Back to Journals » Journal of Inflammation Research » Volume 17
Emerging Insights into Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Induced by Immune Checkpoint Inhibitor and Tumor-Targeted Therapy
Authors Lin M , Gong T, Ruan S, Lv X, Chen R, Su X, Cheng B, Ji C
Received 13 December 2023
Accepted for publication 4 April 2024
Published 17 April 2024 Volume 2024:17 Pages 2337—2351
DOI https://doi.org/10.2147/JIR.S454673
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Min Lin,1,* Ting Gong,2,* Shifan Ruan,1,* Xiaoqing Lv,1 Rongying Chen,1 Xinhong Su,1 Bo Cheng,1 Chao Ji1
1Department of Dermatology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, 350000, People’s Republic of China; 2Department of Central Laboratory, The First Affiliated Hospital of Fujian Medical University, Fuzhou, 350000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Cheng; Chao Ji, Department of Dermatology, The First Affiliated Hospital of Fujian Medical University, 20 Chazhong Road, Taijiang District, Fuzhou, 350000, People’s Republic of China, Tel +86 13859024296 ; +86 18651619908, Email [email protected]; [email protected]
Objective: Anticancer drugs have revolutionized tumor therapy, with cutaneous toxicities such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) being common immune-related adverse events. The debate over the efficacy of systemic corticosteroids in treating these conditions persists, while tumor necrosis factor (TNF)-alpha inhibitors show promise. This study aims to evaluate the effectiveness and safety of combination therapy involving the TNF-α inhibitor adalimumab for SJS/TEN induced by anticancer drugs.
Methods: A literature review of SJS/TEN cases induced by anticancer drugs from 1992 to 2023 was conducted, alongside an analysis of patients admitted to the First Affiliated Hospital of Fujian Medical University during the same period. Clinical characteristics, skin healing time, mortality, and adverse events were evaluated in two treatment groups: SJS/TEN patients treated with targeted anticancer therapies and immunotherapies.
Results: Among the 27 patients studied (18 with SJS or SJS-TEN overlapping and 9 with TEN), combination therapy with adalimumab significantly reduced mucocutaneous reepithelization time and healing duration compared to corticosteroid monotherapy. Patients receiving adalimumab combined with corticosteroids had lower actual mortality rates than those on corticosteroid monotherapy. The combination therapy also showed a trend towards reducing standardized mortality rates based on the Score of Toxic Epidermal Necrolysis (SCORTEN).
Conclusion: The findings suggest that adalimumab in combination with corticosteroids provides significant clinical benefits and is safer than corticosteroids alone for treating SJS/TEN induced by targeted anticancer therapies and immunotherapies. This study contributes valuable insights into potential treatment strategies for severe cutaneous adverse reactions to anticancer drugs, highlighting the importance of exploring alternative therapies such as TNF-α inhibitors in managing these conditions effectively.
Keywords: anti-TNF-α, adalimumab, treatment, Stevens-Johnson syndrome, toxic epidermal necrolysis
Introduction
Anticancer drugs, including anticancer chemotherapy, targeted therapy, and immune checkpoint inhibitors (ICIs), represent potent therapeutic options for various cancers. Their approval marked a significant milestone in the history of cancer therapy, sparking a transformative paradigm shift. However, the use of these drugs has been associated with numerous reported cases of adverse reactions. In addition to their antitumor effects, the broad and relative immune activation mechanism of anticancer drugs can lead to an amplified immune response against natural cellular functions, resulting in a spectrum of autoimmune-like toxicities known as immune-related adverse events (irAEs). Dermatologic irAEs are the most commonly reported and often occur early in the course of treatment. It has been reported that up to 30 to 50% of patients treated with anticancer drugs have experienced adverse dermatologic reactions.1–3 Fortunately, these cutaneous irAEs are typically mild and often do not necessitate a discontinuation of immunotherapy. Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) are rare complications associated with anticancer drug therapy. However, they can result in substantial mortality and severe long-term effects, significantly reducing the quality of life for affected patients.4–9
While the precise biological mechanisms driving SJS/TEN remain incompletely understood, this process may entail the hyperactivation of CD8+ cytotoxic T lymphocytes and the ensuing occurrence of cytokine storms.10,11 Elevated concentrations of granulysin, a cytolytic protein, and tumor necrosis factor (TNF)-α, an inflammatory cytokine, are detectable in the plasma and blister fluids of afflicted individuals.12 Currently, alongside the administration of supportive care, the standard initial therapeutic modalities for SJS and TEN involve the use of systemic corticosteroids, intravenous immunoglobulin, and cyclosporine.13,14 However, the use of systemic corticosteroids remains a subject of ongoing debate and analysis within the medical community, with continuous evaluation of their risks and benefits.15,16 The utilization of systemic corticosteroids remains a contentious topic, evoking ongoing discussion and analysis within the medical community as their risks and benefits are subject to continuous evaluation. Meanwhile, emerging as a potential beacon of promise, tumor necrosis factor-α (TNF-α) inhibitors come to the forefront, offering a noteworthy avenue of exploration and investigation due to their prospective effectiveness.17,18 While systemic corticosteroids are commonly used in the treatment of SJS/TEN, the exploration of combination therapy, which involves the additional use of TNF-α inhibitors and other biologic agents, presents itself as a potential strategy. This approach may provide a better-tolerated and more effective method for halting disease progression and reducing corticosteroid-related adverse events, especially in patients with more severe and rapidly progressing clinical manifestations.18,19 In our recent randomized controlled study, which compared adalimumab and systemic steroids, we obtained findings indicating that TNF-α inhibitors may confer potential benefits in the management of SJS and TEN. These results contribute to the growing body of evidence supporting the efficacy of TNF-α inhibitors in this context.8
The progress made in cancer detection and the evolution of anticancer drug therapy have contributed to a rising occurrence of cutaneous adverse reactions following the administration of anticancer medications.20 Patients afflicted with malignancies commonly present with concurrent comorbidities, multi-systemic involvement, and are subjected to multiple concomitant medications and treatment modalities.21,22 These factors collectively contribute to the heightened intricacies and challenges encountered in the management of SJS and TEN within the context of cancer patients. Hence, it is imperative to assess the therapeutic advantages of corticosteroid monotherapy and the combination of TNF-α inhibitors, with the aim of investigating whether anti-TNF-α and corticosteroid combination therapy could expedite the treatment of patients afflicted by SJS and TEN. In this study, we reviewed a literature review on SJS/TEN induced by targeted anticancer therapies and immunotherapies spanning the years 1992 to 2023. Simultaneously, we conducted a meticulous analysis of patients who were diagnosed with SJS/TEN s induced by anticancer drugs and received care at the First Affiliated Hospital of Fujian Medical University during the same period. We conducted a retrospective analysis of patients with SJS and TEN who received treatment involving adalimumab in conjunction with systemic corticosteroids, in comparison to those treated with systemic corticosteroids as monotherapy.
Methods
Patients
We reviewed cases of SJS/TEN induced by anticancer drugs from PubMed, EMBASE, Web of Science, SCOPUS, and OVID from 1992 to September 2023 using the search terms “Stevens-Johnson syndrome”, “toxic epidermal necrolysis”, “anticancer targeted therapy” and “immune checkpoint inhibitors”.23–88 We included primary case reports, case series, reports from clinical trials, and post marketing surveillance data. Histopathological diagnosis of SJS/TEN was not mandatory for inclusion. We excluded cases involving multiple concomitant medications used during the same period and those with questionable diagnoses.
In addition, we conducted a retrospective analysis by reviewing the registration database of SJS/TEN patients who were admitted to the First Affiliated Hospital of Fujian Medical University (FJMU) in mainland China. The diagnosis of SJS/TEN was based on the RegiSCAR criteria, including a characteristic progressive blistering exanthema of purpuric macules and targetoid lesions with mucosal involvement and skin detachment (Supplemental Figure 1).89,90 The classification criteria for the severity of skin detachment were as follows: SJS was characterized by epidermal detachment affecting less than 10% of the total body surface area, while SJS/TEN overlap syndrome and TEN were defined by epidermal detachment involving between 10% and 30%, and greater than 30% of the total body surface area, respectively.91 In the course of this study, our cohort encompassed patients diagnosed with SJS/TEN who were subjected to either solitary systemic corticosteroid treatment or a combination regimen with adalimumab and systemic corticosteroids. Notably, adalimumab was introduced as a supplementary therapeutic approach for individuals manifesting ongoing symptoms despite their initial systemic corticosteroid therapy.
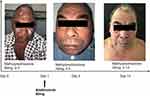 |
Figure 1 Representative photographs illustrate disease progression. A patient with TEN before (day 0) and after (day 14) the combination therapy. |
The population study was carried out following the principles stated in the Declaration of Helsinki and received approval from the Ethics Committee of the First Affiliated Hospital of Fujian Medical University, Fuzhou, China, in accordance with Chinese law (no. [2021]261). Written informed consent was obtained from each study subject before their inclusion into the study.
Clinical Data Collection of the SJS/TEN Patients Induced by Anticancer Drugs in Our Hospital
The drug-related causality for the enrolled patients was assessed employing the ALDEN algorithm, with only those cases deemed as having a probable or definite causality (ALDEN score of 4 or higher) being included in the category of drug-induced SJS/TEN (Supplemental Table 1).92 Patient demographics, encompassing age and gender, as well as clinical variables such as the extent of skin detachment measured in total body surface area (TBSA), causative medications, prior history of malignancy, presence of mucosal involvement, the calculation of the Score of Toxic Epidermal Necrolysis (SCORTEN) (Supplemental Table 2), laboratory parameters, concurrent complications, the average corticosteroid dosage, the administered treatment modalities, and recorded mortality rates were systematically extracted from the patients’ medical records. All patients included in this study received systemic corticosteroid treatment, specifically methylprednisolone, with an initial dose ranging from 1 to 1.5 mg/kg/day. Additionally, patients were provided with symptomatic supportive care, which encompassed wound management, pain relief, intravenous fluid administration, nutritional evaluation, and electrolyte regulation. The corticosteroid dosages were quantified and standardized by converting them into methylprednisolone-equivalent doses for uniformity and ease of comparison. Upon the observation of reepithelization, a gradual reduction in the dosage of methylprednisolone was initiated. The specific dosage and tapering schedule of methylprednisolone were individualized based on the progress of reepithelization, as well as the presence of comorbidities and complications in the patients, including conditions such as diabetes, hypertension, and infections. Furthermore, we assessed the timing of therapeutic interventions, specifically the introduction of corticosteroids and adalimumab. This was quantified by calculating the duration between the first administration of adalimumab and the index day, which represents the onset date of SJS/TEN. Additionally, we documented the time taken to initiate systemic corticosteroids in both groups, measured as the elapsed time between the first corticosteroid administration and the index day.
Clinical Outcomes and Complications of the SJS/TEN Patients Induced by Anticancer Drugs in Our Hospital
The time to reepithelization (days) was defined as the period from the commencement of treatment until the absence of skin detachment and erosions, as indicated by the absence of a positive Nikolsky’s sign. Simultaneously, it marked the initiation of skin islands’ regeneration to replace the damaged superficial epithelia in localized skin or mucous lesions. Time to complete pain relief (days) was defined by assessing the level of pain through daily Visual Analog Scale (VAS) scores. VAS scores ranged from 0 (indicating no pain) to 10 (representing insupportable pain). The time to achieve a VAS score of zero, along with the highest recorded VAS score for each patient, was employed to gauge the duration of complete pain relief. The time to skin healing (days) was established as the duration spanning from the initiation of treatment to the point when complete reepithelization became visible in all skin lesions. The time to mucous healing (days) was defined as the duration from the commencement of treatment to the stage when full reepithelization could be discerned in all mucous lesions, encompassing those affecting the eye, oral, and genital mucous membranes. During the hospitalization period, vigilant monitoring was conducted to assess and address common adverse effects resulting from the administered treatments. During the hospitalization period, vigilant monitoring was conducted to assess and address common adverse effects resulting from the administered treatments. These effects encompassed elevated blood pressure, increased blood glucose levels, gastrointestinal bleeding, electrolyte imbalances, and mucocutaneous infections. A comprehensive follow-up process was implemented for all patients in both treatment groups, spanning a duration of 2 weeks at least, to track and manage these associated adverse effects. The observed mortality rate was determined by tallying the total number of documented deaths within the patient cohort. The Standardized Mortality Rate (SMR) was calculated by dividing the cumulative count of observed deaths by the anticipated deaths, which were estimated based on SCORTEN scores within each respective group. To evaluate the severity and duration of skin healing during hospitalization, photographs of all enrolled patients or data sourced from the registration database were procured for assessment.
Statistical Analysis
Different statistical methods were used to analyze the results. The data are presented as mean ± standard deviation (SD). Continuous variables are presented as both the mean ± standard deviation and medians with interquartile ranges. Proportions between groups were compared with chi-square. Student’s t-test and Mann–Whitney-Wilcoxon nonparametric test were used to evaluate the statistical differences among the groups. All P values are two-tailed, and values less than 0.05 were considered statistically significant. GraphPad Prism 9 software (GraphPad Software Inc., San Diego, CA, U.S.A.). ImageJ software (Version 1.47, National Institutes of Health, Bethesda, MD, USA) was used to quantify the optical density (pixels/mm2) or the intensity of images.
Results
Characteristics of the Included Studies
Between 1992 and 2023, 135 cases of SJS/TEN were collected from the database. Additionally, we analyzed the admission database from the First Affiliated Hospital of Fujian Medical University. 112 cases met our inclusion criteria, including 78 cases from the literature and 34 cases from the hospital. Among them, 58 (50.89%) were diagnosed with SJS, 8 (7.15%) with SJS-TEN, and 46 (41.96%) with TEN. The demographic and characteristics are summarized in Table 1. The age at the onset of SJS/TEN ranged from 22 to 86 years. The mean age for all SJS/TEN cases was over 55 years, with SJS at 59.55 years, SJS-TEN at 61.38 years, and TEN at 60.14 years. The majority of the patients are male (62.5% vs 37.5%). In these groups, there is no difference in tumor metastasis. The onset of epidermal necrolysis can range from 1 day to 2 years. The average disease incubation period is 43.00±82.16 days. The majority of the scholarly literature on SJS/TEN induced by anticancer drugs originates from the Asian region. All deceased patients were TEN (p<0.0001).
 |
Table 1 Demographic and Clinical Characteristics of the SJS/TEN Patients Induced by Targeted Anticancer Therapies and Immunotherapies |
Culprit Drugs and Tumor Type
A total of 112 patients with SJS (n = 58), SJS/TEN (n = 8), or TEN (n = 46), associated with 14 targeted anticancer drugs, were identified. These drugs included PD-1 inhibitors (pembrolizumab, nivolumab, camrelizumab, sintilimab, tislelizumab, serplulimab, treprinumab, pralsetinib), CTLA4 inhibitors (ipilimumab), recombinant IL-2, KIT and BCR-ABL inhibitors, EGFR inhibitors, BRAF inhibitor, anti-CD20, anti-CD30, and others (Table 2). Imatinib, pembrolizumab and nivolumab were the top three medications most commonly associated with SJS/TEN, with PD-1 inhibitors being the drug group that most frequently induced SJS/TEN. In the PD-1 inhibitor category, pembrolizumab and nivolumab constitute the majority, accounting for 28.89% and 24.44%, respectively, followed by camrelizumab at 15.56%. The most frequently associated cancer types were melanoma (n=21), lung cancer (n=20), and chronic myelogenous leukemia (n=54). Other malignancies (n=19) included gastrointestinal tumors, liver cancer, Lymphoma, squamous cell carcinoma, renal cell carcinoma, myeloma, nasopharyngeal carcinoma, prostate cancer, malignant tumor of the tonsil, glioblastoma, urothelium carcinoma, breast carcinoma (Supplemental Table 3). Lymphoma patients are all SJS. However, overall, no trends were observed between tumor and rash type. Furthermore, we also analyzed the tolerability follow-up for rechallenge or alternative treatments in patients with severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapy (Supplemental Table 4). These patients still develop rashes after rechallenging with suspected allergenic drugs.
 |
Table 2 Culprit Drugs of the SJS/TEN Patients Induced by Targeted Anticancer Therapies and Immunotherapies |
Demographic Data, Characteristics and Culprit Drugs of the SJS/TEN Patients Induced by Anticancer Drugs in Our Hospital
This study included 2 clinical groups with a total of 27 SJS/TEN patients. Notably, 13 of these patients (48.1%) were administered methylprednisolone as a monotherapeutic intervention, with an average dosage of 0.54±0.21 mg/kg/day. In addition, 14 SJS/TEN patients (51.9%) were treated with a biologic TNF-α inhibitor (adalimumab) at a dose of 40 or 80 mg every two weeks combined with systemic corticosteroids (0.64±0.20 mg/kg/d). In this study, corticosteroids were initially introduced through intravenous infusion for the enrolled cases, following which the dosages were gradually transitioned to oral formulations, specifically methylprednisolone or prednisone. The study protocol and participants’ disposition in this study are shown in Supplemental Figure 2 and Table 3. The mean age of the patients in the combination therapy group was 59.64±19.49 years and 61.46±15.77 years the conventional therapy group (P=0.737) and slight male predominance. The analysis revealed noteworthy distinctions between patients who underwent corticosteroid monotherapy and those who received a combination of corticosteroids and adalimumab therapies. Specifically, lower SCORTEN scores, fewer sites exhibiting mucous erosion, and a reduced average percentage of TBSA denuded were observed in the former group, as detailed in Table 3. The median score of toxic epidermal necrolysis (SCORTEN) in the patients of the two groups was 3 (IQR: 2.25–3), and 2 (IQR: 2–3), respectively (P=0.204). Two groups were well matched at baseline for clinical and demographic characteristics.
 |
Table 3 Demographic and Clinical Characteristics of the Patients in Two Groups |
The potential drugs responsible for adverse effects within these two groups are detailed in (Supplemental Table 5). We classified causality into five distinct groups. Notably, PD-1 inhibitors emerged as the most prominent culprits, accounting for 63% of the identified cases. Within the PD-1 inhibitor category, Pembrolizumab and camrelizumab constituted the majority, contributing 23.53% each, followed by Sintilimab at 17.65%. Among the monoclonal antibodies targeting CD20, Rituximab was identified as the culprit drug.
Assessment of Clinical Outcomes of the SJS/TEN Patients Induced by Anticancer Drugs in Our Hospital
In the context of this study, an analysis was conducted to assess the timeframes necessary for reepithelization, mucous membrane healing, complete skin healing, and the attainment of complete pain relief among the subset of patients who survived the condition of SJS/TEN in both groups (n = 22). The median time to reepithelization was 6.0 days (IQR: 5.0–7.0) and 7.5 days (IQR: 5.0–9.0) in groups 1 and 2, respectively (Table 4). The median time to skin and mucous healing in patients receiving combination therapy (17.5 days, 19.0 days) was markedly shorter than in patients treated with conventional therapy (20.0 days, 22.0 days). Combination therapy significantly shortened the time to skin (P=0.043) and mucous healing (P=0.037) in comparison with conventional therapy (P=0.019). Moreover, the median time to obtain complete pain relief in the patients was 15.0 days (IQR: 13.5–15.0) and 17.5 days (IQR: 15.5–19.8) in the two groups, respectively (P=0.018). It is worth noting that significantly shorter corticosteroid tapering courses (P=0.039). However, the initial dosage of methylprednisolone and the total dosage of methylprednisolone (mg/kg) were no differences. Representative patient photographs showing disease progression before and after the combination therapy were shown in Figure 1.
 |
Table 4 Clinical Outcomes, Methylprednisolone Use, and Common Adverse Events of SJS/TEN Patients in Two Cohorts |
Assessment of Safety and Mortality Rate of the SJS/TEN Patients Induced by Anticancer Drugs in Our Hospital
The incidence of common adverse events associated with systemic corticosteroid administration, encompassing hypertension, hyperglycemia, gastrointestinal (GI) bleeding, electrolyte disturbances, and mucocutaneous infections, was examined within the two treatment groups (Supplemental Table 6). The group receiving combination therapy exhibited a significantly lower occurrence of electrolyte disturbances (P=0.034) when contrasted with the conventional therapy group. Notably, no statistically significant disparities were observed between the two groups in terms of elevated blood pressure (P=0.303), elevated blood glucose levels (P=0.686), GI hemorrhage (P=0.957), and mucocutaneous infections (P=0.580).
To assess survival rates, we conducted an analysis involving the observed mortality rate and the Standardized Mortality Ratio (SMR) adjusted for the severity of SJS/TEN (Table 5). The SCORTEN-predicted mortality rates for the treatment groups receiving adalimumab in combination with corticosteroids and corticosteroid monotherapy were 0.31% and 0.24%, respectively. It is noteworthy that in all groups, the actual mortality rates were lower than those predicted by SCORTEN. Furthermore, the SMR for the treatments among adalimumab combined with corticosteroids and monotherapy corticosteroid treatment groups were 0.96 and 0.46, respectively. Throughout the study duration, five patients succumbed to SJS/TEN, with three of them having undergone corticosteroid monotherapy and the remaining two having received adalimumab in combination with corticosteroids. The causes of death among the 5 patients are listed in Supplemental Table 7. The deceased patients died owing to pulmonary fungal infection, sepsis, infectious shock, cardiopulmonary failure respiratory failure and infectious shock.
 |
Table 5 SCORTEN, Expected Predicted Mortality Rates, Actual Mortality Rate, and SMR of SJS/TEN Patients in Two Groups |
Discussion
Targeted anticancer therapies and immunotherapies, representing a potent new category of pharmaceutical agents, have emerged as pivotal treatment modalities for a variety of cancer types. Their approval marked a profound and transformative epoch in the landscape of cancer therapy. Nevertheless, these innovative treatments have been accompanied by a significant number of reported adverse reactions and side effects. Patients who develop SJS/TEN due to targeted anticancer therapies and immunotherapies appear to experience a lower quality of life compared to non-oncological SJS/TEN patients, as they often exhibit poor treatment compliance, reduced treatment continuity, and heightened levels of psychosocial distress stemming from the skin toxicity.93
In this study, we enrolled a total 112 patients diagnosed with SJS/TEN induced by anticancer drugs from a tertiary medical center and literatures during 1992 to 2023. We evaluated underlying condition, causation, treatment, and clinical outcome. Mean age of SJS/TEN was 60.14 years. The average age of SJS/TEN is 60.14 years. This result is inconsistent with previous reports of SJS/TEN caused by non-targeted drugs, where the average age was 43 years.94 SJS/TEN exhibited a male predominance (male-to-female ratio 1.67: 1). This observation was different from an earlier study which showed equally affected by male and female.95 The average incubation period of the disease is 43 days, which is different from SJS/TEN caused by non-anticancer drugs, where the average incubation period is 4–20 days. There is speculation in some studies that the interference with checkpoint molecules may disrupt the delicate balance between peripheral tolerance and the protective role of keratinocytes against damage.96 This may result from the difference in the incubation period of SJS/TEN induced by anticancer drugs compared to that caused by non-anticancer drugs.
In some studies, it has been observed immunotherapy-induced SJS/TEN exhibit a gene expression profile similar to classical SJS/TEN. Both conditions exhibit elevated expression of inflammatory chemokines, cytotoxic mediators like perforin and granzyme B, and apoptosis-promoting molecules such as Fas Ligand.96 Especially, TNF-α has been identified in the plasma and blister fluids of patients affected by SJS/TEN. It appears to act as a potential inducer of keratinocyte apoptosis in the context of SJS/TEN.17,97,98 Despite extensive research efforts over the years, the molecular mechanisms underlying SJS/TEN remain incompletely elucidated, and a universally accepted “gold standard” for their treatment has yet to be established.
Currently, the common treatments include supportive care, systemic corticosteroids, immunoglobulins, cyclosporine A, plasmapheresis and supportive care. Nevertheless, the clinical efficacy of these therapeutic approaches continues to be a subject of debate.10,99,100 Furthermore, prior investigations have reported an elevated risk of mortality in SJS/TEN among patients administered higher doses of methylprednisolone.101 Combination therapy involving immunosuppressants is a frequently employed strategy in the clinical management of immune-related or autoimmune disorders. The concurrent application of treatments with distinct and complementary mechanisms of action holds the potential to augment therapeutic efficacy while mitigating associated toxicities.102,103 There is a growing body of evidence supporting the use of TNF-α blockade in the treatment of patients with SJS/TEN.8,104 To date, there have been several case reports as well as case-control and randomized control studies examining the use of adalimumab in the treatment of SJS/TEN. However, there has been no investigation to date that has assessed the potential advantages of a combined therapy involving anti-TNF-α agents and systemic corticosteroids for patients suffering from SJS/TEN induced by targeted anticancer therapies and immunotherapies.
Additionally, our group conducted an observational retrospective study to evaluate the efficacy of adalimumab combined with steroids for SJS/TEN. Compared with steroid monotherapy, we found that additional adalimumab to the steroid treatment could significantly reduce the time of skin healing as well as the incidence of adverse events.8 Despite this, there is still limited knowledge on the effects of adalimumab treatment in SJS/TEN induced by targeted anticancer therapies and immunotherapies patients.
In this study, the skin healing time of patients with SJS/TEN induced by targeted anticancer therapies and immunotherapies was significantly reduced when treated with a combination of Adalimumab and systemic corticosteroids. Notably, the majority of cases in the combination therapy group involving adalimumab achieved healing within 18 days, indicating the potential advantages of using adalimumab over corticosteroids for skin healing in the context of SJS/TEN, which was consistent with the previous studies.8,18 In addition, a notably lower incidence of electrolyte disturbance complications was observed among patients who received combination therapy involving adalimumab and systemic corticosteroids. Combining treatments with distinct and complementary mechanisms of action has the potential to augment therapeutic efficacy while simultaneously diminishing the associated toxicity.105–107 The adoption of combination therapy involving anti-TNF-α biologics and systemic corticosteroids has the potential to amplify the anti-inflammatory impact and, in turn, mitigate the adverse effects associated with systemic corticosteroid treatment.
Most importantly, our investigation revealed that within all groups, the observed mortality rates were lower than the projected rates derived from SCORTEN scoring. Notably, patients treated with adalimumab in conjunction with systemic corticosteroids exhibited an even lower mortality rate (0.14% compared to 0.23%). Furthermore, the SMR within the group receiving adalimumab in combination with systemic corticosteroids was notably lower than that observed in patients subjected to corticosteroid monotherapy (0.46 compared to 0.96). Despite the combined therapy groups, particularly those involving adalimumab combined with corticosteroids, having higher average SCORTEN scores in comparison to the systemic corticosteroid monotherapy group, the actual mortality rates within the combined treatment groups were lower than those within the corticosteroid monotherapy group. In the context of this study, adalimumab was administered as an adjunctive therapy to patients whose conditions exhibited progression despite systemic corticosteroid treatment. Notably, these patients still achieved a notably elevated survival rate, implying that the early utilization of anti-TNF-α biologics, such as adalimumab, in conjunction with systemic corticosteroids during the acute or, at the very least, the most advanced stages of SJS/TEN induced by targeted anticancer therapies and immunotherapies could potentially yield more favorable treatment outcomes.
The therapeutic efficacy of anti-TNF-α biologics, exemplified by agents like adalimumab, extends beyond the mere blockade of the inflammatory cytotoxic pathway through the targeting of TNF-α. It also involves the stimulation of regulatory T cell generation within the peripheral blood.17 Furthermore, adalimumab is administered via a subcutaneous route at a singular dose in the treatment of SJS/TEN, rendering it a less frequent and safer alternative when compared to other TNF-α inhibitors.17,108,109 In the context of the present study, the inclusion of adalimumab demonstrated a significant capability to expedite the recovery of skin lesions, arrest disease progression, and mitigate the occurrence of diverse adverse events associated with steroid administration.
Nonetheless, this retrospective study is subject to several limitations. Firstly, the study is constrained by the relatively small number of cases included in the analysis. Secondly, these findings are derived from retrospective data, which inherently carry the potential for confounding variables. Additionally, it’s important to note that the results may primarily apply to Asian patients, and further validation is necessary to ascertain their applicability to patient populations from different geographic regions. Subsequent investigations involving larger sample sizes from multiple healthcare centers across various countries are imperative to thoroughly assess the potential advantages associated with the combination therapy employing anti-TNF-α biologics in conjunction with systemic corticosteroids for patients with SJS/TEN induced by targeted anticancer therapies and immunotherapies. Finally, the mechanism between immune checkpoint inhibitor and tumor-targeted therapy inducing SJS/TEN may be different, which needs to be further investigated.
Conclusion
Our study has contributed fresh insights into the utilization of a combined therapeutic approach involving anti-TNF-α biologics and systemic corticosteroids for the management of SJS/TEN patients induced by targeted anticancer therapies and immunotherapies. Notably, this combination therapy demonstrated superior effectiveness in expediting cutaneous healing and reducing mortality rates among SJS/TEN patients. The combination treatment holds the potential to mitigate the occurrence of common adverse events associated with the administration of higher doses of systemic corticosteroids in the context of SJS/TEN.
Data Sharing Statement
Data presented in this study are available on request from the corresponding author.
Ethics Approval and Consent to Participate
The population study was carried out following the principles stated in the Declaration of Helsinki and received approval from the Ethics Committee of the First Affiliated Hospital of Fujian Medical University, Fuzhou, China, in accordance with Chinese law (no. [2021] 261). Written informed consent was obtained from each study subject before their inclusion into the study. The patient in Figure 1 provided the written informed consent for the images to be published.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
1. Fujian Provincial Health Technology Project (No. 2020CXA034), 2. Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2020Y9120), 3. Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2021Y9150), 4. Startup Fund for scientific research, Fujian Medical University (No. 2020QH2029).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560–575. doi:10.3978/j.issn.2218-6751.2015.06.06
2. Chirasuthat P, Chayavichitsilp P. Atezolizumab-induced Stevens-Johnson syndrome in a patient with non-small cell lung carcinoma. Case Rep Dermatol. 2018;10(2):198–202. doi:10.1159/000492172
3. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346–1353. doi:10.1001/jamaoncol.2016.1051
4. Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, Litvinov IV. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: a dermatology perspective on management [Formula: see text]. J Cutan Med Surg. 2021;25(1):59–76. doi:10.1177/1203475420943260
5. Zhu J, Chen G, He Z, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: a safety analysis of clinical trials and FDA pharmacovigilance database. EClinicalMedicine. 2021;37:100951. doi:10.1016/j.eclinm.2021.100951
6. Chiu YM, Chiu HY. Lifetime risk, life expectancy, loss-of-life expectancy, and lifetime healthcare expenditure for Stevens-Johnson syndrome/toxic epidermal necrolysis in Taiwan: follow-up of a nationwide cohort from 2008 to 2019. Br J Dermatol. 2023;189:553–560. doi:10.1093/bjd/ljad234
7. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau J-C. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92–96. doi:10.1001/archderm.1993.01680220104023
8. Gong T, Zhang P, Ruan S-F, et al. APOA4 as a novel predictor of prognosis in Stevens-Johnson syndrome/toxic epidermal necrolysis: a proteomics analysis from two prospective cohorts. J Am Acad Dermatol. 2023;89(1):45–52. doi:10.1016/j.jaad.2023.02.058
9. Diphoorn J, Cazzaniga S, Gamba C, et al. Incidence, causative factors and mortality rates of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in northern Italy: data from the REACT registry. Pharmacoepidemiol Drug Saf. 2016;25(2):196–203. doi:10.1002/pds.3937
10. Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. 2018;54(1):147–176. doi:10.1007/s12016-017-8654-z
11. Gibson A, Deshpande P, Campbell CN, et al. Updates on the immunopathology and genomics of severe cutaneous adverse drug reactions. J Allergy Clin Immunol. 2023;151(2):289–300.e4. doi:10.1016/j.jaci.2022.12.005
12. Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343–1350. doi:10.1038/nm.1884
13. Law EH, Leung M. Corticosteroids in Stevens-Johnson Syndrome/toxic epidermal necrolysis: current evidence and implications for future research. Ann Pharmacother. 2015;49(3):335–342. doi:10.1177/1060028014560012
14. Yamane Y, Matsukura S, Watanabe Y, et al. Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients--treatment and outcome. Allergol Int. 2016;65(1):74–81. doi:10.1016/j.alit.2015.09.001
15. Hirahara K, Kano Y, Sato Y, et al. Methylprednisolone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis: clinical evaluation and analysis of biomarkers. J Am Acad Dermatol. 2013;69(3):496–498. doi:10.1016/j.jaad.2013.04.007
16. Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157(10):1182–1190. doi:10.1001/jamadermatol.2021.3154
17. Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3):985–996. doi:10.1172/JCI93349
18. Zhang J, Lu CW, Chen CB, et al. Evaluation of combination therapy with etanercept and systemic corticosteroids for Stevens-Johnson syndrome and toxic epidermal necrolysis: a multicenter observational study. J Allergy Clin Immunol Pract. 2022;10(5):1295–1304.e6. doi:10.1016/j.jaip.2022.01.038
19. Chang HC, Wang TJ, Lin MH, Chen TJ. A review of the systemic treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Biomedicines. 2022;10:9. doi:10.3390/biomedicines10092105
20. Zhang J, Zhang P, Xu QY, Zhu YT, Chen W, Ji C. Pembrolizumab associated Stevens-Johnson syndrome with porokeratosis in a patient in the setting of primary hepatocellular carcinoma. Australas J Dermatol. 2022;63(1):e71–e74. doi:10.1111/ajd.13704
21. Héritier S, Emile JF, Barkaoui MA, et al. BRAF mutation correlates with high-risk Langerhans cell histiocytosis and increased resistance to first-line therapy. J Clin Oncol. 2016;34(25):3023–3030. doi:10.1200/JCO.2015.65.9508
22. Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–255. doi:10.1093/annonc/mdx642
23. Honda Y, Hattori Y, Katsura S, et al. Stevens-Johnson syndrome-like erosive dermatitis possibly related to Afatinib. Eur J Dermatol. 2016;26(4):413–414. doi:10.1684/ejd.2016.2807
24. Doesch J, Debus D, Meyer C, et al. Afatinib-associated Stevens-Johnson syndrome in an EGFR-mutated lung cancer patient. Lung Cancer. 2016;95:35–38. doi:10.1016/j.lungcan.2016.02.015
25. Lee SS, Chu PY. Toxic epidermal necrolysis caused by cetuximab plus minocycline in head and neck cancer. Am J Otolaryngol. 2010;31(4):288–290. doi:10.1016/j.amjoto.2009.02.021
26. Pantano F, Silletta M, Iovieno A, et al. Stevens-Johnson syndrome associated with reduced tear production complicating the use of cetuximab and panitunumab. Int J Colorectal Dis. 2009;24(10):1247–1248. doi:10.1007/s00384-009-0676-4
27. Urosevic-Maiwald M, Harr T, French LE, Dummer R. Stevens-Johnson syndrome and toxic epidermal necrolysis overlap in a patient receiving cetuximab and radiotherapy for head and neck cancer. Int J Dermatol. 2012;51(7):864–867. doi:10.1111/j.1365-4632.2011.05356.x
28. Lin WL, Lin WC, Yang JY, et al. Fatal toxic epidermal necrolysis associated with cetuximab in a patient with colon cancer. J Clin Oncol. 2008;26(16):2779–2780. doi:10.1200/JCO.2007.15.7883
29. Huang JJ, Ma SX, Hou X, et al. Toxic epidermal necrolysis related to AP (pemetrexed plus cisplatin) and gefitinib combination therapy in a patient with metastatic non-small cell lung cancer. Chin J Cancer. 2015;34(2):94–98. doi:10.5732/cjc.014.10151
30. Wnorowski AM, de Souza A, Chachoua A, Cohen DE. The management of EGFR inhibitor adverse events: a case series and treatment paradigm. Int J Dermatol. 2012;51(2):223–232. doi:10.1111/j.1365-4632.2011.05082.x
31. Ladizinski B, Sankey C. A topical matter: toxic epidermal necrolysis. Am J Med. 2014;127(10):931–932. doi:10.1016/j.amjmed.2014.06.013
32. Yoon J, Oh CW, Kim CY. Stevens-Johnson syndrome induced by vandetanib. Ann Dermatol. 2011;23(Suppl 3):S343–5. doi:10.5021/ad.2011.23.S3.S343
33. Bois E, Holle LM, Farooq U. Late onset imatinib-induced Stevens-Johnson syndrome. J Oncol Pharm Pract. 2014;20(6):476–478. doi:10.1177/1078155213518226
34. Severino G, Chillotti C, De Lisa R, Del Zompo M, Ardau R. Adverse reactions during imatinib and lansoprazole treatment in gastrointestinal stromal tumors. Ann Pharmacother. 2005;39(1):162–164. doi:10.1345/aph.1E127
35. Rule SA, O’Brien SG, Crossman LC. Managing cutaneous reactions to imatinib therapy. Blood. 2002;100(9):3434–3435. doi:10.1182/blood-2002-08-2431
36. Hsiao LT, Chung HM, Lin JT, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002;117(3):620–622. doi:10.1046/j.1365-2141.2002.03499.x
37. Hsieh HJ, Chan AL, Lin SJ. Stevens-Johnson syndrome induced by combination of imatinib and allopurinol. Chemotherapy. 2009;55(4):197–199. doi:10.1159/000218097
38. Jha P, Himanshu D, Jain N, Singh AK. Imatinib-induced Stevens-Johnson syndrome. BMJ Case Rep. 2013;2013:1.
39. Schaich M, Schäkel K, Illmer T, Ehninger G, Bornhäuser M. Severe epidermal necrolysis after treatment with imatinib and consecutive allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2003;82(5):303–304. doi:10.1007/s00277-003-0643-z
40. Mahapatra M, Mishra P, Kumar R. Imatinib-induced Stevens-Johnson syndrome: recurrence after re-challenge with a lower dose. Ann Hematol. 2007;86(7):537–538. doi:10.1007/s00277-007-0265-y
41. Pavithran K, Thomas M. Imatinib induced Stevens-Johnson syndrome: lack of recurrence following re-challenge with a lower dose. Indian J Dermatol Venereol Leprol. 2005;71(4):288–289. doi:10.4103/0378-6323.16628
42. Sanchez-Gonzalez B, Pascual-Ramirez JC, Fernandez-Abellan P, Belinchon-Romero I, Rivas C, Vegara-Aguilera G. Severe skin reaction to imatinib in a case of Philadelphia-positive acute lymphoblastic leukemia. Blood. 2003;101(6):2446. doi:10.1182/blood-2002-12-3696
43. O’Brien SG, Rule SA. Position paper on imatinib mesylate in chronic myeloid leukaemia. Br J Haematol. 2002;119(1):268–272. doi:10.1046/j.1365-2141.2002.39201.x
44. Sohn KH, Oh SY, Lim KW, Kim MY, Lee SY, Kang HR. Sorafenib induces delayed-onset cutaneous hypersensitivity: a case series. Allergy Asthma Immunol Res. 2015;7(3):304–307. doi:10.4168/aair.2015.7.3.304
45. Choi MK, Woo HY, Heo J, et al. Toxic epidermal necrolysis associated with sorafenib and tosufloxacin in a patient with hepatocellular carcinoma. Ann Dermatol. 2011;23(Suppl 3):S404–7. doi:10.5021/ad.2011.23.S3.S404
46. Ikeda M, Fujita T, Amoh Y, Mii S, Matsumoto K, Iwamura M. Stevens-Johnson syndrome induced by sorafenib for metastatic renal cell carcinoma. Urol Int. 2013;91(4):482–483. doi:10.1159/000351918
47. Fang B, Song Y, Ma J, Zhao RC. Severe epidermal necrolysis after bortezomib treatment for multiple myeloma. Acta Haematol. 2007;118(2):65–67. doi:10.1159/000102604
48. Rui X, Meidan W, Gongqiang W, et al. Chemotherapy-induced toxic epidermal necrolysis in a patient with multiple myeloma, a case report and literature review. Front Oncol. 2023;13:1227448. doi:10.3389/fonc.2023.1227448
49. Castaneda CP, Brandenburg NA, Bwire R, Burton GH, Zeldis JB. Erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis in lenalidomide-treated patients. J Clin Oncol. 2009;27(1):156–157. doi:10.1200/JCO.2008.20.3737
50. Lowndes S, Darby A, Mead G, Lister A. Stevens-Johnson syndrome after treatment with rituximab. Ann Oncol. 2002;13(12):1948–1950. doi:10.1093/annonc/mdf350
51. Fallon MJ, Heck JN. Fatal Stevens-Johnson syndrome/toxic epidermal necrolysis induced by allopurinol-rituximab-bendamustine therapy. J Oncol Pharm Pract. 2015;21(5):388–392. doi:10.1177/1078155214533368
52. Parise L, Kahle J, Schlaak M, Mauch C, Kurschat P. Reply to Rituxan is not associated with Stevens-Johnson syndrome. Ann Oncol. 2012;23(3):807. doi:10.1093/annonc/mdr629
53. Del Principe MI, Sconocchia G, Buccisano F, et al. Extensive toxic epidermal necrolysis following brentuximab vedotin administration. Ann Hematol. 2015;94(2):355–356. doi:10.1007/s00277-014-2148-3
54. Kılıç S, Özkaya E, Baykal C, Vatansever S. Vemurafenib-induced toxic epidermal necrolysis: is it an emerging side-effect of the drug? J Eur Acad Dermatol Venereol. 2017;31(8):e354–e355. doi:10.1111/jdv.14150
55. Minor DR, Rodvien R, Kashani-Sabet M. Successful desensitization in a case of Stevens-Johnson syndrome due to vemurafenib. Melanoma Res. 2012;22(5):410–411. doi:10.1097/CMR.0b013e3283573437
56. Bellón T, Lerma V, González-Valle O, González Herrada C, de Abajo FJ. Vemurafenib-induced toxic epidermal necrolysis: possible cross-reactivity with other sulfonamide compounds. Br J Dermatol. 2016;174(3):621–624. doi:10.1111/bjd.14201
57. Arenbergerova M, Mrazova I, Horazdovsky J, Sticova E, Fialova A, Arenberger P. Toxic epidermal necrolysis induced by vemurafenib after nivolumab failure. J Eur Acad Dermatol Venereol. 2017;31(5):e253–e254. doi:10.1111/jdv.14010
58. Jeudy G, Dalac-Rat S, Bonniaud B, et al. Successful switch to dabrafenib after vemurafenib-induced toxic epidermal necrolysis. Br J Dermatol. 2015;172(5):1454–1455. doi:10.1111/bjd.13522
59. Sinha R, Lecamwasam K, Purshouse K, Reed J, Middleton MR, Fearfield L. Toxic epidermal necrolysis in a patient receiving vemurafenib for treatment of metastatic malignant melanoma. Br J Dermatol. 2014;170(4):997–999. doi:10.1111/bjd.12796
60. Wiener JS, Tucker JA, Walther PJ. Interleukin-2-induced dermatotoxicity resembling toxic epidermal necrolysis. South Med J. 1992;85(6):656–659. doi:10.1097/00007611-199206000-00020
61. Polder K, Wang C, Duvic M, et al. Toxic epidermal necrolysis associated with denileukin diftitox (DAB389IL-2) administration in a patient with follicular large cell lymphoma. Leuk Lymphoma. 2005;46(12):1807–1811. doi:10.1080/10428190500233764
62. Dika E, Ravaioli GM, Fanti PA, et al. Cutaneous adverse effects during ipilimumab treatment for metastatic melanoma: a prospective study. Eur J Dermatol. 2017;27(3):266–270. doi:10.1684/ejd.2017.3023
63. Lee O, Masood M, Nutan F. Case series of Stevens-Johnson syndrome and toxic epidermal necrolysis with nivolumab and nivolumab/ ipilimumab combination therapy in metastatic melanoma. J Drugs Dermatol. 2022;21(5):529–530. doi:10.36849/JDD.6559
64. Nayar N, Briscoe K, Fernandez Penas P. Toxic epidermal necrolysis-like reaction with satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016;39(3):149–152. doi:10.1097/CJI.0000000000000112
65. Vivar KL, Deschaine M, Messina J, et al. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. 2017;44(4):381–384. doi:10.1111/cup.12876
66. Lin YT, Yang JC, Chu CY. Esomeprazole-induced Stevens-Johnson syndrome in a patient who underwent nivolumab therapy for advanced lung adenocarcinoma. Lung Cancer. 2020;148:177–178. doi:10.1016/j.lungcan.2020.09.001
67. Kim MC, Khan HN. Nivolumab-induced toxic epidermal necrolysis: rare but fatal complication of immune checkpoint inhibitor therapy. Cureus. 2021;13(5):e15017. doi:10.7759/cureus.15017
68. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer. 2017;109:58–61. doi:10.1016/j.lungcan.2017.04.020
69. Dasanu CA. Late-onset Stevens-Johnson syndrome due to nivolumab use for hepatocellular carcinoma. J Oncol Pharm Pract. 2019;25(8):2052–2055. doi:10.1177/1078155219830166
70. Salati M, Pifferi M, Baldessari C, et al. Stevens-Johnson syndrome during nivolumab treatment of NSCLC. Ann Oncol. 2018;29(1):283–284. doi:10.1093/annonc/mdx640
71. Shah KM, Rancour EA, Al-Omari A, Rahnama-Moghadam S. Striking enhancement at the site of radiation for nivolumab-induced Stevens-Johnson syndrome. Dermatol Online J. 2018;24:6.
72. Rodríguez-Otero N, Chamorro-Pérez J, Fernández-Lozano C, et al. Nivolumab-induced Stevens-Johnson syndrome: not only due to PD-1 inhibition. J Allergy Clin Immunol Pract. 2023;11(9):2936–2938.e1. doi:10.1016/j.jaip.2023.06.008
73. Rouyer L, Bursztejn AC, Charbit L, Schmutz JL, Moawad S. Stevens-Johnson syndrome associated with radiation recall dermatitis in a patient treated with nivolumab. Eur J Dermatol. 2018;28(3):380–381. doi:10.1684/ejd.2018.3295
74. Hwang A, Iskandar A, Dasanu CA. Stevens-Johnson syndrome manifesting late in the course of pembrolizumab therapy. J Oncol Pharm Pract. 2019;25(6):1520–1522. doi:10.1177/1078155218791314
75. Kian W, Zemel M, Elobra F, et al. Intravenous immunoglobulin efficacy on pembrolizumab induced severe toxic epidermal necrolysis. Anticancer Drugs. 2022;33(1):e738–e740. doi:10.1097/CAD.0000000000001162
76. Ryu S, Jun I, Kim TI, Seo KY, Kim EK. Pembrolizumab-induced Stevens-Johnson syndrome with severe ocular complications. Ocul Immunol Inflamm. 2022;30(6):1533–1535. doi:10.1080/09273948.2021.1896006
77. Neema S, Sathu S, Vasudevan B, Shreshta S, Bhatt S, K L. Pembrolizumab-induced toxic epidermal necrolysis: a rare cause of severe adverse drug reaction. Indian J Dermatol Venereol Leprol. 2023;89(4):589–591. doi:10.25259/IJDVL_612_2022
78. Saw S, Lee HY, Ng QS. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur J Cancer. 2017;81:237–239. doi:10.1016/j.ejca.2017.03.026
79. Bhardwaj M, Chiu MN, Pilkhwal Sah S. Adverse cutaneous toxicities by PD-1/PD-L1 immune checkpoint inhibitors: pathogenesis, treatment, and surveillance. Cutan Ocul Toxicol. 2022;41(1):73–90. doi:10.1080/15569527.2022.2034842
80. Cai ZR, Lecours J, Adam JP, et al. Toxic epidermal necrolysis associated with pembrolizumab. J Oncol Pharm Pract. 2020;26(5):1259–1265. doi:10.1177/1078155219890659
81. Liniker E, Menzies AM, Kong BY, et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology. 2016;5(9):e1214788. doi:10.1080/2162402X.2016.1214788
82. Goldinger SM, Stieger P, Meier B, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. 2016;22(16):4023–4029. doi:10.1158/1078-0432.CCR-15-2872
83. Li G, Gong S, Wang N, Yao X. Toxic epidermal necrolysis induced by sintilimab in a patient with advanced non-small cell lung cancer and comorbid pulmonary tuberculosis: a case report. Front Immunol. 2022;13:989966. doi:10.3389/fimmu.2022.989966
84. Zhang L, Wang L, Cheng Q, Lei X, Wu J, Chen N. Lichenoid dermatitis following PD-1 inhibitor-induced toxic epidermal necrolysis: a case report and literature review. Immunotherapy. 2023;15(15):1249–1256. doi:10.2217/imt-2023-0081
85. Sato I, Mizuno H, Kataoka N, et al. Osimertinib-associated toxic epidermal necrolysis in a lung cancer patient harboring an EGFR mutation-a case report and a review of the literature. Medicina. 2020;56:8.
86. Yang W, Xu X, Xia D, Wang H, Jiang J, Yang G. Toxic epidermal necrolysis associated with chemoimmunotherapy for lymphoma: case report and literature review. Immunotherapy. 2022;14(5):275–282. doi:10.2217/imt-2021-0074
87. Sommers KR, Kong KM, Bui DT, Fruehauf JP, Holcombe RF. Stevens-Johnson syndrome/toxic epidermal necrolysis in a patient receiving concurrent radiation and gemcitabine. Anticancer Drugs. 2003;14(8):659–662. doi:10.1097/00001813-200309000-00012
88. Sendur MA, Kilickap S. Stevens–Johnson syndrome after treatment with capecitabine. Clin Oncol. 2008;20(2):202–203. doi:10.1016/j.clon.2007.11.005
89. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi:10.1038/clpt.1981.154
90. Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schröder W, Roujeau JC. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002;138(8):1019–1024. doi:10.1001/archderm.138.8.1019
91. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115(2):149–153. doi:10.1046/j.1523-1747.2000.00061.x
92. Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88(1):60–68. doi:10.1038/clpt.2009.252
93. Rosen AC, Case EC, Dusza SW, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am J Clin Dermatol. 2013;14(4):327–333. doi:10.1007/s40257-013-0021-0
94. Yang SC, Hu S, Zhang SZ, et al. The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in China. J Immunol Res. 2018;2018:4320195. doi:10.1155/2018/4320195
95. Tan SK, Tay YK. Profile and pattern of Stevens-Johnson syndrome and toxic epidermal necrolysis in a general hospital in Singapore: treatment outcomes. Acta Derm Venereol. 2012;92(1):62–66. doi:10.2340/00015555-1169
96. Teng YS, Yu S. Molecular mechanisms of cutaneous immune-related adverse events (irAEs) induced by immune checkpoint inhibitors. Curr Oncol. 2023;30(7):6805–6819. doi:10.3390/curroncol30070498
97. Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and IFN-γ are potential inducers of Fas-mediated keratinocyte apoptosis through activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133(2):489–498. doi:10.1038/jid.2012.330
98. Hunger RE, Hunziker T, Buettiker U, Braathen LR, Yawalkar N. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116(4):923–924. doi:10.1016/j.jaci.2005.06.029
99. Tsai TY, Huang IH, Chao YC, et al. Treating toxic epidermal necrolysis with systemic immunomodulating therapies: a systematic review and network meta-analysis. J Am Acad Dermatol. 2021;84(2):390–397. doi:10.1016/j.jaad.2020.08.122
100. Zimmermann S, Sekula P, Venhoff M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(6):514–522. doi:10.1001/jamadermatol.2016.5668
101. Hynes AY, Kafkala C, Daoud YJ, Foster CS. Controversy in the use of high-dose systemic steroids in the acute care of patients with Stevens-Johnson syndrome. Int Ophthalmol Clin. 2005;45(4):25–48. doi:10.1097/01.iio.0000177430.89645.6d
102. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
103. Shah JV, Parekh JM, Shah PA, Shah PV, Sanyal M, Shrivastav PS. Application of an LC-MS/MS method for the analysis of amlodipine, valsartan and hydrochlorothiazide in polypill for a bioequivalence study. J Pharm Anal. 2017;7(5):309–316. doi:10.1016/j.jpha.2017.06.001
104. Osawa K, Kiniwa Y, Shimosato Y, et al. Toxic epidermal necrolysis caused by apalutamide: a case report of treatment using etanercept with conventional steroid therapy. Acta Derm Venereol. 2022;102:adv00723. doi:10.2340/actadv.v102.2243
105. Ben-Eltriki M, Deb S, Guns ES. Calcitriol in combination therapy for prostate cancer: pharmacokinetic and pharmacodynamic interactions. J Cancer. 2016;7(4):391–407. doi:10.7150/jca.13470
106. Mamza J, Mehta R, Donnelly R, Idris I. Comparative efficacy of adding sitagliptin to metformin, sulfonylurea or dual therapy: a propensity score-weighted cohort study. Diabetes Ther. 2015;6(2):213–226. doi:10.1007/s13300-015-0110-6
107. Renert-Yuval Y, Guttman-Yassky E. The changing landscape of alopecia areata: the therapeutic paradigm. Adv Ther. 2017;34(7):1594–1609. doi:10.1007/s12325-017-0542-7
108. Lichtenstein L, Ron Y, Kivity S, et al. Infliximab-related infusion reactions: systematic review. J Crohns Colitis. 2015;9(9):806–815. doi:10.1093/ecco-jcc/jjv096
109. Kherlopian A, Mewton E, Fong G, Fischer G. Highlighting Adalimumab as a treatment option for systemic treatment of toxic epidermal necrolysis: a case series from a tertiary specialised burns centre. Australas J Dermatol. 2022;63(4):497–504. doi:10.1111/ajd.13911
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
