steroids.2012.02.013
28. Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9(9):631–643. doi:10.1038/nrc2713
29. Montaser RZ, Coley HM. Crosstalk between ERalpha and receptor tyrosine kinase signalling and implications for the development of anti-endocrine resistance. Cancers. 2018;10:6. doi:10.3390/cancers10060209
30. Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, Wang ZY. A positive feedback loop of ER-alpha36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene. 2011;30(7):770–780. doi:10.1038/onc.2010.458
31. Kang L, Guo Y, Zhang X, Meng J, Wang ZY. A positive cross-regulation of HER2 and ER-alpha36 controls ALDH1 positive breast cancer cells. J Steroid Biochem Mol Biol. 2011;127(3–5):262–268. doi:10.1016/j.jsbmb.2011.08.011
32. Gu W, Dong N, Wang P, Shi C, Yang J, Wang J. Tamoxifen resistance and metastasis of human breast cancer cells were mediated by the membrane-associated estrogen receptor ER-alpha36 signaling in vitro. Cell Biol Toxicol. 2017;33(2):183–195. doi:10.1007/s10565-016-9365-6
33. Li G, Zhang J, Jin K, . Estrogen receptor-alpha36 is involved in development of acquired tamoxifen resistance via regulating the growth status switch in breast cancer cells. Mol Oncol. 2013;7(3):611–624.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.