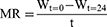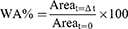Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Effects of Human Fibroblast-Derived Multi-Peptide Factors on the Proliferation and Migration of Nitrogen Plasma-Treated Human Dermal Fibroblasts
Authors Lee SY, Kim DY , Suh SB, Suh JY, Cho SB
Received 23 July 2022
Accepted for publication 4 November 2022
Published 15 November 2022 Volume 2022:15 Pages 2465—2475
DOI https://doi.org/10.2147/CCID.S383483
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Song Yi Lee,1,* Do Yeon Kim,1,* Sang Bum Suh,1 Ji Youn Suh,1 Sung Bin Cho2
1BNV Biolab, Seoul, Korea; 2Yonsei Seran Dermatology and Laser Clinic, Seoul, Korea
*These authors contributed equally to this work
Correspondence: Sung Bin Cho, Yonsei Seran Dermatology and Laser Clinic, 224 Siheung-daero, Seoul, 08628, Korea, Tel +82 2-2135-1375, Fax +82 70-8250-1375, Email [email protected]
Background: Human fibroblast-derived multi-peptide factors (MPFs) promote wound repair by playing crucial roles in cell recruitment, adhesion, attachment, migration, and proliferation.
Methods: Cultured human dermal fibroblasts (HDFs) were directly treated with non-contact low- and high-energy nitrogen plasma and further cultured in various conditioned media. Cell proliferation and wound-healing properties were evaluated.
Results: In Opti-modified Eagle’s medium + GlutaMAX culture, reduced HDF viability was observed 24 h after 2-J/pulse plasma treatment and 12 and 24 h after 3-J/pulse treatment. Meanwhile, in dermal fibroblast-conditioned medium (DFCM) containing MPF culture, reduced HDF viability was observed only 24 h after 3-J/pulse treatment. Under DFCM-MPF culture, the wound area percentage was significantly decreased after 12 and 24 h in untreated HDFs; at 9, 12, and 24 h after 1-J/pulse plasma treatment; at 3, 6, 9, 12, and 24 h after 2-J/pulse plasma treatment; and at 9, 12, and 24 h after 3-J/pulse plasma treatment. Greater migration of HDFs with or without plasma treatment was found in DFCM-MPFs than in other conditioned media.
Conclusion: Low-energy nitrogen plasma treatment promotes HDF proliferation and wound repair. DFCM-MPFs enhanced cell proliferation and improved the wound healing properties of HDFs treated with low- and high-energy plasma.
Keywords: human dermal fibroblasts, multi-peptide factors, dermal fibroblast-conditioned media, plasma, nitrogen, cell proliferation, wound healing, cell migration
Introduction
Human fibroblast-derived multi-peptide factors (MPFs) play crucial roles in cell recruitment, adhesion, attachment, migration, and proliferation in wound repair.1–5 Dermal fibroblasts exhibit highly varying patterns of MPF secretion that differ among species and anatomic sites.1–4 Subpopulations of fibroblasts contribute to distinct aspects of the proliferation and differentiation of the epidermis and dermis.1–4
Fresh cultured dermal substitutes release high levels of vascular endothelial growth factor, basic fibroblast growth factor, hepatocyte growth factor, platelet-derived growth factor-AA, transforming growth factor (TGF)-β1, keratinocyte growth factor, interleukin (IL)-6, and IL-8 in allogeneic cultured dermal substitutes containing fibroblasts.6 Dermal fibroblast-conditioned medium (DFCM) also contains fibroblast-derived MPFs, and proteomic studies have documented the presence of other proteins, including serum albumin, α-fetoprotein, α-2-HS-glycoprotein, α-2-macroglobin, collagen type 1 alpha 1 (COL1A1), COL4A1, COL12A1, decorin, fibronectin, fibulin-1, inter-α-trypsin inhibitor heavy chain H2, lactotransferrin, nucleobindin-1, pentraxin 3, pigment epithelium-derived factor, pregnancy zone protein, TGF-β-induced protein ig-h3, and thrombospondin-1.7,8
Energy-delivering modalities have been used to induce the secretion of MPFs in the dermis for photodamaged skin treatment or skin rejuvenation. These energy sources can also assist in delivering therapeutic drugs to the epidermis or the upper papillary dermis of the skin. Among several modalities, non-thermal atmospheric-pressure nitrogen plasma can facilitate thermal tissue coagulation and modification in the skin in an energy-dependent and chromophore-independent manner.9 Nitrogen plasma exhibits therapeutic efficacy in the treatment of photoaged wrinkles with or without pigmented lesions.10–12
In the present study, we evaluated the effects of DFCM-containing human fibroblast-derived MPFs (DFCM-MPFs) on the proliferation of human dermal fibroblasts (HDFs) pretreated via a non-contact and non-ablative energy-delivering modality. To do so, non-thermal atmospheric-pressure nitrogen plasma pulses at low- and high-energy settings were used for treating HDFs cultured in various media. Cell proliferation and wound healing properties were serially evaluated using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and scratch wound healing assays.
Materials and Methods
Culture of HDFs and Preparation of DFCM-MPFs
Neonatal HDFs (Lonza, Walkersville, MD, USA) were mixed with the fibroblast growth medium (Lonza) in a 1:1 ratio. Cells were seeded in a 75-cm2-T-flask and incubated overnight at 37 ℃ and 5% CO2. Then, the medium was exchanged with Opti-modified Eagle’s medium (Opti-MEM; Gibco BRL, Rockville, MD, USA) to create low-serum culture conditions, and the cells were cultured for 24 h to reach approximately 90% confluency. Then, the cells were washed with Dulbecco’s phosphate-buffered saline (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and treated with 0.15% trypsin (Gibco BRL) for 5 min at 37 ℃ to detach cells from the surface of the culture flask. The medium was added to stop the trypsin action, and the cells were centrifuged for 5 min. Collected cells were resuspended in Opti-MEM and split into 75-cm2-T flasks. The cells were expanded until sufficient number of cells were prepared for the experiment. To prepare DFCM-MPFs, HDFs (passage 5–7) were cultured in 175-cm2-T flasks with Opti-MEM supplemented with 2-mM GlutaMAX, 2-ng/mL recombinant human epidermal growth factor and 2-ng/mL recombinant human fibroblast growth factor (Opti-MEM+G+GFs; all purchased from Lonza) without adding fetal bovine serum. The cells were incubated at 37 ℃ in a 5% CO2 incubator for 24 h, and the medium was collected as DFCM-MPFs.
Treatment of HDFs with Nitrogen Plasma
A non-thermal, atmospheric-pressure generator (PlaDuoTM; Shenb Co., Ltd., Seoul, Korea) was utilized in this study to generate nitrogen plasma pulses from an inert gaseous source. To do so, an unexcited nitrogen source was loaded at the distal end of the handpiece, and 2.45-GHz microwave energy converted the loaded source to plasma within the nozzle. The loading volume and pulse duration were set to 0.2–1.42 mL/pulse and 5–36 ms at 0.5–4 J/pulse. The pulse pressure of nitrogen plasma was 1.0–1.2 bars.
For the cell proliferation assay, HDFs were seeded into a 96-well plate in Opti-MEM and incubated for 24 h at 37 ℃ and 5% CO2. For the wound healing assay, cells were seeded into a culture insert (ibidi culture-insert 2 well; ibidi GmbH, Martinsried, Germany) at a density of 3×104 cells/well and incubated overnight for attachment. Then, HDFs were treated with nitrogen plasma at energy settings of 1, 2, and 3 J, loading volumes of 0.4, 0.68, and 1.12 mL/pulse, and pulse durations of 10, 17, and 28 ms, respectively. The distance from the tip of the disposable nozzle to the bottom of the well was 5 mm, and three stacking pulses/experimental settings at a frequency of 2 Hz were delivered. All experiments were performed in triplicates.
Cell Proliferation and Wound Healing Assays
The viability of nitrogen plasma-treated HDFs was measured using an MTS assay (Cell Titer 96 Aqueous One solution Reagent; Promega, Madison, WI, USA), according to the manufacturer’s instructions. Immediately after nitrogen plasma treatment, the medium in the 96-well plate was exchanged with: 1) Opti-MEM with 2 mM GlutaMAX (Opti-MEM+G), 2) Opti-MEM+G+GFs, or 3) DFCM-MPFs, and cultured for 0, 3, 6, 9, 12, and 24 h. At the specified time, 20 µL of the Cell Titer 96 Aqueous One Solution Reagent was added to each well and incubated at 37 ℃ for 60 min. The quantity of formazan products was determined by measuring the absorbance at 490 nm using a microplate reader (Bio-Rad) in triplicate.
For the wound healing assay, immediately after nitrogen plasma treatment, the cell inserts were removed and the medium was exchanged with: 1) Opti-MEM+G, 2) Opti-MEM+G+GFs, or 3) DFCM-MPFs, and cultured for 0, 3, 6, 9, 12, and 24 h. Photographs of each plate were taken at each time point under a phase-contrast light microscope at ×40 magnification (Olympus CKX53; Olympus Life Science, Tokyo, Japan). Width and area of the wound region were measured using ImageJ software (NIH, Bethesda, MD, USA).13 Then, the rate of cell migration (MR) was calculated as follows:
where W is the average wound width (µm), t is the time span of the wound healing assay (hours).14 Additionally, the wound area percentage (WA%) was calculated as follows:
where Area is the average wound area (µm2) at t=0 (initial wound area) and t=∆t (wound area at specific time points after plasma treatment).14
Statistical Analysis
Values obtained from the cell proliferation and wound healing assays were statistically analyzed using Prism 8.0 (GraphPad Software, San Diego, CA, USA). Multiple comparisons of results from the control and experimental nitrogen plasma treatment groups under varying post-treatment HDF cultivation conditions at different time points were made using two-way analysis of variance with Tukey’s post hoc test. Differences were considered statistically significant at *P < 0.05, **P < 0.001. Error bars indicate the standard deviation.
Results
Effects of Nitrogen Plasma on HDFs
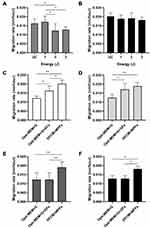
Effects of nitrogen plasma on HDFs were evaluated after delivering nitrogen plasma at an energy of 1, 2, and 3 J/pulse for three stacking pulses and further culturing the untreated control and plasma-treated cells in Opti-MEM+G. MTS assay revealed that nitrogen plasma treatment at 1 J/pulse did not produce any detectable changes in HDF viability at all time points compared to the control (Figure 1A). Nitrogen plasma treatment at 2 J/pulse elicited significantly reduced HDF viability only at 24 h, whereas treatment at 3 J/pulse elicited remarkably reduced cell viability at 12 and 24 h.
WA% in untreated HDFs cultured in Opti-MEM+G was significantly decreased at 24 h than at 0 h (Figure 1B). Meanwhile, WA% was markedly decreased at 9, 12, and 24 h than at baseline with 1-J/pulse plasma treatment; at 6 and 24 h with 2-J/pulse plasma treatment; and at 12 and 24 h with 3-J/pulse plasma treatment (Figure 2). Nonetheless, statistically significant differences in WA% among the different energy settings were found only at 6 h (Figure 1C). The rate of cell migration was also evaluated after delivering nitrogen plasma to cells and further incubating them in Opti-MEM+G for 24 h (Figure 1D). The migration rates of HDFs were calculated as 0.0121 ± 0.00115 mm/h for untreated HDFs, 0.0124 ± 0.00193 mm/h with 1-J/pulse plasma treatment, 0.0122 ± 0.00388 mm/h with 2-J/pulse plasma treatment, and 0.0127 ± 0.00168 mm/h with 3-J/pulse plasma treatment. No statistically significant differences were found between the experimental conditions of the nitrogen plasma treatment.
Proliferation of Plasma-Treated HDFs Cultured in DFCM-MPFs
In the experiments with Opti-MEM+G+GFs, MTS assay revealed that plasma-treated HDFs at 1 and 2 J/pulse did not experience remarkable decrease in cell viability at any time point compared to the untreated control (Figure 3A). The viability of 3-J/pulse plasma-treated HDFs decreased only at 24 h. In the experiments with DFCM-MPF culture, MTS assay showed no remarkable decrease in the cell viability of plasma-treated HDFs at 1 and 2 J/pulse at all time points (Figure 3B). Nonetheless, the viability of 3-J/pulse-treated HDFs cultivated in DFCM-MPFs was still significantly decreased at 24 h.
Untreated controls cultured in Opti-MEM+G+GFs and DFCM-MPFs exhibited significantly increased cell proliferation at 12 h compared to those cultured in Opti-MEM+G (Figure 3C). The proliferation of 1-J/pulse-treated HDFs cultured in Opti-MEM+G+GFs and DFCM-MPFs increased at 24 h, compared to the proliferation of those cultured in Opti-MEM+G (Figure 3D). The proliferation of 2-J/pulse-treated HDFs increased at 12 h in DFCM-MPFs and at 24 h in Opti-MEM+G+GFs and DFCM-MPFs, compared to the proliferation of those cultured in Opti-MEM+G (Figure 3E). The proliferation of 3-J/pulse-treated HDFs increased at 12 h in DFCM-MPFs and at 24 h in Opti-MEM+G+GFs and DFCM-MPFs, compared to the proliferation of those cultured in Opti-MEM+G (Figure 3F).
Wound Healing Properties of Plasma-Treated HDFs Cultured in DFCM-MPFs
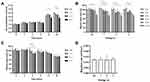
In Opti-MEM+G+GF culture, WA% in untreated HDFs was significantly decreased at 6, 9, 12, and 24 h, compared to that at 0 h (Figures 4A and 5). WA% decreased markedly at 9, 12, and 24 h than at 0 h in 1-J/pulse plasma-treated HDFs; at 6, 9, 12, and 24 h in 2-J/pulse-treated HDFs; and at 9, 12, and 24 h in 3-J/pulse-treated HDFs. No statistically significant differences in WA% among the different energy settings were found at all time points. Under DFCM-MPF culture, WA% in untreated HDFs was significantly decreased at 12 and 24 h than at 0 h (Figures 4B and 6). WA% was markedly decreased at 9, 12, and 24 h than at 0 h in 1-J/pulse-treated HDFs; at 3, 6, 9, 12, and 24 h in 2-J/pulse-treated HDFs; and at 9, 12, and 24 h in 3-J/pulse-treated HDFs. No statistically significant differences in WA% among the different energy settings were found at all time points.
Untreated HDFs exhibited a reduced WA% at 6 h in Opti-MEM+G+GFs and DFCM-MPFs; at 9 h in Opti-MEM+G+GFs; at 12 h in Opti-MEM+G+GFs and DFCM-MPFs, and at 24 h in Opti-MEM+G+GFs and DFCM-MPFs, compared to that of Opti-MEM+G (Figure 4C). HDFs treated with 1-J/pulse showed reduced WA% at 3 h in DFCM-MPFs and at 6, 12, and 24 h in Opti-MEM+G+GFs and DFCM-MPFs than that in Opti-MEM+G (Figure 4D). HDFs treated with 2 and 3 J/pulse exhibited reduced WA% at 3 h in DFCM-MPFs and at 9, 12, and 24 h in Opti-MEM+G+GFs and DFCM-MPFs than that in Opti-MEM+G (Figure 4E and F, respectively).
Migration Rate of Plasma-Treated HDFs Cultured in DFCM-MPFs
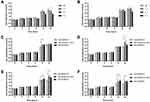
Cell migration rates were evaluated after delivering nitrogen plasma and incubating HDFs in Opti-MEM+G+GFs and DFCM-MPFs for 24 h. The migration rate under Opti-MEM+G+GFs cultivation was 0.0163 ± 0.00266 mm/h for untreated HDFs, 0.0171 ± 0.00319 mm/h for 1-J/pulse-treated HDFs, 0.0175 ± 0.00049 mm/h for 2-J/pulse-treated HDFs, and 0.0165 ± 0.00167 mm/h for 3-J/pulse-treated HDFs, without a statistically significant difference among the treatment settings (P > 0.05) (Figure 7A). Meanwhile, the migration rate under DFCM-MPF cultivation was 0.0202 ± 0.00197 mm/h for untreated HDFs, 0.0188 ± 0.00204 mm/h for 1-J/pulse plasma-treated HDFs, 0.0191 ± 0.00313 mm/h for 2-J/pulse plasma-treated HDFs, and 0.0181 ± 0.00157 mm/h for 3-J/pulse plasma-treated HDFs, without a statistically significant difference among the treatment settings (P > 0.05) (Figure 7B). When comparing the effects of Opti-MEM+G, Opti-MEM+G+GFs, and DFCM-MPFs on the migration rates of plasma-treated HDFs, we found that untreated control and plasma-treated HDFs at all settings exhibited significantly higher migration rates in DFCM-MPFs, followed by Opti-MEM+G+GFs and Opti-MEM+G (Figures 7C–F).
Discussion
In the present study, we treated HDFs cultured in Opti-MEM+G with nitrogen plasma at 1, 2, and 3 J/pulse for three stacking pulses, and compared their cell proliferation and wound healing properties at 0, 3, 6, 12, and 24 h relative to that of the untreated controls. The cell viability of HDFs treated with 1 J/pulse did not decrease at any time point compared to that of the untreated controls. HDFs treated with 2 J/pulse showed decreased cell viability at 24 h, and those treated with 3 J/pulse showed decreased cell viability at both 12 and 24 h. Our experimental conditions differed from those of other studies in that 1) the gaseous source of plasma was nitrogen, 2) nitrogen plasma was delivered to HDFs via jet-type delivery at a pulse pressure of 10–12 bars with a pulse duration of milliseconds, and 3) nitrogen plasma was directly delivered to HDFs cultivated in Opti-MEM+G, Opti-MEM+G+GFs, and DFCM-MPF post-treatment. Accordingly, we suggest that the viability and proliferation patterns of plasma-treated HDFs reflect the direct effects of plasma, but not the indirect effects of plasma-treated media, on HDFs.
Opti-MEM had lower serum and higher hypoxanthine and thymidine levels than other culture media.15 We used Opti-MEM without adding fetal bovine serum to obtain human fibroblast-secreted MPFs in DFCM containing no or minimum serum proteins. Although Opti-MEM was supplemented with synthetic epidermal growth factor and fibroblast growth factor, we believe that fibroblasts exhausted most of the supplementary growth factors to induce MPF secretion. A previous study on the growth factor G1 array revealed that the DFCM-MPF preparation contained markedly increased number and amount of growth factors compared to the control media.16 Moreover, DFCM-MPFs-treated outer root sheath (ORS) cells secreted more growth factors than the control media-treated ORS cells.16
In this study, culture media, including Opti-MEM+G, Opti-MEM+G+GFs, and DFCM-MPFs, were used for the culture of untreated and plasma-treated HDFs. The overall viability of HDFs in DFCM-MPFs in each experimental setting was notably higher than that of HDFs cultured in Opti-MEM+G and similar to that of HDFs cultured in Opti-MEM+G+GF. Accordingly, we suggest that the culture in DFCM-MPFs could increase HDF viability after low- and high-energy plasma treatment. Additionally, our results demonstrated that the use of DFCM-MPFs elicited earlier reduction in WA% at 3 h and greater reduction at 12 and 24 h. In addition, HDFs in DFCM-MPFs showed higher migration rates than those in Opti-MEM+G and Opti-MEM+G+GFs. However, whether DFCM-MPFs protect HDFs from high-energy plasma-induced apoptosis or stimulate HDF proliferation remains to be elucidated. In addition, the mechanisms of action of MPFs, except epidermal and fibroblast growth factors, during wound repair need to be further investigated to determine the efficiency and safety of MPFs in DFCM and synthetic supplementary growth factors in culture media.
A previous in vivo rat study demonstrated that nitrogen plasma treatment results in dermal fibroblast proliferation and neocollagenesis.17 Nitrogen plasma contains nitric oxide (NO), NO2, reactive oxygen species (ROS), and reactive nitrogen species (RNS). NO plays an important role in the proliferation of keratinocytes and fibroblasts.17,18 ROS and RNS stimulate wound repair by upregulating the nuclear factor erythroid-related factor 2/Kelch-like ECH-associated protein 1/BTB domain and CNC homolog 1 pathway.17,19 Meanwhile, plasma treatment with argon promotes murine fibroblast proliferation, increases the intracellular NO, ROS, and O2 levels, activates the nuclear transcription factor-κB pathway, and increases cyclinD1 expression.20 Additionally, indirect argon plasma treatment increases the proliferation and migration of HaCaT keratinocytes or MRC5 fibroblasts to enhance wound closure.21
Based on our results, we recommend the use of combined treatment with low- and high-energy plasma and DFCM-MPFs. Low-energy plasma treatment reportedly improves the refractory melasma lesions in Asian patients, and the pathogenesis of melasma is significantly associated with senescent HDF-secreting factors.22–24 We suggest that a combination of low-energy plasma and DFCM-MPFs cab theoretically promote the rejuvenation of senescent HDFs and photodamaged dermal environments, and that MPFs, which are obtained from young HDFs, can counteract the pathological effects of senescent HDF-secreted factors. High-energy nitrogen plasma is used to treat photoaged skin; however, unavoidable downtime and post-inflammatory hyperpigmentation significantly limit the use of high-energy settings in Asian patients.10–12 We suggest that DFCM-MPF pre- and post-plasma treatment can prevent excessive tissue damage and the risk of side effects associated with high-energy nitrogen plasma treatment, along with promoting wound repair to achieve better clinical outcomes. However, further controlled clinical investigations should be conducted to validate these findings and establish optimized treatment settings.
Conclusion
Our data demonstrated that low-energy, non-thermal, and atmospheric-pressure nitrogen plasma treatment promotes HDF proliferation and wound repair. Cell viability and wound healing properties of untreated HDFs were improved by DFCM-MPF culture. Moreover, when low-energy plasma-treated HDFs were cultured with DFCM-MPFs, cell viability and wound-healing properties were enhanced. High-energy nitrogen plasma treatment decreased HDF viability and delayed wound repair, although post-plasma culture with DFCM-MPFs significantly recovered these properties. Further investigations should be conducted to document the mechanisms of plasma-enhanced cell proliferation and DFCM-MPF-induced promotion of wound repair.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We would like to thank Min Choi (Shenb Co., Ltd., Seoul, Korea) and Bora Kim (Shenb Co., Ltd.) for their help with technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Lawlor KT, Kaur P. Dermal contributions to human interfollicular epidermal architecture and self-renewal. Int J Mol Sci. 2015;16:28098–28107. doi:10.3390/ijms161226078
2. Leary T, Jones PL, Appleby M, Blight A, Parkinson K, Stanley M. Epidermal keratinocyte self-renewal is dependent upon dermal integrity. J Invest Dermatol. 1992;99:422–430. doi:10.1111/1523-1747.ep12616134
3. Tuan TL, Keller LC, Sun D, Nimni ME, Cheung D. Dermal fibroblasts activate keratinocyte outgrowth on collagen gels. J Cell Sci. 1994;107:2285–2289. doi:10.1242/jcs.107.8.2285
4. Sorrell JM, Baber MA, Caplan AI. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol. 2004;200:134–145. doi:10.1002/jcp.10474
5. Maarof M, Lokanathan Y, Ruszymah HI, Saim A, Chowdhury SR. Proteomic analysis of human dermal fibroblast conditioned medium (DFCM). Protein J. 2018;37:589–607. doi:10.1007/s10930-018-9800-z
6. Kubo K, Kuroyanagi Y. A study of cytokines released from fibroblasts in cultured dermal substitute. Artif Organs. 2005;29:845–849. doi:10.1111/j.1525-1594.2005.00138.x
7. Chowdhury SR, Aminuddin BS, Ruszymah BH. Effect of supplementation of dermal fibroblasts conditioned medium on expansion of keratinocytes through enhancing attachment. Indian J Exp Biol. 2012;50:332–339.
8. Manira M, Chowdhury SR, Rosliza A, et al. Concentration dependent effect of dermal fibroblast conditioned medium on in vitro wound healing properties of keratinocytes. Regen Res. 2014;3:30–32.
9. Kim H, Kim HJ, Kim HK, Hong JY, Cho SB. Effects of argon and nitrogen plasma pulses on the skin and skin appendages in an in vivo animal model. Skin Res Technol. 2020;26:81–90. doi:10.1111/srt.12767
10. Elsaie ML, Kammer JN. Evaluation of plasma skin regeneration technology for cutaneous remodeling. J Cosmet Dermatol. 2008;7:309–311. doi:10.1111/j.1473-2165.2008.00411.x
11. Fitzpatrick R, Bernstein E, Iyer S, Brown D, Andrews P, Penny K. A histopathologic evaluation of the Plasma Skin Regeneration System (PSR) versus a standard carbon dioxide resurfacing laser in an animal model. Lasers Surg Med. 2008;40:93–99. doi:10.1002/lsm.20547
12. Sanderson AR, Wu EC, Liaw LH, Garg R, Gangnes RA. The effect of topical anesthetic hydration on the depth of thermal injury from the plasma skin regeneration device. Lasers Surg Med. 2014;46:127–131. doi:10.1002/lsm.22210
13. Suarez-Arnedo A, Figueroa FT, Clavijo C, Arbeláez P, Cruz JC, Carolina M-C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS One. 2020;15:e0232565. doi:10.1371/journal.pone.0232565
14. Grada A, Otero-Vinas M, Prieto-Castrillo F, Obagi Z, Falanga V. Research techniques made simple: analysis of collective cell migration using the wound healing assay. J Invest Dermatol. 2017;137:e11–16. doi:10.1016/j.jid.2016.11.020
15. Hu R, Cao Q, Sun Z, Chen J, Zheng Q, Xiao F. A novel method of neural differentiation of PC12 cells by using Opti-MEM as a basic induction medium. Int J Mol Med. 2018;41:195–201. doi:10.3892/ijmm.2017.3195
16. Shin JM, Lee YY, Kim KM, et al. The potential role of fibroblast-derived multi-peptide factors in activation of growth factors and β-Catenin in hair follicle cells. J Cosmet Dermatol. 2022;2022:1.
17. Babossalam S, Abdollahimajd F, Aghighi M, et al. The effect of nitrogen plasma on the skin and hair follicles: a possible promising future for the treatment of alopecia. Arch Dermatol Res. 2020;312:361–371. doi:10.1007/s00403-019-02020-w
18. Cals-Grierson MM, Ormerod A. Nitric oxide function in the skin. Nitric Oxide. 2004;10:179–193. doi:10.1016/j.niox.2004.04.005
19. Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and Phase II pathways. J Biol Chem. 2015;290:6731–6750. doi:10.1074/jbc.M114.603555
20. Liu JR, Xu GM, Shi XM, Zhang GJ. Low temperature plasma promoting fibroblast proliferation by activating the NF-κB pathway and increasing cyclinD1 expression. Sci Rep. 2017;7:11698. doi:10.1038/s41598-017-12043-w
21. Schmidt A, Bekeschus S, Wende K, Vollmar B, von Woedtke T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp Dermatol. 2017;26:156–162. doi:10.1111/exd.13156
22. Kim H, Kim H, Kim YK, Cho SB. Treatment of refractory melasma with microwave-generated, atmospheric-pressure, non-thermal nitrogen plasma. Med Laser. 2019;8:74–79. doi:10.25289/ML.2019.8.2.74
23. Kim M, Kim SM, Kwon S, Park TJ, Kang HY. Senescent fibroblasts in melasma pathophysiology. Exp Dermatol. 2019;28:719–722. doi:10.1111/exd.13814
24. Yoon JE, Kim Y, Kwon S, et al. Senescent fibroblasts drive ageing pigmentation: a potential therapeutic target for senile lentigo. Theranostics. 2018;8:4620–4632. doi:10.7150/thno.26975
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.