Back to Journals » Drug Design, Development and Therapy » Volume 16
Emerging Therapeutic Strategies of Different Immunotherapy Approaches Combined with PD-1/PD-L1 Blockade in Cervical Cancer
Authors Ge Y, Zhang Y, Zhao KN, Zhu H
Received 14 May 2022
Accepted for publication 28 July 2022
Published 9 September 2022 Volume 2022:16 Pages 3055—3070
DOI https://doi.org/10.2147/DDDT.S374672
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Yanjun Ge,1,* Yuchen Zhang,1,* Kong-Nan Zhao,2,3 Haiyan Zhu1,2
1Department of Gynecology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2School of Basic Medical Science, Wenzhou Medical University, Wenzhou, People’s Republic of China; 3Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, St Lucia, Queensland, Australia
*These authors contributed equally to this work
Correspondence: Haiyan Zhu, Shanghai First Maternity and Infant Hospital, No. 2699 Gaokexi Road, Shanghai, 200092, People’s Republic of China, Tel +86 13758465255, Email [email protected]
Abstract: Currently, therapeutic methods for advanced and recurrent cervical cancer patients are limited and unsatisfactory. Immunotherapy is a promising approach for cancer treatment. However, its investigation and application in cervical cancer remain slow. Although pembrolizumab is a remarkable milestone as the first anti-PD-1 mAb approved by the FDA for treating cervical cancer, it shows relatively low response rate. It is noticed that multiple novel immune checkpoints have emerged in recent years, such as CTLA-4, TIGIT, LAG-3, TIM-3, and A2AR. Accumulated studies have suggested that strategies combining the PD-1/PD-L1 inhibitors and different immunotherapies or biotherapies could enhance the antitumor efficacy in human cancers. In this review article, we provide an overview of anti-PD-1/PD-L1-based immunotherapy in cervical cancer treatment. We further summarize the developmental strategies of different immunotherapies or biotherapies combined with PD-1/PD-L1 blockade for treating cervical cancer. We also discuss how these new combined therapies increase the therapeutic benefit gained from experimental evidence in cervical cancer.
Keywords: cervical cancer, immunotherapy, PD-1/PD-L1, combinatorial strategy, mechanism, clinical practice
Introduction
Cervical cancer (CC) ranks fourth for both incidence and mortality among cancers in women globally,1 indicating that this cancer remains a heavy burden worldwide despite the development and application of prophylactic HPV vaccines and effective screening and early detection methods. Traditional treatments for cervical cancer are surgery, radiation therapy, and chemotherapy.2 For early-stage cervical cancers, radical surgery and radiation therapy can achieve good prognosis. However, for advanced and recurrent cervical cancers, the efficacy of the current treatment modalities is unsatisfactory, resulting in poor survival outcomes.3 Therefore, searching for novel treatment strategies for advanced and recurrent cervical cancers is necessary. Treatments targeting epidermal growth factor receptor (EGFR) or vascular endothelial growth factor (VEGF) are improvements made in cervical cancer therapy in recent years.4,5 But their efficacy is not very satisfying. Chemotherapy combined with the anti-VEGF antibody bevacizumab for cervical cancer showed progression-free survival of only 8.2 months in the phase III randomized trial, GOG 240.6
Immunotherapy is emerging and rapidly developing in recent years. It is expected to harness the host’s immune system to attack tumor cells by stimulating the adaptive immune system through the administration of anti-cancer vaccines, which is known as active immunotherapy, or by using immune compounds such as adoptive cellular transfer (ACT), immune checkpoint inhibitors (ICIs) and cytokines to enhance antitumor immunity, ie passive immunotherapy.7,8 In addition, several other agents such as IDO (indoleamine 2.3-dioxygenase) inhibitors are classified as “immunomodulation”, and they also enhance antitumor immune response through different mechanisms.8 Compared with conventional treatment methods, immunotherapy demonstrates a more durable clinical response once effective, and is better tolerated.
In particular, immune checkpoint inhibitors (ICIs) have made a remarkable step forward in the field of cancer immunotherapy. ICIs are used to block negative immune cell regulatory axes, such as programmed cell death-1 (PD-1) and its ligand PD-L1, which typically impair antitumor immunity when they are activated, aiming to restore the anti-cancer capacity of immune cells. It has been observed that PD-1 blockade induces antitumor responses through enhancing the activity of effector T cells and NK cells in tissues and tumor microenvironments and diminishing the number and suppressive activity of Treg cells, as well as abrogating the inhibition of kinases and recruitment of tyrosine phosphatases that are related to T cell activation.9,10
Pembrolizumab, one of the ICIs, was approved by the FDA in 2018 for treating advanced PD-L1 positive cervical cancer due to its safety and antitumor effects in the KEYNOTE-158 clinical trial on advanced cervical cancer.11 It is also the only immunotherapy drug approved to date for the treatment of cervical cancer. Although PD-1/PD-L1 blockade has shown clinically significant efficacy and prolonged patient survival, the response rate of cervical cancer patients is less than 30%.12 In the KEYNOTE-158 study, an objective response rate (ORR) for pembrolizumab monotherapy was 12.2% in all patients with advanced cervical cancer and 14.6% in patients with PD-L1-positive tumors.11 In the phase I/II CheckMate 358 trial, nivolumab showed an objective response rate of 26.3% in patients with recurrent/metastatic cervical cancer.13 Recently, in the first phase III randomized trial of a checkpoint inhibitor in cervical cancer (GOG 3016, NCT03257267), cemiplimab (PD-1-blocking antibody) showed an objective response rate of 16.4%.14 A retrospective cross-sectional study reported that the estimated percentage of US cancer patients who respond overall to the six ICIs approved for 14 indications in 2018 was only 12.46%.15 Taken together, monotherapy of PD-1/PD-L1 blockade shows unsatisfactory effects. The low response rate may be due to intrinsic resistance to PD-1/PD-L1 blockade drugs, involving lack of neoantigen in tumors with mismatch repair function, T cell malfunction due to T cell exhaustion, and immunosuppressive components of the tumor microenvironment.16–18 Another issue is that some patients who initially gain the benefits of PD-1/PD-L1 blockade therapy may eventually harbor tumor progression or recurrence due to adaptive resistance, such as compensatory activation of alternative immune checkpoint signaling pathways, allowing the tumors to regain the ability of immune escape.17,18 Therefore, new strategies for immunotherapy of cervical cancer should be explored to improve the therapeutic efficacy. In fact, PD-1 blockade therapy combined with conventional therapy or molecular-targeted therapy has shown efficacy. For example, pembrolizumab combined with chemotherapy with or without bevacizumab showed benefits in progression-free and overall survival in patients with persistent, recurrent, or metastatic cervical cancer, as reported in the results of KEYNOTE-826.19 Therefore, combination therapies may be promising. Here, we hold the view that combination of PD-1/PD-L1 blockade therapy and other immunotherapy approaches appears to be a promising direction.
Besides PD-1/PD-L1 blockade therapy, several other immunotherapy approaches with different mechanisms of action are emerging and have shown preliminary effects, such as other immune checkpoint blockade therapies, therapeutic vaccines, adoptive cell therapy and so on. There has been appreciable evidence suggesting that the efficacy of PD-1/PD-L1 blockade therapy may be boosted by other immunotherapy approaches or vice versa, or even mutually, resulting in potent synergistic effects when they are combined. Indeed, Hegde et al listed “optimizing long-term survival with multi-agent cancer immunotherapy combination regimens” as one of the top 10 challenges in cancer immunotherapy,20 suggesting that this strategy is both unknown and promising. Smyth et al proposed four immune nodes that immunotherapy should target, namely, elimination of immune suppression, induction of immunogenic cancer cell death, enhancement of antigen presentation or adjuvanticity and stimulation of the activation and survival of immune effector cells.21 Combinatorial immunotherapies can focus on multiple immune pathways and target more than one node of the above four, thereby fully activating endogenous tumor immunity.
Cervical cancer has unique features involving the tumor genome and tumor microenvironment, indicating a high probability of responding to combinatorial immunotherapies. Firstly, a large proportion of cervical cancers harbor high tumor mutational burdens and T cell-inflamed gene signature, known as immunologically hot.22–24 Unlike tumors with the “immune-desert” or “immune cell-excluded” phenotype, in the immunologically hot tumors there exists an ongoing immune response that is suppressed due to immunoevasive or immunosuppressive pathways in the tumor microenvironment.22,25 For example, the immunosuppressive signal involving TIGIT contributes to immune evasion of cancer cells in these immunologically hot tumors.26 In these tumors, upregulation of metabolites and cytokines associated with immunosuppression, such as adenosine, kynurenine produced by tryptophan catabolism and TGFβ, induces suppression of antitumor immunity.22 Decreased levels of major histocompatibility complex class I (MHC I) on the surface of HPV-infected cells caused by HPV oncoprotein E5 may affect antigen presentation in HPV-related cervical cancer.22,27 These mechanisms, as well as others that will be mentioned and elaborated on in the following text, provide possible avenues for further improving antitumor immunity. Importantly, tumors with high mutational burdens are theoretically highly responsive to PD-1/PD-L1 blockade therapy but practically show low levels of response because of these immunoevasive and immunosuppressive mechanisms.28 Therefore, combining PD-1/PD-L1 blockade with other immunotherapies may enhance the proportion of cervical cancer patients responsive to PD-1/PD-L1 blockade therapy. Secondly, as the main cause of cervical carcinogenesis, human papilloma virus was found to exert influence on host immunity. HPV oncoproteins activate multiple signaling pathways such as PI3K/AKT, MAPK and STAT3/NF-kB.29 This may contribute to dysregulation of the tumor immune microenvironment as these pathways are closely related to immune response. In addition, HPV infection leads to recruitment of suppressive immune cells to the infected sites, impairment of NK cell activity and CTL responses as well as hindrance of antigen presentation machinery.30
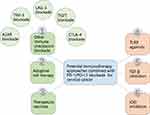
Therefore, this review highlights the potential and promise of the combinatorial strategies of different immunotherapy approaches based on PD-1/PD-L1 blockade to further enhance the host immune response against tumors and increase the proportion of cervical cancer patients benefiting from immunotherapy (Figure 1), as well as the clinical development (Table 1).
 |
Table 1 Ongoing Clinical Trials of PD-1/PD-L1 Blockade Therapy Combined with Other Immunotherapies for Cervical Cancer Only or a Variety of Tumor Types Including Cervical Cancer |
Combination of Anti-PD-1/PD-L1 Therapy with Other Immune Checkpoint Inhibitors
CTLA-4 Blockade
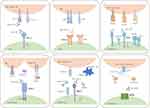
CTLA-4 is an important negative regulator of immune responses and its ligands are CD80 and CD86 (Figure 2). Anti-CTLA-4 antibodies, the start of immune checkpoint blockade therapy, have shown impressive results in a range of cancers such as melanoma, renal cell carcinoma and colorectal carcinoma, either alone or in combination.31 Blocking CTLA-4 activates both CD4+ and CD8+ effector cells and selectively depletes regulatory T cells in tumors, thereby enhancing antitumor immunity and promoting tumor rejection.32,33 Certain types of CTLA-4 genetic polymorphism have been proven to be relevant to an increased risk of cervical cancer.34,35 CTLA-4 is expressed on more than 50% of invasive cervical cancer cells and is associated with the clinical stage of the tumor and lymph node metastasis.36 A clinical trial has found that ipilimumab (anti-CTLA-4) following chemoradiation therapy in locally advanced cervical cancer patients induced an expansion of central and effector memory T cells.37 Accordingly, inhibition of CTLA-4 has the potential to help patients fight cervical cancer.
Blocking PD-1 and CTLA-4 has shown remarkable antitumor activity in a wide range of tumors.38 Several studies have explored the effects of PD-1/PD-L1 and CTLA-4 checkpoint co-inhibitory pathways on immunomodulatory functions and their potential tumor immunotherapeutic effects. Blockade of CTLA-4 may upregulate expression of PD-L1 on tumor cells and immune cells through IFN-γ produced by T cells.38 In a study evaluating the safety and antitumor activity of an anti-CTLA-4 mAb, ipilimumab, in recurrent cervical cancer, PD-1 expression was found to be upregulated in peripheral CD4+ and CD8+ T cells following the use of ipilimumab.39 In a preclinical study, concurrent blockade of PD-1 and CTLA-4 showed synergistic antitumor activity in mouse colorectal tumor models.40 The potential mechanism may be that combination therapy leads to a favorable ratio of effector and regulatory T-cell, increased secretion of pro-inflammatory cytokines, and activation of tumor-specific T cells.40 Another study, using an HPV+ oral tumor model, showed an increased survival benefit under the condition of combining anti-PD-1 therapy with anti-CTLA-4 therapy.41 The addition of CTLA-4 blockade therapy to PD-1 blockade therapy induces an expansive frequency of effector CD4+ T cells that is not sufficient for PD-1 inhibitory monotherapy, which is observed in murine colon carcinoma models.9,42 However, dual blockade may not induce the expansion of phenotypically exhausted CD8+ T cells, which occurs with anti-PD-1 monotherapy, and may lead to an increase in activated terminally differentiated effector CD8+ T cells.42 Therefore, dual blockade of PD-1 and CTLA-4 induces an improved frequency of effector CD8+ and CD4+ T cells in the tumor, resulting in an increased ratio of CD8+ T cell to Treg (regulatory T cell) and CD4+ T cell to Treg ratio, which correlates with high antitumor activity.40,43,44 Collectively, these findings support the potential of dual blockade of PD-1 and CTLA-4 in cervical cancer.
Clinical trials using this combination regimen in cervical cancer are currently underway (NCT02488759, NCT03894215, NCT05033132). In addition, a PD-1/CTLA-4 bispecific antibody (AK104) is undergoing clinical trials for recurrent or metastatic cervical cancer (NCT04380805, NCT04868708). Furthermore, several other clinical trials have added chemotherapy or radiation therapy to the combination of PD-1 and CTLA-4 blockade (NCT03518606, NCT03452332, NCT03277482). Hopefully, these clinical trials will provide evidence for the novel therapy for treating cervical cancer patients.
Immune-related adverse events of the combination should not be neglected. It was reported that for advanced melanoma patients who received ipilimumab plus nivolumab, immune-related adverse drug reactions occurred more frequently and earlier with higher severity, compared with those who received ipilimumab or nivolumab alone.45
TIGIT Blockade
T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT) is an immune checkpoint receptor expressed on natural killer (NK) cells and T cells (Figure 2). It attenuates the functions of these immune cells by binding to its ligand PVR located in antigen-presenting cells or tumor cells.46,47
Dual blockade of TIGIT and PD-1 has the potential to recover and augment the activity of immune cells in cervical cancer. Firstly, a significant increase in various subsets of peripheral blood NK cells and T cells expressing both PD-1 and TIGIT has been reported in patients with cervical cancer.48 Moreover, diverse inhibitory checkpoints including PD-1 and TIGIT tend to co-express in HPV+ head and neck cancer samples.49 Co-blockade of TIGIT and PD-L1 synergistically and specifically enhanced the effector function of CD8(+) T cells, leading to significant clearance of tumors and viruses in murine models of colorectal carcinoma and chronic viral infections.49,50 Therefore, concurrent blockade of TIGIT and PD-1 may significantly reverse the exhausted state and enhance the function of these PD-1+TIGIT+ immune cells, which may not be fully achieved by blocking only a single checkpoint receptor. Secondly, it was found that in mouse models of colon cancer, breast cancer, melanoma and fibrosarcoma, NK cells highly expressed TIGIT, while PD-1 expression was relatively low, resulting in limited efficacy of mono blockade of PD-1 on NK cells.51 Therefore, dual blockade of TIGIT and PD-1 will not only enhance the function of T cells highly expressing PD-1 and TIGIT, but also enhance the function of NK cells by blocking TIGIT.51 This means that this combination can sufficiently reactivate different types of antitumor immune cells through their highly expressed checkpoint receptors. A bispecific nanobody targeting both PD-1 and TIGIT has been shown to have the ability to enhance T cell activity in vitro.52 Combination therapy of anti-PD-1 with anti-TIGIT has shown efficacy in various tumor models, such as colon carcinoma models and glioblastoma models.50,53 In addition, CD96, another member in the TIGIT axis expressed on T cells and NK cells, was recently found to attenuate the function of CD8+ tumor-infiltrating lymphocytes in cooperation with PD-1 in cervical cancer.54 As a result, dual blockade of CD96 and PD-1 further enhanced the function of CD8+ tumor-infiltrating lymphocytes and inhibited tumor growth in cervical cancer murine models.54 In terms of clinical application, the anti-TIGIT mAb tiragolumab in combination with the anti-PD-L1 mAb atezolizumab has been approved by FDA for treating the metastatic non-small cell lung cancer (NSCLC). A phase II trial involving NSCLC patients reported that combination treatment of tiragolumab and atezolizumab achieved an overall response rate (ORR) of 37% and a median progression-free survival (PFS) of 5.6 months compared with 21% and 3.9 months for the atezolizumab treatment alone.46 However, in the combination group 69% of the patients had immune-related adverse events (most frequently rash and infusion) compared with 47% in the atezolizumab group.46 Although there have been only a few preclinical and clinical investigations involving this combination in cervical cancer, encouraging results can be expected.
LAG-3 Blockade
LAG-3 is another immune checkpoint receptor whose ligands are MHC-II on APCs and LSECtin on tumor cells (Figure 2).55,56 LAG-3 is highly expressed in a variety of HPV-related malignancies, especially in cervical cancer with an expression rate as high as 75%.53,55,57 LAG-3 blockade has been reported to exhibit excellent performance in enhancing immune activity.57 Specifically, the blockade of LAG-3 enhanced the proliferation of CD4+ and CD8+ T cells and secretion of IFN-γ and TNF-α more conspicuously than the PD-1 blockade in vitro.57 Furthermore, LAG-3 blockade increased markedly WT1 tumor antigen-specific T cells, whereas PD-1 blockade only slightly increased.57 This significant difference between the two blockades is probably due to WT1 being expressed in various solid tumor cells, including cervical cancer cells, to play an oncogenic role during carcinogenesis.58,59 Therefore, adding LAG-3 blockade to PD-1 blockade therapy may further activate antitumor immunity through the advantages of LAG-3 blockade over PD-1 blockade and significantly increase treatment efficacy. In addition, LAG-3 blockade inhibits Treg cells that perform inhibitory functions, and its influences on Treg cells were observed in mouse models with loss of both LAG-3 and PD-1.60,61 Several studies have revealed that dual blockade of LAG-3 and PD-1 resulted in augmented T cell proliferation, an enhanced proportion of effector T cells, and improved T cell killing capacity compared with PD-1 blockade alone, thereby suppressing tumor growth.57,61 In addition, dual blockade of LAG-3 and PD-1 resulted in upregulation of IFN-γ expression, and blockade of the two checkpoints achieved this through diffirent types of T cells--LAG3 blockade through naïve T cells and central memory T cells while PD-1 blockade through effector memory T cells.57,62 Increased production of TNF-α may also be obtained under conditions of dual blockade.62 Recently, a phase 2–3 clinical trial reported that the combination of relatlimab (a LAG-3-blocking antibody) and nivolumab achieved a median progression-free survival of 10.1 months compared with 4.6 months for nivolumab monotherapy in untreated advanced melanoma.63 Another clinical trial has shown antitumor activity of the combination of ieramilimab (anti-LAG-3) and spartalizumab (anti-PD-1) in solid tumors, with 3 (2%) complete responses and 10 (8%) partial responses, compared with no complete response and partial response in ieramilimab monotherapy.64 MGD013, a bispecific DART® protein that binds to PD-1 and LAG-3, is going through a clinical trial for patients with a variety of tumors, including cervical cancer (NCT03219268).
TIM-3 Blockade
T cell immunoglobulin mucin 3 (TIM-3) plays an important role in immunosuppression following binding to its ligand Gal-9 (Figure 2).65 High expression of TIM-3 and its ligand was found in cervical and vulvar squamous neoplasia.65 Overexpression of TIM-3 is associated with HPV-positive status and may be related to poor tumor differentiation and shorter survival time in cervical cancer.66,67 Co-expression of TIM-3/Gal-9 and PD-1/PD-L1 occurs frequently in cervical cancer.48,65 Tim-3+PD-1+ TILs (tumor infiltrating cells) exhibit an exhausted phenotype characterized by decreased secretion of IL-2, TNF, and IFN-γ.68 Therefore, concurrent blockade of TIM-3 and PD-1 may result in a reversal of immune cell exhaustion and an increase in cytokines, thereby enhancing the antitumor immune response against cervical cancer. This combination regimen has shown potential benefit in several advanced solid tumors.69,70 One clinical trial reported that a combination of LY3300054 (anti-PD-L1) and LY3321367 (anti-TIM-3) showed antitumor activity against PD-1/PD-L1 inhibitor-naïve MSI-H/dMMR solid tumors.69 Another clinical trial also showed preliminary efficacy of sabatolimab(anti-TIM-3) plus spartalizumab(anti-PD-1) for advanced solid tumors.70 However, studies involving cervical cancer are sparse. Thus, the efficacy of the dual blockade approach in cervical cancer needs to be explored.
A2AR Blockade
A2A receptor (A2AR) is one of four subtypes of adenosine receptor and belongs to the G-protein coupled receptors (Figure 2).71 A2AR is highly expressed on the immune cells and is involved in the regulation of immune functions after activation.72 The adenosine pathway involves CD39/CD73/A2AR and was found to impede NK cell maturation and enhance the immunosuppressive function of regulatory T cells.72,73 A2AR blockade downregulated PD-1 expression on CD8+ effector T cells (Teff) and FoxP3+ CD4+ regulatory T cells (Tregs) and the draining lymph nodes of a tumor, which was found in colon cancer mouse models.74 Dual blockade therapy with A2AR and PD-1 contributed to tumor regression and prolonged survival in colon cancer models.74 A phase I clinical trial has shown antitumor activity of the combination of ciforadenant (a small-molecule A2AR antagonist) and atezolizumab (anti-PD-L1) in renal cell cancer.75 In addition, CD73 expression was found to be associated with limited efficacy of anti-PD-1 therapy,76,77 and CD73 inhibitors were shown to enhance antitumor activity with PD-1 blockade in mouse tumor models of breast cancer and colon cancer.78,79 A clinical trial involving cervical cancer patients is underway to evaluate the therapeutic efficacy of the combination of a CD73 monoclonal antibody with an oral A2AR antagonist or an anti-PD1 antibody (NCT03454451).
Combination of Anti-PD-1/PD-L1 Therapy with Biotherapies
With Adoptive Cell Therapy
Adoptive cell therapy (ACT) is an immunotherapeutic approach that involves isolating autologous immune cells from a patient, manipulating them specifically ex vivo, and then infusing them back into the patient with the expectation that these manipulated cells will attack and eliminate tumor cells.80,81 In chimeric antigen receptor and T-cell receptor (CAR-T/TCR-T) immunotherapy, which is the main modality of ACT, T cells are genetically modified to express a special chimeric antigen receptor (CAR) or T-cell receptor (TCR) to achieve specific and precise recognition of tumor cells.80,81 There have been several clinical trials assessing the safety and efficacy of TCR-T cell therapy in cervical cancer, with significant regression of the tumors.82,83 Although to date CAR-T cell therapy for cervical cancer has not been clinically evaluated, preclinical studies have shown preliminary efficacy and more preclinical assessments are underway.84,85 In another strategy of ACT, tumor-infiltrating T cells (TIL), T cells are isolated from tumor samples, selected, amplified, and then reinfused.80 Clinical studies have demonstrated efficacy of TIL in HPV-associated cancers.86,87
The rationale for combining the use of adoptive cell therapy with PD-1 blockade can be illustrated from two perspectives. Firstly, monotherapy with PD-1 blockade has shown unsatisfactory efficacy in certain poorly immunogenic cancer types, as exhaustion-reversed effector T cells still cannot recognize these cancer cells well.17,88 Therefore, the addition of manipulated T cells with good tumor recognition ability may enhance antitumor responses and treatment efficacy of these cancer types.80 Secondly, CAR-T therapy is less effective in solid tumors than in hematological tumors, possibly due to the negative function of inhibitory checkpoints on CAR-T cells.89–92 Therefore, applying PD-1 blockade to CAR-T therapy will augment the activity of CAR-T cells and improve the efficacy of this therapeutic approach in solid tumors, including cervical cancer.
There are several strategies for blocking PD-1 function on CAR-T cells (Table 2). One strategy is to engineer CAR-T cells to secrete PD-1-blocking antibodies that then act on the CAR-T cells themselves, which has been shown to enhance antitumor efficacy.90,93,94 Besides PD-1-blocking antibodies, CAR-T cells modified to secrete soluble PD-1 (sPD-1) were also shown to improve antitumor efficacy through blockade of PD-L1 present on cancer cells.95,96 Another strategy is to genetically modify CAR-T cells to overexpress a PD-1 dominant negative receptor that competes with normal PD-1 to bind to PD-L1 on tumor cells but does not show inhibitory effects, thereby attenuating the effects of the PD-1 signaling pathway.97 In addition, the use of CRISPR/Cas9 gene-editing approaches could disrupt PD-1 on CAR-T cells, and stronger antitumor immunity has been observed in vitro and in vivo.98,99 Tang et al constructed a chimeric activated receptor named PD1-CAR, which consists of the extracellular domain of PD1 and the transmembrane and intracellular domains of the positive costimulatory molecules CD28 and 4–1BB. T cells expressing PD1-CAR retained the capacity to bind to PDL1 and were activated to specifically target PD-L1+ tumor cells.100 CD8+ T cells transfected with PD1-CAR (CAR-T-PD1 cells) showed higher antitumor activity against cervical cancer in a mouse model, while CAR-T-PD1 cells activated by HPV16mE7-pulsed and SOCS1-silenced DCs showed even more significant increases in cytokine secretion, cytotoxic activity and survival rate.89 In addition to intrinsic PD-1 blockade of adoptively transferred T cells, which license the cells with checkpoint blockade without extra antibody administration, one study used TIL therapy in combination with the PD-1 monoclonal antibody nivolumab to treat patients with metastatic cervical cancer with low microsatellite instability and low PD-L1 expression, and observed an improved prognosis.101 Besides T cells, NK cell therapy also has the potential to cooperate with anti-PD-1 therapy to improve antitumor efficacy. Jeffrey et al developed a manufacturing system for production of NK cells derived from induced pluripotent stem cells (iPSC-derived NK cells), which can recruit and activate T cells to tumors and make them responsive to PD-1 blockade due to their potential to overcome checkpoint blockade resistance, thereby enhancing cytokine production and tumor elimination in ovarian cancer models.102 These results suggest that the use of a combination of adoptive cell therapy and PD-1 blockade therapy in solid tumors, including cervical cancer, is promising, although more studies are needed to further validate this. There have been clinical trials demonstrating the antitumor efficacy of the combination of CAR-T cell therapy and PD-1 blockade in solid tumors.103,104 A clinical trial involving patients with recurrent, metastatic and persistent cervical cancer is underway, with a cohort receiving TIL therapy and pembrolizumab (NCT03108495). In addition, an ongoing clinical trial is evaluating the efficacy of HPV-E6-specific TCR-T cells with anti-PD-1 autocrine elements in the treatment of HPV-positive head and neck carcinoma and cervical cancer (NCT03578406).
 |
Table 2 Strategies Combining Adoptive Cell Therapy with PD-1 Blockade |
With Therapeutic Vaccines
Therapeutic vaccines are designed to activate antigen-specific immunity and then kill tumor cells through a variety of vaccine platform technologies. The therapeutic vaccines have included live vector vaccines, peptide/protein-based vaccines, DNA vaccines, cell-based vaccines, and combinatorial strategies such as prime-boost regimen.105,106 For treating HPV-associated cervical cancer, the E6 and E7 oncoproteins of HPV are the ideal vaccine targets because they are constitutively expressed on malignant cells following HPV infection and play a pivotal role in the carcinogenesis and maintenance of malignant phenotype in cervical cancer.105–108 The desired effect of HPV-targeted vaccines is to elicit a robust immune response produced primarily by Th1 cells and cytotoxic lymphocytes, which may be the key elements to clear HPV-induced cervical cancer.105,106,109 Although clinical studies have demonstrated the efficacy of the therapeutic HPV vaccine in cervical intraepithelial neoplasia (CIN),110,111 efficacy is unsatisfactory in invasive cervical cancers and no therapeutic vaccines have been approved in clinical practice yet. The efficacy is limited due to negative regulatory role of various factors in the tumor immunosuppressive environment, including the negative function exerted by the PD-1 axis on vaccine-activated immune cells, resulting in the exhaustion of these cells that are expected to play a role in the eradication of cancer cells.112–114 Therefore, combining PD-1 blockade with therapeutic vaccines may maintain the immune activity of vaccine-activated cells, thus allowing them to efficiently kill tumor cells.
Lee et al developed a non-oncogenic HPV 16 E6/E7 vaccine called Ad5 [E1-, E2b-]- E6/E7 immunizations.115 They have demonstrated that this therapeutic vaccine induced HPV-E6/E7 specific cell-mediated immune responses. In a mouse model of HPV-E6/E7 TC-1 tumors, co-administration of anti-PD-1 antibody with Ad5 [E1-, E2b-]-E6/E7 showed tumor regression rates of up to 57% compared to 29% in mice treated with Ad5 [E1-, E2b-]-E6/E7 alone.116 In addition, they observed that the expression of PD-1 and LAG-3 on TILs and PD-L1 on tumor cells was reduced when Ad5 [E1-, E2b-]-E6/E7 was bound to anti-PD-1 antibodies, implying a reduction in the exhaustive phenotype of effector T cells.116 Hung et al developed a pBI-11 DNA vaccine targeting E6/E7 of HPV16 and HPV18, and found that combination of the pBI-11 DNA vaccination boosted by TA-HPV (tissue-antigen HPV vaccine) with PD-1 antibody blockade induced E7-specific CD8+ T cell immune responses and higher antitumor effects as well as better survival, whereas treatment with anti-PD-1 antibody alone without a prior immune response did not show significant antitumor effects when treating mice bearing HPV-E6/E7 TC-1 tumors.117 This suggests that the efficacy of PD-1 blockade might be improved with the combination of therapeutic vaccines.
A phase II clinical trial showed that nivolumab in combination with ISA 101, a synthetic long-peptide HPV-16 vaccine, was superior to PD-1 blockade alone in treating patients with incurable HPV-16-positive cancers, having 33% of ORR and 17.5 months of median overall survival (NCT02426892).118 A single-arm, phase II trial that has a combination of GX-188E therapeutic DNA vaccine and pembrolizumab for treating recurrent and advanced cervical cancer is ongoing.119 Interim analysis declared preliminary anti-tumor activity, with 42% of patients showing an overall response at 24 weeks.119 In addition, some other clinical trials focusing on this combination regimen involving cervical cancer patients are underway (NCT04800978, NCT03946358).
Combination of Anti-PD-1/PD-L1 Therapy with Other Immunotherapies
With TLR9 Agonists
TLR9, a member of Toll-like receptors, is a pattern recognition receptor expressed on the innate immune cells, including dendritic cells, macrophages, and natural killer cells.120,121 TLR9 agonists elicit the secretion of cytokines such as interferon (IFN) that contribute to antigen presentation to naive T cells and induce antigen-specific adaptive immune responses.120–122 The combination of TLR9 agonists and anti-PD-1 therapy has been observed to have superior antitumor effects at both injection sites and distant non-injected sites, revealing systemic antitumor immunity.122–124 CMP-001, a virus-like particle (VLP) encapsulating an immunostimulatory CpG-A oligodeoxynucleotide (ODN) TLR9 agonist, combined with PD-1 blockade elicited durable regression of injected and distant tumors and prolonged survival of the HPV+ tumor mouse model, compared to anti-PD-1 alone.122 The mechanism underlying the therapeutic effects was elucidated by increased recruitment of activated T cells to the draining lymph nodes and enhanced circulating TNFα and IL-6 levels when the combination treatment was administered.122 Another study, by Torrejon et al, revealed that TLR9 agonist can overcome anti-PD-1 resistance caused by JAK1/2 loss of function mutations and subsequent lack of IFN signaling by inducing a potent type I IFN systemic response.124 Similarly, after intratumoral injection of TLR9 agonist SD-101 combined with anti-PD-1 in mice with JAK1 and JAK2 knockout tumors, antitumor effects were observed at both the injection site and the contralateral non-injection site, and survival rates were higher.124 In addition, TLR9 agonists have been reported to improve the response rate to anti-PD-1 therapy because they upregulate PD-L1 expression in hepatocellular carcinoma cells through STAT3(Tyr705) phosphorylation.125 Therefore, the addition of TLR9 agonists to PD-1 blockade has the potential to enhance antitumor effects via multiple mechanisms and achieve systemic antitumor responses, indicating promising clinical applications. Results of a phase Ib clinical trial of the TLR9 agonist DV281 plus nivolumab showed potential efficacy in NSCLC.126
With TGF-β Inhibition
TGF-β promotes tumor progression, invasiveness and metastasis in the late stages of tumor (mainly TGF-β1 and TGF-β2, while TGF-β3 may have a protective function).127,128 The activation of TGF-β pathway is associated with the reduced chemo-sensitivity in gynecologic cancer.129 Combined inhibition of PD-L1 and TGF-β may enhance antitumor activity due to independent and complementary immunosuppressive effects on the two pathways.130 In a study by Strauss et al, a fusion protein composed of a mAb against PD-L1 fused to a TGF-β “trap” was used to treat the heavily pretreated patients with advanced solid tumors, including cervical cancer.130 Results showed that one cervical cancer patient was ongoing confirmed to have a complete response, and one other was in near partial response.
With IDO Inhibition
Indoleamine 2.3-dioxygenase (IDO) is a crucial enzyme in the catabolism of tryptophan to kynurenine.131 Tryptophan depletion induces an increase in T cell apoptosis and a decrease in T cell proliferation through suppression of mTORC1 and eIF-2 phosphorylation, respectively, while kynurenine accumulation leads to a promotion of Treg differentiation, resulting in a diminished antitumor immune response.131 Thus, IDO inhibition enhances antitumor immunity and promotes cancer elimination to generate encouraging results of suppressing various types of tumor in animal models and clinical trials.131
Considering the remarkable effects of IDO inhibition, combining it with PD-1 blockade may produce high treatment efficacy in cervical cancer, as evidence has shown. As detected in cervical squamous neoplasia, expression of tumoral IDO was up to 75%.132 In addition, the co-expression of IDO and PD-L1 on cervical cancer cells was 63%.132 These data provide evidence to support the immunotherapy of concurrent inhibition of IDO and PD-1 in the majority of cervical cancer patients. Furthermore, it was observed that IDO mRNA expression increased after the treatment of anti-PD-1 in a murine melanoma model, which may be part of resistance mechanisms of anti-PD-1 therapy and thus can be overcome with the combination regimen.133 Spranger et al found that dual blockade of IDO and PD-1/PD-L1 induced tumor rejection by restoring IL-2 production and proliferation of tumor-infiltrating CD8(+) T cells in the tumor microenvironment without attracting new T cells from secondary lymphoid structures.133 Given these studies revealing the possible potential mechanisms of improving combination therapy efficacy, the undergoing clinical trial will be promising. A phase I clinical study has shown the efficacy of a combination of the IDO inhibitor navoximod and the anti-PD-L1 mAb atezolizumab in multiple types of cancer, including cervical cancer.134 Another clinical trial is ongoing to evaluate the efficacy and safety of pembrolizumab plus epacadostat, an orally available IDO1 inhibitor, in recurrent and metastatic head and neck squamous cell carcinoma (HNSCC), an HPV-related cancer type (NCT03358472).
Conclusions and Perspectives
The combination of different immunotherapeutic approaches is rational and promising, with an increased ability to mobilize the host immune system to recognize, fight and ultimately destroy malignant cells. As mentioned above, some combination strategies have shown higher efficacy in cancers other than in cervical cancer. The limited preliminary efficacy in cervical cancer shown in the published studies is still far from clinical application. In addition, some other immunotherapeutic strategies involving some immune-related enzymes and chemokines as well as co-stimulatory molecules (such as ICOS) are not listed here, as their combinations with PD-1/PD-L1 blockade are rarely investigated in cervical cancer and need more attention and research. In spite of this, combinatorial immunotherapies hold great potential in cervical cancer treatment due to unique features in the tumor microenvironment of HPV-induced cancers. Therefore, preclinical and clinical studies are greatly required for evaluating the antitumor efficacy and safety of these combinatorial immunotherapy approaches in cervical cancer.
Despite the potential advantages of a combinatorial strategy, there are challenges and problems. Firstly, concurrent administration of two immunotherapy approaches may increase the frequency of immune-related adverse events and even lead to special ones that never occur in monotherapy. In fact, higher frequency and greater severity as well as earlier onsets of adverse drug reactions have been observed when combining two immune checkpoint inhibitors, and even, in some severe cases, the drugs had to be terminated to control the immune toxicities.45 Secondly, it remains a challenge how to identify patients who are suitable for a certain combination regimen and have a high possibility of gaining benefits. Mismatch repair deficiency, elevated tumor mutation burden, high microsatellite instability, increased intratumoral plasma cells and elevated Notch signaling have shown the potential to predict clinical benefit of PD-1 blockade therapies.135–139 In addition, vaginal and gut microbiota also influence immune checkpoint protein profiles.140,141 PD-L1 and LAG-3 in the cervicovaginal microenvironment are negatively associated with abundance of Lactobacillus while positively correlated with dysbiotic bacteria in the vagina.140 Bifidobacterium in the gut are positively correlated with response to anti-PD-L1 treatment in cancer patients.141 Therefore, to select appropriate patients for combinatorial immunotherapy, it may be valuable to test gene expression profiling, molecular profiling, immune profiling or microbiota as the bases for selection.142 Thirdly, when is the best time to use combinatorial immunotherapies and how they can be added to the existing treatment algorithms need to be investigated. Accordingly, future studies should focus on resolving these issues, which are important for the entry of combination therapies into clinical practice, in addition to further validating their efficacy, durability and safety in patients with cervical cancer.
Acknowledgment
Yanjun Ge and Yuchen Zhang contributed equally to this work and share first authorship.
Funding
This work is supported by Research Project of Shanghai Municipal Health Care Commission [grant number 202040129].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Serkies K, Jassem J. Systemic therapy for cervical carcinoma - current status. Chin J Cancer Res. 2018;30(2):209–221. doi:10.21147/j.issn.1000-9604.2018.02.04
3. Feng CH, Mell LK, Sharabi AB, McHale M, Mayadev JS. Immunotherapy with radiotherapy and chemoradiotherapy for cervical cancer. Semin Radiat Oncol. 2020;30(4):273–280. doi:10.1016/j.semradonc.2020.05.003
4. Tomao F, Di Tucci C, Imperiale L, et al. Cervical cancer: are there potential new targets? An update on preclinical and clinical results. Curr Drug Targets. 2014;15(12):1107–1120. doi:10.2174/1389450115666141010145547
5. Minion LE, Tewari KS. Cervical cancer - State of the science: from angiogenesis blockade to checkpoint inhibition. Gynecol Oncol. 2018;148(3):609–621. doi:10.1016/j.ygyno.2018.01.009
6. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, Phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390(10103):1654–1663. doi:10.1016/S0140-6736(17)31607-0
7. Abbott M, Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Semin Oncol Nurs. 2019;35(5):150923. doi:10.1016/j.soncn.2019.08.002
8. Ventriglia J, Paciolla I, Pisano C, et al. Immunotherapy in ovarian, endometrial and cervical cancer: state of the art and future perspectives. Cancer Treat Rev. 2017;59:109–116. doi:10.1016/j.ctrv.2017.07.008
9. Wei SC, Levine JH, Cogdill AP, et al. Distinct cellular mechanisms underlie Anti-CTLA-4 and Anti-PD-1 checkpoint blockade. Cell. 2017;170(6):1120–1133.e17. doi:10.1016/j.cell.2017.07.024
10. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
11. Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17):1470–1478. doi:10.1200/JCO.18.01265
12. Ferrall L, Lin KY, Roden RBS, Hung CF, Wu TC. Cervical cancer immunotherapy: factsand hopes. Clin Cancer Res. 2021;27(18):4953–4973. doi:10.1038/s41591-020-01225-1
13. Naumann RW, Hollebecque A, Meyer T, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II checkmate 358 trial. J Clin Oncol. 2019;37(31):2825–2834. doi:10.1200/JCO.19.00739
14. Tewari KS, Monk BJ, Vergote I, et al. Survival with cemiplimab in recurrent cervical cancer. N Engl J Med. 2022;386(6):544–555. doi:10.1056/NEJMoa2112187
15. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. doi:10.1001/jamanetworkopen.2019.2535
16. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi:10.1056/NEJMoa1500596
17. Li B, Chan HL, Chen P. Immune checkpoint inhibitors: basics and challenges. Curr Med Chem. 2019;26(17):3009–3025. doi:10.2174/0929867324666170804143706
18. Lei Q, Wang D, Sun K, Wang L, Zhang Y. Resistance mechanisms of Anti-PD1/PDL1 therapy in solid tumors. Front Cell Dev Biol. 2020;8:672. doi:10.3389/fcell.2020.00672
19. Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385(20):1856–1867. doi:10.1056/NEJMoa2112435
20. Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. doi:10.1016/j.immuni.2019.12.011
21. Smyth MJ, Ngiow SF, Ribas A, Teng MWL. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13(3):143–158. doi:10.1038/nrclinonc.2015.209
22. O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16(3):151–167. doi:10.1038/s41571-018-0142-8
23. Chen YP, Zhang Y, Lv JW, et al. Genomic analysis of tumor microenvironment immune types across 14 solid cancer types: immunotherapeutic implications. Theranostics. 2017;7(14):3585–3594. doi:10.7150/thno.21471
24. Prat A, Navarro A, Paré L, et al. Immune-related gene expression profiling after PD-1 blockade in non-small cell lung carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res. 2017;77(13):3540–3550. doi:10.1158/0008-5472.CAN-16-3556
25. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi:10.1038/nature21349
26. Manieri NA, Chiang EY, Grogan JL. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol. 2017;38(1):20–28. doi:10.1016/j.it.2016.10.002
27. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. doi:10.1038/nrc2886
28. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–2501. doi:10.1056/NEJMc1713444
29. Zhang L, Zhao Y, Tu Q, Xue X, Zhu X, Zhao KN. The roles of programmed cell death ligand-1/ programmed cell death-1 (PD-L1/PD-1) in HPV-induced cervical cancer and potential for their use in blockade therapy. Curr Med Chem. 2021;28(5):893–909. doi:10.2174/0929867327666200128105459
30. Yuan Y, Cai X, Shen F, Ma F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021;497:243–254. doi:10.1016/j.canlet.2020.10.034
31. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12(3):738. doi:10.3390/cancers12030738
32. Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist. 2008;13(Suppl 4):2–9. doi:10.1634/theoncologist.13-S4-2
33. Liu Y, Zheng P. How does an Anti-CTLA-4 antibody promote cancer immunity? Trends Immunol. 2018;39(12):953–956. doi:10.1016/j.it.2018.10.009
34. Hu S, Pu D, Xia X, Guo B, Zhang C. CTLA-4 rs5742909 polymorphism and cervical cancer risk: a meta-analysis. Medicine. 2020;99(11):e19433. doi:10.1097/MD.0000000000019433
35. Qiu H, Tang W, Yin P, Cheng F, Wang L. Cytotoxic T‑lymphocyte-associated antigen‑4 polymorphisms and susceptibility to cervical cancer: a meta‑analysis. Mol Med Rep. 2013;8(6):1785–1794. doi:10.3892/mmr.2013.1721
36. Karpathiou G, Chauleur C, Mobarki M, Peoc’h M. The immune checkpoints CTLA-4 and PD-L1 in carcinomas of the uterine cervix. Pathol Res Pract. 2020;216(1):152782. doi:10.1016/j.prp.2019.152782
37. Da Silva DM, Enserro DM, Mayadev JS, et al. Immune activation in patients with locally advanced cervical cancer treated with ipilimumab following definitive chemoradiation (GOG-9929). Clin Cancer Res. 2020;26(21):5621–5630. doi:10.1158/1078-0432.CCR-20-0776
38. Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–573. doi:10.1146/annurev-immunol-032414-112049
39. Lheureux S, Butler MO, Clarke B, et al. Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. JAMA Oncol. 2018;4(7):e173776. doi:10.1001/jamaoncol.2017.3776
40. Selby MJ, Engelhardt JJ, Johnston RJ, et al. Preclinical development of ipilimumab and nivolumab combination immunotherapy: mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS One. 2016;11(9):e0161779. doi:10.1371/journal.pone.0161779
41. Dorta-Estremera S, Hegde VL, Slay RB, et al. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer. J Immunother Cancer. 2019;7(1):252. doi:10.1186/s40425-019-0728-4
42. Wei SC, Anang NAAS, Sharma R, et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U S A. 2019;116(45):22699–22709. doi:10.1073/pnas.1821218116
43. Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–4280. doi:10.1073/pnas.0915174107
44. Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–1945. doi:10.1172/JCI27745
45. Hassel JC, Heinzerling L, Aberle J, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017;57:36–49. doi:10.1016/j.ctrv.2017.05.003
46. Caruso, C. Tiragolumab impresses in multiple trials. Cancer Discov. 2020;10(8):1086–1087. doi:10.1158/2159-8290.CD-NB2020-063
47. Harjunpää H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020;200(2):108–119. doi:10.1111/cei.13407
48. Solorzano-Ibarra F, Alejandre-Gonzalez AG, Ortiz-Lazareno PC, et al. Immune checkpoint expression on peripheral cytotoxic lymphocytes in cervical cancer patients: moving beyond the PD-1/PD-L1 axis. Clin Exp Immunol. 2021;204(1):78–95. doi:10.1111/cei.13561
49. Gameiro SF, Ghasemi F, Barrett JW, et al. Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology. 2018;7(10):e1498439. doi:10.1080/2162402X.2018.1498439
50. Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923–937. doi:10.1016/j.ccell.2014.10.018
51. Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–732. doi:10.1038/s41590-018-0132-0
52. Ma L, Gai J, Qiao P, et al. A novel bispecific nanobody with PD-L1/TIGIT dual immune checkpoint blockade. Biochem Biophys Res Commun. 2020;531(2):144–151. doi:10.1016/j.bbrc.2020.07.072
53. Hung AL, Maxwell R, Theodros D, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018;7(8):e1466769. doi:10.1080/2162402X.2018.1466769
54. Wang Y, Wang C, Qiu J, et al. Targeting CD96 overcomes PD-1 blockade resistance by enhancing CD8+ TIL function in cervical cancer. J Immunother Cancer. 2022;10(3):e003667. doi:10.1136/jitc-2021-003667
55. Qi Y, Chen L, Liu Q, Kong X, Fang Y, Wang J. Research progress concerning dual blockade of lymphocyte-activation gene 3 and programmed death-1/programmed death-1 ligand-1 blockade in cancer immunotherapy: preclinical and clinical evidence of this potentially more effective immunotherapy strategy. Front Immunol. 2021;11:563258. doi:10.3389/fimmu.2020.563258
56. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi:10.1016/j.immuni.2016.05.001
57. Lichtenegger FS, Rothe M, Schnorfeil FM, et al. Targeting LAG-3 and PD-1 to enhance T cell activation by antigen-presenting cells. Front Immunol. 2018;9:385. doi:10.3389/fimmu.2018.00385
58. Sugiyama H. Wilms’ tumor gene WT1: its oncogenic function and clinical application. Int J Hematol. 2001;73(2):177–187. doi:10.1007/BF02981935
59. Oji Y, Ogawa H, Tamaki H, et al. Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn J Cancer Res. 1999;90(2):194–204. doi:10.1111/j.1349-7006.1999.tb00733.x
60. Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Rev Immunol. 2015;15(1):45–56. doi:10.1038/nri3790
61. Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi:10.1158/0008-5472.CAN-11-1620
62. Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875–7880. doi:10.1073/pnas.1003345107
63. Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34. doi:10.1056/NEJMoa2109970
64. Schöffski P, Tan DSW, Martín M, et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J Immunother Cancer. 2022;10(2):e003776. doi:10.1136/jitc-2021-003776
65. Curley J, Conaway MR, Chinn Z, Duska L, Stoler M, Mills AM. Looking past PD-L1: expression of immune checkpoint TIM-3 and its ligand galectin-9 in cervical and vulvar squamous neoplasia. Mod Pathol. 2020;33(6):1182–1192. doi:10.1038/s41379-019-0433-3
66. Wuerdemann N, Pütz K, Eckel H, et al. LAG-3, TIM-3 and vista expression on tumor-infiltrating lymphocytes in oropharyngeal squamous cell carcinoma-potential biomarkers for targeted therapy concepts. Int J Mol Sci. 2020;22(1):E379. doi:10.3390/ijms22010379
67. Chen Z, Dong D, Zhu Y, Pang N, Ding J. The role of Tim-3/Galectin-9 pathway in T-cell function and prognosis of patients with human papilloma virus-associated cervical carcinoma. FASEB J. 2021;35(3):e21401. doi:10.1096/fj.202000528RR
68. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi:10.1084/jem.20100643
69. Hollebecque A, Chung HC, de Miguel MJ, et al. Safety and antitumor activity of α-PD-L1 antibody as monotherapy or in combination with α-TIM-3 antibody in patients with microsatellite instability-high/mismatch repair-deficient tumors. Clin Cancer Res. 2021;27(23):6393–6404. doi:10.1158/1078-0432.CCR-21-0261
70. Curigliano G, Gelderblom H, Mach N, et al. Phase I/Ib clinical trial of sabatolimab, an Anti-TIM-3 antibody, alone and in combination with spartalizumab, an Anti-PD-1 antibody, in advanced solid tumors. Clin Cancer Res. 2021;27(13):3620–3629. doi:10.1158/1078-0432.CCR-20-4746
71. Al-Attraqchi OHA, Attimarad M, Venugopala KN, Nair A, Al-Attraqchi NHA. Adenosine A2A receptor as a potential drug target - current status and future perspectives. Curr Pharm Des. 2019;25(25):2716–2740. doi:10.2174/1381612825666190716113444
72. Young A, Ngiow SF, Gao Y, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78(4):1003–1016. doi:10.1158/0008-5472.CAN-17-2826
73. Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:190. doi:10.3389/fimmu.2012.00190
74. Leone RD, Sun IM, Oh MH, et al. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol Immunother. 2018;67(8):1271–1284. doi:10.1007/s00262-018-2186-0
75. Fong L, Hotson A, Powderly JD, et al. Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discov. 2020;10(1):40–53. doi:10.1158/2159-8290.CD-19-0980
76. Turiello R, Capone M, Giannarelli D, et al. Serum CD73 is a prognostic factor in patients with metastatic melanoma and is associated with response to anti-PD-1 therapy. J Immunother Cancer. 2020;8(2):e001689. doi:10.1136/jitc-2020-001689
77. Beavis PA, Slaney CY, Milenkovski N, et al. CD73: a potential biomarker for anti-PD-1 therapy. Oncoimmunology. 2015;4(11):e1046675. doi:10.1080/2162402X.2015.1046675
78. Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19(20):5626–5635. doi:10.1158/1078-0432.CCR-13-0545
79. Liu S, Li D, Liu J, et al. A Novel CD73 inhibitor SHR170008 suppresses adenosine in tumor and enhances anti-tumor activity with PD-1 blockade in a mouse model of breast cancer. Onco Targets Ther. 2021;14:4561–4574. doi:10.2147/OTT.S326178
80. Ö M, Jensen KM, Chamberlain CA, Donia M, Svane IM. Principles of adoptive T cell therapy in cancer. Semin Immunopathol. 2019;41(1):49–58. doi:10.1007/s00281-018-0703-z
81. Ivica NA, Young CM. Tracking the CAR-T revolution: analysis of clinical trials of CAR-T and TCR-T therapies for the treatment of cancer (1997–2020). Healthcare. 2021;9(8):1062. doi:10.3390/healthcare9081062
82. Doran SL, Stevanović S, Adhikary S, et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J Clin Oncol. 2019;37(30):2759–2768. doi:10.1200/JCO.18.02424
83. Nagarsheth NB, Norberg SM, Sinkoe AL, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med. 2021;27(3):419–425.
84. Zhang Y, Li X, Zhang J, Mao L. Novel cellular immunotherapy using NKG2D CAR-T for the treatment of cervical cancer. Biomed Pharmacother. 2020;131:110562. doi:10.1016/j.biopha.2020.110562
85. He Y, Li XM, Yin CH, Wu YM. Killing cervical cancer cells by specific chimeric antigen receptor-modified T cells. J Reprod Immunol. 2020;139:103115. doi:10.1016/j.jri.2020.103115
86. Stevanović S, Helman SR, Wunderlich JR, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25(5):1486–1493. doi:10.1158/1078-0432.CCR-18-2722
87. Stevanović S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543–1550. doi:10.1200/JCO.2014.58.9093
88. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi:10.1126/science.aaa8172
89. Zheng J, Huang J, Ma W, Yang W, Hu B. The antitumor activity of CAR-T-PD1 cells enhanced by HPV16mE7-pulsed and SOCS1-silenced DCs in cervical cancer models. Cancer Manag Res. 2021;13:6045–6053. doi:10.2147/CMAR.S321402
90. Chen X, Yang S, Li S, et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-β trap enhances antitumor efficacy of CAR-T cell therapy. Mol Ther Oncolytics. 2021;21:144–157. doi:10.1016/j.omto.2021.03.014
91. John LB, Devaud C, Duong CPM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19(20):5636–5646. doi:10.1158/1078-0432.CCR-13-0458
92. John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2(10):e26286. doi:10.4161/onci.26286
93. Rafiq S, Yeku OO, Jackson HJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36(9):847–856. doi:10.1038/nbt.4195
94. Li S, Siriwon N, Zhang X, et al. Enhanced cancer immunotherapy by chimeric antigen receptor- modified T cells engineered to secrete checkpoint inhibitors. Clin Cancer Res. 2017;23(22):6982–6992. doi:10.1158/1078-0432.CCR-17-0867
95. Zhang A, Sun Y, Wang S, et al. Secretion of human soluble programmed cell death protein 1 by chimeric antigen receptor-modified T cells enhances anti-tumor efficacy. Cytotherapy. 2020;22(12):734–743. doi:10.1016/j.jcyt.2020.05.007
96. Khan M, Zhao Z, Arooj S, Fu Y, Liao G. Soluble PD-1: predictive, prognostic, and therapeutic value for cancer immunotherapy. Front Immunol. 2020;11:587460. doi:10.3389/fimmu.2020.587460
97. Chen N, Morello A, Tano Z, Adusumilli PS. CAR T-cell intrinsic PD-1 checkpoint blockade: a two-in-one approach for solid tumor immunotherapy. Oncoimmunology. 2016;6(2):e1273302. doi:10.1080/2162402X.2016.1273302
98. Rupp LJ, Schumann K, Roybal KT, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. doi:10.1038/s41598-017-00462-8
99. Guo X, Jiang H, Shi B, et al. Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol. 2018;9:1118. doi:10.3389/fphar.2018.01118
100. Tang X, Li Q, Zhu Y, et al. The advantages of PD1 activating chimeric receptor (PD1-ACR) engineered lymphocytes for PDL1(+) cancer therapy. Am J Transl Res. 2015;7(3):460–473.
101. Yin H, Guo W, Sun X, Li R, Feng C, Tan Y. TILs and Anti-PD1 therapy: an alternative combination therapy for PDL1 negative metastatic cervical cancer. J Immunol Res. 2020;2020:8345235. doi:10.1155/2020/8345235
102. Cichocki F, Bjordahl R, Gaidarova S, et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci Transl Med. 2020;12(568):eaaz5618. doi:10.1126/scitranslmed.aaz5618
103. Adusumilli PS, Zauderer MG, Riviere I, et al. A Phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 2021;11(11):2748–2763. doi:10.1158/2159-8290.CD-21-0407
104. Wang Z, Li N, Feng K, et al. Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell Mol Immunol. 2021;18(9):2188–2198. doi:10.1038/s41423-021-00749-x
105. Chabeda A, Yanez RJR, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018;5:46–58. doi:10.1016/j.pvr.2017.12.006
106. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88–102. doi:10.1016/j.canlet.2019.11.039
107. Mahdavi A, Monk BJ. Vaccines against human papillomavirus and cervical cancer: promises and challenges. Oncologist. 2005;10(7):528–538. doi:10.1634/theoncologist.10-7-528
108. Clark KT, Trimble CL. Current status of therapeutic HPV vaccines. Gynecol Oncol. 2020;156(2):503–510. doi:10.1016/j.ygyno.2019.12.017
109. Morrow MP, Yan J, Sardesai NY. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev Vaccines. 2013;12(3):271–283. doi:10.1586/erv.13.23
110. Harper DM, Nieminen P, Donders G, et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: randomized controlled phase II trial with 2.5 years of follow-up. Gynecol Oncol. 2019;153(3):521–529. doi:10.1016/j.ygyno.2019.03.250
111. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078–2088. doi:10.1016/S0140-6736(15)00239-1
112. Bowen WS, Svrivastava AK, Batra L, Barsoumian H, Shirwan H. Current challenges for cancer vaccine adjuvant development. Expert Rev Vaccines. 2018;17(3):207–215. doi:10.1080/14760584.2018.1434000
113. Kim CG, Sang YB, Lee JH, Chon HJ. Combining cancer vaccines with immunotherapy: establishing a new immunological approach. Int J Mol Sci. 2021;22(15):8035. doi:10.3390/ijms22158035
114. Wang J, Mamuti M, Wang H. Therapeutic vaccines for cancer immunotherapy. ACS Biomater Sci Eng. 2020;6(11):6036–6052. doi:10.1021/acsbiomaterials.0c01201
115. Wieking BG, Vermeer DW, Spanos WC, et al. A non-oncogenic HPV 16 E6/E7 vaccine enhances treatment of HPV expressing tumors. Cancer Gene Ther. 2012;19(10):667–674. doi:10.1038/cgt.2012.55
116. Rice AE, Latchman YE, Balint JP, Lee JH, Gabitzsch ES, Jones FR. An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression. Cancer Gene Ther. 2015;22(9):454–462. doi:10.1038/cgt.2015.40
117. Peng S, Ferrall L, Gaillard S, et al. Development of DNA vaccine targeting E6 and E7 proteins of human papillomavirus 16 (HPV16) and HPV18 for immunotherapy in combination with recombinant vaccinia boost and PD-1 antibody. mBio. 2021;12(1):e03224–20. doi:10.1128/mBio.03224-20
118. Massarelli E, William W, Johnson F, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73. doi:10.1001/jamaoncol.2018.4051
119. Youn JW, Hur SY, Woo JW, et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: interim results of a single-arm, phase 2 trial. Lancet Oncol. 2020;21(12):1653–1660. doi:10.1016/S1470-2045(20)30486-1
120. Hemmi H, Akira S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–135.
121. Karapetyan L, Luke JJ, Davar D. Toll-like receptor 9 agonists in cancer. Onco Targets Ther. 2020;13:10039–10060. doi:10.2147/OTT.S247050
122. Cheng Y, Lemke-Miltner CD, Wongpattaraworakul W, et al. In situ immunization of a TLR9 agonist virus-like particle enhances anti-PD1 therapy. J Immunother Cancer. 2020;8(2):e000940. doi:10.1136/jitc-2020-000940
123. Sato-Kaneko F, Yao S, Ahmadi A, et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight. 2017;2(18):93397. doi:10.1172/jci.insight.93397
124. Torrejon DY, Abril-Rodriguez G, Champhekar AS, et al. Overcoming genetically based resistance mechanisms to PD-1 blockade. Cancer Discov. 2020;10(8):1140–1157. doi:10.1158/2159-8290.CD-19-1409
125. Zhou B, Yan J, Guo L, et al. Hepatoma cell-intrinsic TLR9 activation induces immune escape through PD-L1 upregulation in hepatocellular carcinoma. Theranostics. 2020;10(14):6530–6543. doi:10.7150/thno.44417
126. Garon EB, Spira AI, Johnson M, et al. A phase Ib open-label, multicenter study of inhaled DV281, a TLR9 agonist, in combination with nivolumab in patients with advanced or metastatic non-small cell lung cancer. Clin Cancer Res. 2021;27(16):4566–4573. doi:10.1158/1078-0432.CCR-21-0263
127. Syed V. TGF-β signaling in cancer. J Cell Biochem. 2016;117(6):1279–1287. doi:10.1002/jcb.25496
128. Zhu H, Luo H, Shen Z, Hu X, Sun L, Zhu X. Transforming growth factor-β1 in carcinogenesis, progression, and therapy in cervical cancer. Tumour Biol. 2016;37(6):7075–7083. doi:10.1007/s13277-016-5028-8
129. Zhu H, Gu X, Xia L, et al. A novel TGFβ trap blocks chemotherapeutics-induced TGFβ1 signaling and enhances their anticancer activity in gynecologic cancers. Clin Cancer Res. 2018;24(12):2780–2793. doi:10.1158/1078-0432.CCR-17-3112
130. Strauss J, Heery CR, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res. 2018;24(6):1287–1295. doi:10.1158/1078-0432.CCR-17-2653
131. Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer. 2017;76:167–182. doi:10.1016/j.ejca.2017.01.011
132. Chinn Z, Stoler MH, Mills AM. PD-L1 and IDO expression in cervical and vulvar invasive and intraepithelial squamous neoplasias: implications for combination immunotherapy. Histopathology. 2019;74(2):256–268. doi:10.1111/his.13723
133. Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:1–4. doi:10.1186/2051-1426-2-3
134. Jung KH, LoRusso P, Burris H, et al. Phase I study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) administered with PD-L1 inhibitor (atezolizumab) in advanced solid tumors. Clin Cancer Res. 2019;25(11):3220–3228. doi:10.1158/1078-0432.CCR-18-2740
135. Patil NS, Nabet BY, Müller S, et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. 2022;40(3):289–300.e4. doi:10.1016/j.ccell.2022.02.002
136. Roper N, Velez MJ, Chiappori A, et al. Notch signaling and efficacy of PD-1/PD-L1 blockade in relapsed small cell lung cancer. Nat Commun. 2021;12(1):3880. doi:10.1038/s41467-021-24164-y
137. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12(1):54. doi:10.1186/s13045-019-0738-1
138. Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. doi:10.1200/JCO.2017.75.3384
139. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi:10.1126/science.aan6733
140. Łaniewski P, Cui H, Roe DJ, Chase DM, Herbst-Kralovetz MM. Vaginal microbiota, genital inflammation, and neoplasia impact immune checkpoint protein profiles in the cervicovaginal microenvironment. NPJ Precis Oncol. 2020;4:22. doi:10.1038/s41698-020-0126-x
141. Pourmollaei S, Barzegari A, Farshbaf-Khalili A, et al. Anticancer effect of bacteria on cervical cancer: molecular aspects and therapeutic implications. Life Sci. 2020;246:117413. doi:10.1016/j.lfs.2020.117413
142. Meric-Bernstam F, Larkin J, Tabernero J, Bonini C. Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet. 2021;397(10278):1010–1022. doi:10.1016/S0140-6736(20)32598-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.