Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Effects of Early Pulmonary Rehabilitation on Hospitalized Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis
Authors Lu HY, Chen CF, Lee DL, Tsai YJ, Lin PC
Received 12 November 2022
Accepted for publication 30 April 2023
Published 15 May 2023 Volume 2023:18 Pages 881—893
DOI https://doi.org/10.2147/COPD.S397361
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Hsin- Yueh Lu,1,* Chiu-Fan Chen,2,3,* David Lin Lee,2,3 Yi-Ju Tsai,4 Pei-Chin Lin4,5
1Division of Respiratory Therapy, Department of Internal Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan; 2Division of Chest Medicine, Department of Internal Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan; 3School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Hsinchu City, Taiwan; 4Department of Medical Education and Research, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan; 5Department of Pharmacy, School of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan
*These authors contributed equally to this work
Correspondence: Pei-Chin Lin, Department of Medical Education and Research, Kaohsiung Veterans General Hospital, No. 386, Dazhong 1st Road, Zuoying District, Kaohsiung, 813, Taiwan, Tel +886-7-3422121 ext 71584, Email [email protected]
Background: Pulmonary rehabilitation (PR) effectively improves symptoms and exercise ability in patients with stable chronic obstructive pulmonary disease (COPD). However, the effectiveness and timing of early PR on hospitalized patients with acute exacerbation of COPD (AECOPD) is still debated.
Methods: This study conducted a meta-analysis to compare the outcome benefits between early PR and usual care for patient hospitalized due to AECOPD. A systematic search was performed for retrieving randomized control trials (RCTs) from the PubMed, Embase, and Cochrane library until November 2021. RCTs reporting early PR for AECOPD with hospitalization, either during admission or within four weeks of discharge, were enrolled for systematic review and meta-analysis.
Results: Twenty RCTs (1274 participants) were included. Early PR showed significantly improved readmission rate (ten trials, risk ratio 0.68, 95% confidence interval (CI) 0.50– 0.92), 6-minute walking distance (6MWD, twelve trials, MD 59.73, 95% CI 36.34– 83.12), St George’s Respiratory Questionnaire score (eight trials, MD − 10.65, 95% CI − 14.78 to − 6.52), Borg score (eight trials, MD − 0.79, 95% CI − 1.26 to − 0.32), and modified Medical Research Council dyspnea scale (eight trials, MD − 0.38, 95% CI − 0.5 to − 0.25). However, the trend of mortality (six trials, risk ratio 0.72, 95% CI 0.39– 1.34) benefit was not significant. The subgroup analysis showed non-significant trends of better effect in early PR during admission than those after discharge for outcomes of 6MWD, quality of life, and dyspnea. However, non-significant trends of less benefits on mortality and readmission rate were found in early PR during the admission.
Conclusion: Overall, early PR is beneficial for AECOPD with hospitalization, and there was no significant outcome difference between PR initiated during admission or within 4 weeks of discharge.
Keywords: chronic obstructive pulmonary disease, exacerbation, hospitalization, pulmonary rehabilitation
Introduction
Chronic obstructive pulmonary disease (COPD), a clinical syndrome characterized by chronic respiratory symptoms and irreversible airflow limitation, is the third leading cause of death worldwide, with 3.2 million deaths in 2017 and a worldwide prevalence of 10.1%.1,2 Acute exacerbation (AE) is a serious complication of COPD and has many adverse effects, including worsening of the quality of life, accelerating disease progression, and increased hospital admissions and mortality.3–7 AE is an important poor prognostic factor for COPD and is also the most common cause of admission in COPD patients.8 It was reported that severe AE resulted in three times higher mortality in COPD patients than in those without severe AE.7
Pulmonary rehabilitation (PR) is considered a cornerstone for COPD management. According to current guidelines and literature, PR provides additional benefits on respiratory symptoms, exercise ability, quality of life (QoL), readmission rate, acute exacerbation, and survival in stable COPD patients than the standard medical therapy.9,10 The recent American Thoracic Society (ATS) and European Respiratory Society (ERS) guideline also suggests that PR should be initiated within three weeks following discharge from acute exacerbations of chronic obstructive pulmonary disease (AECOPD).11 PR is a comprehensive intervention and is designed to improve the physical and psychological condition of patients with the chronic respiratory disease.12 Exercise training is a key component of PR and includes endurance training and resistance training. Neuromuscular electrical stimulation is an alternative rehabilitation method for patients too weak to perform exercise.12 Other components of PR include respiratory training, breathing retraining, patient education, smoking cessation, nutrition, and psychological support.10,13
The previous two meta-analyses, conducted in 2016 by Puhan et al14 and in 2018 by Ryrsø et al,15 showed that early pulmonary rehabilitation (PR) had significant benefits for patients with AECOPD. However, there were discrepancies in the inclusion of studies and the outcome of mortality. In fact, some of the studies included were not ideal, including some non-COPD patients or non-AECOPD patients, or some studies included control groups that actually received late rehabilitation exercise. In addition, in the 2018 study by Ryrsø et al,15 the subgroup analysis was designed to compare early PR between patients receiving PR from admission to within one week of discharge and those receiving PR from 1 to 4 weeks after discharge, which I personally think is not an ideal way to compare. The recent ATS and ERS guideline also suggested early PR initiated during hospitalization for AECOPD showed increased patient’s exercise capacity; however, increased mortality was also reported.11 Owing to the limited evidence at that time, further evaluation is required to clarify the ideal PR timing after AECOPD with hospitalization. Therefore, the purpose of this meta-analysis is to collect and update the literature in a more pure and objective way to compare the outcomes of AECOPD patients receiving early rehabilitation and those who receive no rehabilitation and to analyze the differences in outcomes between early rehabilitation during admission and after discharge.
Materials and Methods
Search Strategy and Eligibility Criteria
The whole study followed the rule of Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.16 Systematic search was performed to retrieve relevant RCTs from the PubMed, Embase, and Cochrane library from their inception to November 2021, without language restriction. Searches were updated for any new research that also fulfilled the eligibility criteria until November 2021. Articles were also searched from the reference lists of relevant review articles. The keywords, which include both the text word and MeSH term, used for searching included COPD, exacerbation, PR, and physical therapy. The full search strategy is presented in Figure 1. The inclusion criteria were (1) patients admitted to the hospital owing to AECOPD (2) RCTs compared the effect of early PR during their hospitalization or within four weeks after discharge (intervention group) with standard medical therapy without PR (control group). (3) RCTs reporting at least one of the outcomes of mortality, hospital readmission, exercise capacity, dyspnea and health-related QoL. The PR must have main component of exercise training and may also contain respiratory exercise, education, smoking cessation, and nutritional support. Studies using neuromuscular electrical stimulation as an alternative PR intervention were also included. Studies with control group having any exercise training were excluded.
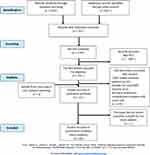 |
Figure 1 Flow diagram of inclusion and exclusion of RCTs for meta- analyses. |
Study Selection
Two authors independently reviewed all articles for identifying qualified studies (HY Lu and CF Chen). Titles and abstract were screened for relevant studies. The search of RCTs was not limited to any language. Duplicate studies were removed from systematic review searches. If there was unclear information, concerned authors were contacted through email.
Data Extraction
Data were extracted and arranged in excel sheets, including author’s name, publish year, country, study design, patients’ characteristics, interventions and outcomes. All the data were independently checked by two authors (HY Lu and CF Chen). If there were disagreements of extract data, it would be discussed by a third author (PC Lin) to reach a consensus.
Outcome Measures
The primary outcome was readmission rate. The secondary outcomes were as follows: mortality, 6-minute walking distance (6MWD) for exercise capacity, Modified Medical Research Council (mMRC) scale and Borg Scale for dyspnea evaluation, and St. George’s Respiratory Questionnaire (SGRQ) for QoL score.
Risk of Bias in Individual Studies
Cochrane risk of bias (RoB) tool17 was used for assessing the quality of included studies. The RoB tool evaluates six domains, including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. The RoBs were independently judged by two authors (HY Lu and CF Chen). If there were disagreements on RoBs judgement, a third author (PC Lin) will join the discussion to reach a consensus. The reviewer’s assessment can be of a high risk of bias, some concerns of bias, and a low risk of bias. If a study has at least one domain judged as high risk of bias, or there are multiple domains judged as some concerns of bias, the overall result would be a high risk of bias. If all domains were judged as low risk of bias, the overall result would be a low risk of bias. If a study was judged to have some concerns or was unclear, the overall result of the risk of bias would be an unclear risk of bias.
Statistical Analyses
Review Manager 5.3 software (Cochrane Library Software, Oxford, UK) was used for the meta-analysis. Weighted mean difference (WMD) was used to pool outcome for continuous outcomes. For dichotomous data such as mortality and readmission rates, relative risk and 95% confidence interval (CI) were used to pool the effect size. Random-effect model was chosen due to the considerable difference of PR method of the included RCTs. Heterogeneity was tested using I2 statistics:17 P < 0.1 or I2> 50% were considered as significant heterogeneity. Subgroup analysis was performed to compare the outcome difference between PR during admission and PR after discharge. Funnel plots were used to check for the presence of publication bias. Egger’s test was used to evaluate the significance of publication bias. Both of them were analyzed by The Comprehensive Meta-Analysis Version 3 software (Borenstein et al 2013).
Results
Study Selection
The flowchart of studies selection is shown in Figure 1. Until November 2021, a total of 1400 studies were searched from the electronic database, and 102 duplicates were removed. Non-RCT studies were excluded from the remaining 1298 studies, leaving 211 studies. We continued to select eligible studies based on the article’s title, abstract, and full text among these 211 studies. Among these, 10 studies included stable COPD patients, two studies included patients with other chronic respiratory diseases in additional to COPD, and 179 studies had pulmonary rehabilitation intervention 1 month after AECOPD, and therefore, these studies were excluded. Finally, 21 articles were included in the qualitative synthesis, and 20 were included in the quantitative synthesis. There were 1274 patients in total (Figure 1).
Characteristic of Included Studies
The summary of included 20 studies is shown in Tables 1 and S1. In the 20 RCTs included in the analysis, all patients were hospitalized because of AECOPD,18–37 and 3 studies included patients with mean FEV1 predicted ≧ 50%.34–36 Most studies included patients with mean/median age of ≧ 60 years (9 studies with mean/median age of 60–69 years, and 9 studies with age of ≧ 70 years), and only 2 studies included patients with mean age between 50 and 59 years.22,35 Most of the articles were male predominant, and only two studies were female predominant,21,29 and the other two studies did not report the gender of their patients.22,34 In the included studies, the baseline characteristics between the intervention group and the control group were all similar. There were two kinds of early PR studies. Ten studies performed PR during hospitalization.18–21,23,24,28,31,33,37 Among these, 2 studies continued the PR program after discharge.19,21 The other 10 studies initiated the PR program after discharge.22,25–27,29,30,32,34–36 The duration of PR program of enrolled studies ranged from 4 days to 3 months. The frequency of the PR program ranged from 2 times per week to 2 times per day. In the intervention group (PR group) of each study, training methods included endurance exercise, resistance exercise, or neuromuscular electrostimulation. The control group (usual care group) received standard hospital care without exercise training (Table 1).
 |
Table 1 Characteristics of Randomized Controlled Trials Enrolled for Meta-Analysis |
Risk of Bias Within Studies
In the 20 RCT studies, there was only one low risk of bias study,32 sixteen high risks of bias studies,18–23,25–31,33,36,37 and three unclear risks of bias studies24,34,35 (Figure 2). Overall, there was high risk of biases in performance bias, detection biases, attrition bias, reporting bias and other bias. For selection bias, including random sequence generation and allocation concealment, there were some concerns and was determined as unclear risk of bias.
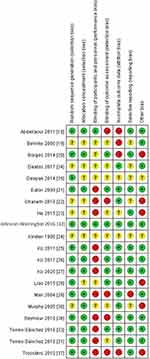 |
Figure 2 Summary of risk of bias. |
Outcome Assessments
Primary Outcome: Readmission Rate
Hospital readmission rate was reported in 10 RCTs (n = 733). The readmission rate was 34.9% (127/364) in early PR group, and 52.6% (194/369) in usual care group. There was a significant improvement in the readmission rate in early PR when compared with usual care (random-effect, Risk ratio (RR) 0.68, 95% CI 0.50–0.92, I2 = 56%). However, there was substantial heterogeneity (Figure 3).
 |
Figure 3 Hospital readmission forest plot: early PR versus usual care.19,21,25–27,29,30,32,36,37 |
Secondary Outcomes
Compared with usual care, early PR was associated with a significant improvement of 6MWD (13 RCTs, n = 797, random-effect, Mean difference (MD) 59.73 meter, 95% confidence interval (CI) 36.34–83.12; I2 = 88%; Figure 4), a better SGRQ total score (9 RCTs, n = 639, random-effect, MD −10.65; 95% CI −14.78 to −6.52; I2 = 51%; Figure 5), a lower Borg score (8 RCTs, n = 390, random-effect, MD −0.79, 95% CI: −1.26 to −0.32; I2 = 56%; Figure 6), a lower mMRC score (8 RCTs, n = 568, random-effect, MD −0.38, 95% CI −0.50 to −0.25; I2 = 0%; Figure 7), and a similar mortality (6 RCTs, n = 521, random-effect, RR 0.72, 95% CI 0.39–1.34; I2 = 0%; Figure 8). Six of the 20 RCTs evaluated adverse outcomes,18,21,23,29,32,37 and all reported no adverse event during the study period.
 |
Figure 4 6MWD forest plot: early PR versus usual care.18,20–28,34,35,37 |
 |
Figure 5 SGRQ total score forest plot: early PR versus usual care.20,25–27,29,30,34–36 |
 |
Figure 6 Borg score forest plot: early PR versus usual care.19,21,24,25,28,30,31,33 |
 |
Figure 7 mMRC forest plot: early PR versus usual care.21,23,25–27,30,34,35 |
 |
Figure 8 Mortality forest plot: early PR versus usual care.19,25–27,29,32 |
Subgroup Analysis
A subgroup analysis was conducted for comparison of PR during admission, PR after discharge, and both (mixed inpatient and outpatient PR) (Table 2 and Figures S1–6), and showed no significant difference in mortality (RR 0.14 vs 0.77, P = 0.54), readmission rate (RR 1.26 vs 0.65, P = 0.2), 6MWD (MD 81.82 vs 39.42 meters, P = 0.18), SGRQ score change (MD −2.8 vs −11.12, P = 0.32), Borg score change (MD −0.87 vs −0.30, P = 0.48), and mMRC change (MD −0.7 vs −0.35, P = 0.26). However, heterogeneity did not reduce in most subgroups (except for a partial reduction in heterogeneity in the Borg score).
 |
Table 2 Summary of Subgroup Analysis |
Publication Bias
No publication bias was observed by visual examination of funnel plots and tests using Egger’s test (P = 0.37) (Figure 9).
 |
Figure 9 Funnel plot for evaluation of publication bias. |
Discussion
The result of this meta-analysis showed that early PR during admission or within four weeks after discharge for severe AECOPD had statistically significant beneficial effect on readmission rate, 6MWD, SGRQ, Borg score, and mMRC than usual care without PR. However, the trend of mortality benefit was not significant. Significant heterogeneity was found in readmission rate, 6MWD, SGRQ and Borg score. Heterogenicity was low in mortality and mMRC. When considering the minimal clinically important difference (MCID), both 6MWD and SGRQ had achieved clinically significant benefit (MCID for 6MWD = 26 meters, SGRQ = 4 units, Borg score = 1 unit).38 In the subgroup analysis, there was no significant outcome difference between PR during admission and PR after discharge. Adverse event after PR was either reported as none or not been reported in enrolled PR studies.
Recent meta-analysis on 2018 by Ryrsø et al concluded that early PR after AECOPD significantly improved mortality, hospital duration, readmission, SGRQ, and 6MWD.15 However, in this meta-analysis, a study evaluating COPD patients without AE within four weeks was included,39 and two studies comparing early PR and late PR were also included (not a true control group).40,41 Another previous Cochrane systematic review on 2016 by Puhan et al also showed that PR after AECOPD significantly improved QoL, exercise capacity, and readmission rate, but the mortality benefit was not significant.14 However, in their meta-analysis some studies with control groups performing exercise training (not true control group) were also included.42,43 In contrast, our meta-analysis made a more focused comparison, with all hospitalized AECOPD patients, and all control groups received standard medical therapy without exercise training. In our study, five additional studies were included that were not analyzed by previous meta-analyses.18,22,27,31,32 The comparison of differences in the enrolled studies between the above two meta-analyses14,15 and our study is presented in Table S2.
Previous ATS/ERS guidelines on 2017 concluded that early PR during hospitalization increases mortality but according to limited evidence.11 This recommendation was based on a study published in 2014 by Greening, which showed early PR within 48 h of admission had higher 12-month mortality than the usual care group.42 However, in that study, the usual care group also received outpatient PR three months after discharge, and therefore, was not a true control group. Besides, the study participants included patients with COPD (approximately 80%), asthma, interstitial lung disease, and bronchiectasis. The author also reported a significantly lower rate of outpatient PR (three months after discharge) in the early PR group than in the usual care group.42 However, in our meta-analysis, the subgroup analysis of the mortality outcome showed that only one study included both inpatient PR and outpatient PR, while all the others included only outpatient PR. Therefore, there is still inadequate evidence about the effectiveness of inpatient PR on mortality, and it is not possible to draw a conclusion currently.
The issue that whether early PR should be initiated during admission of AECOPD is still controversial. In the meta-analysis on 2018 by Ryrsø et al, subgroup analysis was also done to compare different timing of PR (during admission or within 1 week after discharge vs between 1 and 4 weeks after discharge).15 And their result showed no significant difference in mortality, readmission rate, SGRQ, and 6MWD between the 2 subgroups.15 However, we suggest this subgroup analysis was not ideal, because PR during admission and those PR within 1 week after discharge were combined in a same group. Therefore, it cannot clearly answer the problem of whether early PR should be initiated during admission or not. In our study, the subgroup analysis compared early PR during admission vs those PR within 4 weeks after discharge. All the enrolled studies had true control groups (without any exercise training). However, our subgroup analysis result still showed no significant outcome difference between 2 groups.
There are some limitations to this study. The first is clinical heterogeneity. There was considerable variance in exercise training programs between each PR study (Table 1), which includes endurance training (walking and cycling), resistance training (upper and lower limbs), or both. In one study, neuromuscular electrostimulation was used instead of exercise training. Despite the difference in exercise training programs, the goal of exercise training was similar. In addition, the start timing of PR (from second admission day to within 2~3 weeks after discharge), frequency of PR (from 2 times/day to 2 times/week), and duration of PR were also variable (from four days to six months). These variations should be considered carefully when interpreting the results. Second, blinding of participants and personnel was not possible in most studies, and the performance bias and detection bias were inevitable. Third, an insufficient sample size-related imprecision was observed in the mortality outcome. Beside, statistic heterogeneity was observed in the readmission, 6MWD, SGRO total score, Borg score outcomes in this study.
Conclusion
In conclusion, this meta-analysis showed that early PR for AECOPD with hospitalization had a significant beneficial effect on hospital readmission rate, exercise capacity, QoL, and dyspnea. The benefit of early PR on mortality is still controversial. The subgroup analysis showed that the beneficial effects are similar between PR initiated during admission and those initiated within four weeks after discharge. However, heterogeneity was present, and more studies with larger sample size are still needed to verify this conclusion. Early PR timing should also be decided according to the stability of AECOPD patient and the PR program facility of the hospital.
Abbreviations
6MWD, 6-minute walking distance; AE, acute exacerbation; CI, confidence interval; COPD, chronic obstructive pulmonary disease; MD, mean difference; mMRC, modified Medical Research Council; PR, pulmonary rehabilitation; QoL, quality of life; RCT, randomized control trial; RoB, risk of bias; RR, relative risk; SGRQ, St. George’s Respiratory Questionnaire; WMD, weighted mean difference.
Acknowledgments
This research received the grants from Kaohsiung Veterans General Hospital and the Ministry of Science and Technology of Taiwan [grant numbers: VGHKS109-D03-2, MOST 109-2511-H-075B-001-MY2].
Disclosure
Dr Pei-Chin Lin reports grants from Kaohsiung Veterans General Hospital and the Ministry of Science and Technology of Taiwan. The other authors report no conflicts of interest in this work.
References
1. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;393:e44.
2. Celli BR, Wedzicha JA, Drazen JM. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1257–1266. doi:10.1056/NEJMra1900500
3. Global Initiative for Chronic Obstructive Lung Disease. 2020 Global strategy for prevention, diagnosis and management of COPD (2020 report). Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
4. Miravitlles M, Ferrer M, Pont A, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59:387–395. doi:10.1136/thx.2003.008730
5. Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi:10.1136/thorax.57.10.847
6. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi:10.1056/NEJMoa0909883
7. Beeh KM, Glaab T, Stowasser S, et al. Characterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trial. Respir Res. 2013;14:116. doi:10.1186/1465-9921-14-116
8. Rosenberg SR, Kalhan R, Mannino DM. Epidemiology of chronic obstructive pulmonary disease: prevalence, morbidity, mortality, and risk factors. Semin Respir Crit Care Med. 2015;36:457–469. doi:10.1055/s-0035-1555607
9. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2:Cd003793.
10. Rochester CL, Vogiatzis I, Holland AE, et al. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–1386. doi:10.1164/rccm.201510-1966ST
11. Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49:1600791. doi:10.1183/13993003.00791-2016
12. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi:10.1164/rccm.201309-1634ST
13. Celli BR, Stoller JK, Hollingsworth H. Pulmonary rehabilitation; 2020. Available from: https://www.uptodate.com/contents/pulmonary-rehabilitation.
14. Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;12:Cd005305. doi:10.1002/14651858.CD005305.pub4
15. Ryrsø CK, Godtfredsen NS, Kofod LM, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulm Med. 2018;18:154. doi:10.1186/s12890-018-0718-1
16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi:10.1371/journal.pmed.1000097
17. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142. doi:10.1002/14651858.ED000142
18. Abdellaoui A, Préfaut C, Gouzi F, et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J. 2011;38:781–788. doi:10.1183/09031936.00167110
19. Behnke M, Taube C, Kirsten D, et al. Home-based exercise is capable of preserving hospital-based improvements in severe chronic obstructive pulmonary disease. Respir Med. 2000;94:1184–1191. doi:10.1053/rmed.2000.0949
20. Borges RC, Carvalho CR. Impact of resistance training in chronic obstructive pulmonary disease patients during periods of acute exacerbation. Arch Phys Med Rehabil. 2014;95:1638–1645. doi:10.1016/j.apmr.2014.05.007
21. Eaton T, Young P, Fergusson W, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology. 2009;14:230–238. doi:10.1111/j.1440-1843.2008.01418.x
22. Ghanem M, Elaal EA, Mehany M, et al. Home-based pulmonary rehabilitation program: effect on exercise tolerance and quality of life in chronic obstructive pulmonary disease patients. Ann Thorac Med. 2010;5(1):18–25. doi:10.4103/1817-1737.58955
23. He M, Yu S, Wang L, et al. Efficiency and safety of pulmonary rehabilitation in acute exacerbation of chronic obstructive pulmonary disease. Med Sci Monit. 2015;21:806–812.
24. Kirsten DK, Taube C, Lehnigk B, et al. Exercise training improves recovery in patients with COPD after an acute exacerbation. Respir Med. 1998;92(10):1191–1198. doi:10.1016/S0954-6111(98)90420-6
25. Ko FW, Dai DL, Ngai J, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology. 2011;16:617–624. doi:10.1111/j.1440-1843.2010.01921.x
26. Ko FW, Cheung NK, Rainer TH, et al. Comprehensive care programme for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2017;72:122–128. doi:10.1136/thoraxjnl-2016-208396
27. Ko FW, Tam W, Siu E, et al. Effect of short-course exercise training on the frequency of exacerbations and physical activity in patients with COPD: a randomized controlled trial. Respirology. 2021;26:72–79. doi:10.1111/resp.13872
28. Liao LY, Chen KM, Chung WS, et al. Efficacy of a respiratory rehabilitation exercise training package in hospitalized elderly patients with acute exacerbation of COPD: a randomized control trial. Int J Chron Obstruct Pulmon Dis. 2015;10:1703–1709. doi:10.2147/COPD.S90673
29. Man WD, Polkey MI, Donaldson N, et al. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ. 2004;329(7476):1209. doi:10.1136/bmj.38258.662720.3A
30. Murphy N, Bell C, Costello RW. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respir Med. 2005;99:1297–1302. doi:10.1016/j.rmed.2005.02.033
31. Torres-Sánchez I, Valenza MC, Cebriá IIM, et al. Effects of different physical therapy programs on perceived health status in acute exacerbation of chronic obstructive pulmonary disease patients: a randomized clinical trial. DisabilRehabil. 2018;40:2025–2031.
32. Johnson-Warrington V, Rees K, Gelder C, et al. Can a supported self-management program for COPD upon hospital discharge reduce readmissions? A randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2016;11:1161–1169. doi:10.2147/COPD.S91253
33. Torres-Sánchez I, Valenza MC, Sáez-Roca G, et al. Results of a multimodal program during hospitalization in obese COPD exacerbated patients. COPD. 2016;13:19–25. doi:10.3109/15412555.2015.1043428
34. Daabis R, Hassan M, Zidan M. Endurance and strength training in pulmonary rehabilitation for COPD patients. Egypt J Chest Dis Tuberc. 2017;66:231–236. doi:10.1016/j.ejcdt.2016.07.003
35. Deepak TH, Mohapatra PR, Janmeja AK, et al. Outcome of pulmonary rehabilitation in patients after acute exacerbation of chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2014;56(1):7–12.
36. Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65:423–428. doi:10.1136/thx.2009.124164
37. Troosters T, Probst VS, Crul T, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:1072–1077. doi:10.1164/rccm.200908-1203OC
38. Jones PW, Beeh KM, Chapman KR, et al. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189:250–255. doi:10.1164/rccm.201310-1863PP
39. Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med. 2000;109:207–212. doi:10.1016/S0002-9343(00)00472-1
40. Puhan MA, Spaar A, Frey M, et al. Early versus late pulmonary rehabilitation in chronic obstructive pulmonary disease patients with acute exacerbations: a randomized trial. Respiration. 2012;83:499–506. doi:10.1159/000329884
41. Revitt O, Sewell L, Singh S. Early versus delayed pulmonary rehabilitation: a randomized controlled trial - Can we do it? Chron Respir Dis. 2018;15:323–326. doi:10.1177/1479972318757469
42. Greening NJ, Williams JE, Hussain SF, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ. 2014;349:g4315. doi:10.1136/bmj.g4315
43. Nava S. Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854. doi:10.1016/S0003-9993(98)90369-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
