Back to Journals » Journal of Pain Research » Volume 15
Effect of Perineural Dexamethasone with Ropivacaine in Continuous Serratus Anterior Plane Block for Postoperative Analgesia in Patients Undergoing Video-Assisted Thoracoscopic Surgery
Authors Chen JQ, Chen JR, Wang S, Gao W, Gu H, Yang XL , Hu JC , Chai XQ, Wang D
Received 30 April 2022
Accepted for publication 5 August 2022
Published 13 August 2022 Volume 2022:15 Pages 2315—2325
DOI https://doi.org/10.2147/JPR.S372071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ellen M Soffin
Jia-qi Chen,* Jie-ru Chen,* Sheng Wang, Wei Gao, Hai Gu, Xin-lu Yang, Ji-cheng Hu, Xiao-qing Chai, Di Wang
Pain Clinic, Department of Anesthesiology, The First Affiliated Hospital of USTC (Anhui Provincial Hospital), Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Di Wang; Xiao-qing Chai, Department of Anesthesiology, The First Affiliated Hospital of USTC (Anhui Provincial Hospital), Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, 230001, People’s Republic of China, Tel/Fax +86-551-62283963 ; +86-551-62283912, Email [email protected]; [email protected]
Purpose: The goal of this study was to evaluate the analgesic efficiency of dexamethasone with ropivacaine in continuous serratus anterior plane block (cSAPB) after video-assisted thoracoscopic surgery (VATS).
Patients and Methods: Sixty-six patients who underwent VATS were randomized into two groups. All patients received cSAPB postoperatively, and patients in Group RD received 20 mL of 0.375% ropivacaine plus 0.1 mg/kg dexamethasone followed by an infusion of 0.2% ropivacaine plus 0.02 mg/kg/hour dexamethasone at a rate of 5 mL/h in patient-controlled analgesia (PCA) pump. Patients in Group R received 20 mL of 0.375% ropivacaine with normal saline followed by an infusion of 5 mL/h of 0.2% ropivacaine in PCA pump. Fifty milligrams of tramadol was given as rescue medication when the visual analog scale (VAS) score was ≥ 4 at rest. The primary outcomes were the sum of pressing number within 48 hours postoperatively and the time to the first patient-controlled bolus. The secondary outcomes were VAS scores, the incidence of rescue analgesia, wound infection and nausea/vomiting.
Results: Within 48 hours postoperatively, the sum of pressing number was more in Group R (18.33 ± 3.149 vs 16.09 ± 3.292, P = 0.006), and the Log Rank Test showed a significant difference in time to the first patient-controlled bolus (P = 0.006). After the PCA infusion finished, there were significantly lower VAS scores in Group RD at 60 and 72 hours postoperatively (P < 0.001). Additionally, the incidence of rescue analgesia in Group R was significantly more than that in Group RD (P < 0.001). No incision infection was observed in any patient.
Conclusion: The cSAPB with ropivacaine plus dexamethasone prolonged the duration of analgesia and motor blockade, reduced pain intensity and rescued analgesia requirements after the end of PCA infusion for patients undergoing VATS, which provide further improvement to continuous perineural block.
Keywords: continuous serratus anterior plane block, video-assisted thoracoscopic surgery, dexamethasone, levobupivacaine
Introduction
Video-assisted thoracoscopic surgery (VATS) has emerged as a lower stress response and smaller surgical incision compared with thoracotomy, which has become more acceptable in recent years.1 However, many researches indicated that the analgesic requirements of VATS should not be underestimated compared with open thoracotomy.2 Uncontrolled pain can lead to postoperative pulmonary complications (PPCs), including atelectasis and pneumonia, longer hospital stays, and chronic post-surgical pain (CPSP) syndrome.3 In addition, the more severe of postoperative acute pain, the more likely to develop into CPSP.4 Proper management of acute pain is crucial not only to avoid early PPCs but also to prevent chronic pain syndrome.
Thoracic epidural analgesia (TEA) and thoracic paravertebral block (TPVB) are commonly used regional anesthetic techniques for thoracic surgery.5 TEA still has some risks, including epidural hematoma, nerve injury, hemodynamic instability, and urine retention.6 The TPVB technique also carries several complications, including dural puncture, pneumothorax, and hematoma.7 The research suggested that TEA may easily fail due to technical difficulties in catheter placement or dislodgement (nearly 30%).8 Otherwise, it is harder to place or fix the catheter for continuous analgesia in TPVB.9
In 2013, Blanco et al10 injected local anesthetic (LA) into the serratus anterior plane layer, which was termed the serratus anterior plane block (SAPB). It is a promising regional technique for chest wall analgesia. Considering the side effects and difficulties in the performance of TEA and TPVB, SAPB can provide a simple operation, few complications, and stable hemodynamics in patients undergoing VATS.11 Due to the single administration pattern and the pharmacokinetic characteristics of the LAs, the American Pain Society strongly recommends the use of continuous regional analgesic techniques for postoperative pain management.12 Continuous SAPB (cSAPB), in which a catheter is placed in the serratus anterior plane layer and combined with a removable infusion pump, can prolong the duration of SAPB and facilitate the patient’s mobility and recovery.13,14
In addition to using catheterization to achieve continuous infusion, adding adjuvants to LAs is also a common method to increase the duration and quality of regional analgesia.15 Adjuvants commonly used in regional block techniques include dexamethasone, alpha-2 agonists, NMDA antagonists, epinephrine, and nonsteroidal anti-inflammatory drugs. Many studies have demonstrated that dexamethasone, as an adjuvant, combined with LAs may also reinforce and prolong the peripheral nerve block.16
It has been confirmed that dexamethasone combined with LAs can improve analgesic effects during brachial plexus block, transversus abdominis plane block, and epidural block.17 However, there is no report about the effect of dexamethasone with ropivacaine for cSAPB in VATS. Therefore, this study is intended to observe the analgesic efficiency of dexamethasone with ropivacaine in cSAPB after thoracoscopic surgery.
Patients and Methods
Study Design and Subjects
This prospective, randomized, double-blind and controlled trial was approved by the Ethics Committee of the First Affiliated Hospital, University of Science and Technology of China (USTC), and the trial was registered in the Chinese Clinical Trial Registry (ChiCTR2100053518). Seventy-two patients undergoing elective VATS were recruited from November 2021 to March 2022. Patients were given a detailed explanation of the study protocol and were informed about the potential benefits and side effects of the technique.
Participants
To be eligible, patients must have been aged 18–70 years old, had a body mass index (BMI) of 18.5–30 kg/m2, were of both sexes, were American Society of Anesthesiologists (ASA) class I–III, voluntarily participate and had the ability to precisely complete a pain assessment. Exclusion criteria were coagulation dysfunction, diabetes, history of opioid dependence, allergy to the study drugs, systemic or puncture site infection, peripheral nervous system disease or SAPB-impacted area nerve damage, hepatic or renal insufficiency, cardiac insufficiency (New York Heart Association classes III–IV), lung function impairment (forced expiratory volume <50% predicted value within 1 s), chronic pain history or on lasting analgesic therapy before the surgery and thoracoscopic surgery intraoperatively converted to open thoracotomy procedure. All enrolled patients signed informed consent forms.
General Anesthesia
A standardized anesthetic technique was used in both groups. General anaesthesia was induced with 0.05 mg/kg midazolam, 2 mg/kg propofol, 0.4 μg/kg sufentanil, and 1.0 mg/kg rocuronium. This was followed by the insertion of an endobronchial tube. Anaesthesia was maintained with a continuous infusion of propofol (4–8 mg/kg/h) and remifentanil (0.05–0.2 μg/kg/min) to achieve a target bispectral index (BIS) value between 40 and 50. Intraoperative rocuronium boluses were administered as needed. All patients were treated with a protective pulmonary ventilation strategy during surgery and mechanically ventilated to maintain end-tidal carbon dioxide pressure (ETCO2) between 35 and 45 mmHg. One hundred milligrams of flurbiprofen and 0.1 μg/kg of sufentanil were intravenously given to patients 30 minutes before chest closure. All surgical operations were performed by the same surgeon team, without any wound infiltrations and administration of LAs by surgeons.
Randomization and Blinding
Eligible patients were randomized by a computer-generated random number table into two groups: Group R (ropivacaine group) and Group RD (ropivacaine combined with dexamethasone group). Allocation concealment was implemented by using sequentially numbered, opaque, sealed envelopes. The envelopes were opened just before the procedure by the staff nurse who prepared the drug for the block and was not involved further in the study. Postoperative assessments were performed by the staff member who was unaware of the group allocation. The data analysis was also performed by independent research staff who were not informed of the group assignment.
Interventions
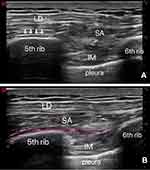
In our hospital, only one skin incision, 4 cm in length, was made in the fourth to fifth intercostal space between the anterior axillary line and the mid-axillary line. All enrolled patients received a cSAPB according to the following protocol. At the end of surgery, the patient was still unawaked and kept in the surgical position (lateral position). Under all aseptic precautions, a linear ultrasound transducer (4–15 Hz, L15-4B, Navis, Wisonic, Shenzhen, China) was placed over the fourth to sixth ribs between the anterior axillary line and posterior axillary line in a sagittal plane. The latissimus dorsi (superficial) and serratus anterior muscles (inferior) were recognized above the ribs. The epidural needle (1.6 mm outer diameter, 80 mm length, Tuoren, China) was inserted using an in-plane approach when the probe was placed in a coronal plane. Furthermore, when the needle reached the surface of the rib, 3 mL of saline was injected to open the potential interfacial space between the rib and serratus anterior muscle (Figure 1). Afterwards, an epidural catheter (0.5 mm inner diameter, 113 mm length, Tuoren, China) was passed through, and a 4.5 cm epidural catheter was left inside the serratus anterior muscle plane (Figure 2).
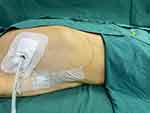 |
Figure 2 The chest wall containing the catheter after cSAPB. |
After a confirmed negative aspiration, Group RD received a bolus of 20 mL of 0.375% ropivacaine plus 0.1 mg/kg dexamethasone followed by an infusion of 0.2% ropivacaine plus 0.02 mg/kg/hour dexamethasone at a rate of 5 mL/h in the patient-controlled analgesia (PCA) pump. Group R received a bolus of 20 mL of 0.375% ropivacaine with normal saline (the same volume as dexamethasone in Group RD) followed by an infusion of 5 mL/h of 0.2% ropivacaine in the PCA pump. A patient-controlled bolus was 5 mL with a 30-minute lockout in both groups. The capacity of the PCA pump was 150 mL (ZZB-150, Apon ®, Jiangsu, China). The PCA pump will give an alarm and prompt when it closed to the end so that we can refill it again. In addition, all patients received 100 mg flurbiprofen intravenously every 12 hours for 48 hours after surgery. Furthermore, 50 mg of tramadol, as a rescue medication, was used when the visual analog scale (VAS) score was reported to be ≥4 at rest or upon patient request.
Outcomes
Postoperatively, the pain intensity assessment was performed after patients were extubated and fully conscious. The assessment was implemented by an independent anesthesiologist who was not involved in the administration of intervention or intraoperative management using a VAS (0–10 cm), in which 0 cm means no pain and 10 cm is the worst pain. All the patients were instructed before surgery in the use of VAS and PCA pump devices. VAS was assessed at rest and on coughing at 2, 6, 12, 24, 36 and 48 hours postoperatively. In addition, after the end of PCA infusion, VAS was still assessed at 60, 72, 84, 96, 108, and 120 hours postoperatively to record the most severe pain score every 12 hours. Patients were guided to conduct pain assessment and record the VAS scores by themselves if they were discharged during the assessment period. They were followed-up through telephone consultation, including VAS scores and number of rescue medications.
The primary outcomes were the sum of pressing number within 48 hours after surgery and the time to the first patient-controlled bolus. The secondary outcomes were the VAS scores, the incidence of rescue analgesia, wound infection and nausea/vomiting after surgery. Other perioperative data, such as the intraoperative propofol and remifentanil requirement, duration of surgery, chest tube duration and hospital stay, were also collected.
Sample Capacity
Few studies have tested the effect of adding dexamethasone to LAs in cSAPB. However, based on our preexperiment outcome, the sum of pressing number of PCA pump within 48 hours after operation in Group R was 18.3 ± 2.8 and that in Group RD was 16.1 ± 1.9. With a power (β) of 0.95 and a 5% significance level, a minimum sample size of 32 patients for each group was estimated. Considering a 10% rate of potential dropout, a total of 36 patients for each group were finally included. The minimum clinical difference of the VAS score between the groups was estimated to be 1.0–1.3 cm.18,19
Statistical Analysis
We used statistical software SPSS, version 20.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis. Quantitative variables were expressed as the means (with SD) or medians (with interquartile range) depending on the (normally or skewed) distribution of data, and qualitative data were expressed as frequencies and percentages. For quantitative data, comparisons between the two groups were performed using independent sample t-tests or Mann–Whitney U-tests. Qualitative data were compared by the Chi-square test or Fisher’s exact test. The time to request for the first patient-controlled bolus was analysed using Kaplan–Meier survival estimates and the log‑rank test. A P value of <0.05 was considered to be statistically significant.
Results
The consolidated standards of reporting trials (CONSORT) flow chart is presented in Figure 3. Seventy-two patients were assessed for eligibility, four patients refused to participate, and two patients changed the operation method. Finally, sixty-six patients were analysed of thirty-three each. Preoperative blood glucose was measured within one week before the operation, and postoperative blood glucose was measured 48 hours after surgery. The basic blood glucose levels were comparable between the two groups, and there was a significant difference after surgery. Apart from this, the two groups were comparable in demographic and other clinical characteristics (Table 1). The duration of the first patient-controlled bolus was 16 (14–33.5) hours in Group R, while in Group RD, it was 36 (20–48) hours (P = 0.004). Afterwards, the Log rank test showed a significant difference (P = 0.006) between the two groups for time to the first patient-controlled bolus (Figure 4). The sum of pressing number was more in Group R (18.33 ± 3.149 vs 16.09 ± 3.292, P = 0.006) than in Group RD. The rescue analgesia requirement of the two groups was comparable when the PCA pump was infused (within 48 hours postoperatively). After the end of PCA infusion, rescue analgesia in Group R was significantly more than that in Group RD (P < 0.001). Table 2 shows the postoperative VAS scores within 48 hours in both groups. There were statistical differences from 12 to 48 hours postoperatively at rest and on coughing between the two groups. After the PCA infusion was finished, the VAS scores were also assessed every 12 hours, and there were significantly lower VAS scores in Group RD than in Group R at 60 and 72 hours postoperatively (Table 3). The descriptive information of VAS scores at individual time points between groups is displayed (Figure 5). There was no significant difference in the rate of nausea/vomiting, chest tube duration or hospital stay. No incision infection was seen in any patient (Table 4).
 |
Table 1 Demographic and Clinical Characteristics of Patients (n = 66) |
 |
Table 2 Intensity of Pain Within 48 Hours Postoperatively Assessed by VAS |
 |
Table 3 Intensity of Pain After PCA Infusion Assessed by VAS |
 |
Table 4 Other Secondary Outcomes of Patients |
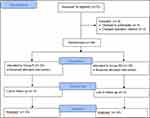 |
Figure 3 CONSORT diagram showing the flow of patients in the trial. |
 |
Figure 5 VAS scores at individual time points between the two groups. |
Discussion
In reviewing the literature, we found that dexamethasone has been used as an adjuvant to LAs in regional blocks since long, but broad research revealed no study where dexamethasone has been used in continuous perineural block, such as cSAPB after VATS. In this study, the sum of PCA pressing number was decreased, while the time to the first patient-controlled bolus was significantly increased after adding dexamethasone within 48 hours postoperatively. The results of the study demonstrated that the addition of dexamethasone to ropivacaine prolonged the duration of analgesia and improved the analgesia effect for VATS.
Anatomically, the serratus anterior is a quadrilateral muscle located on the lateral chest. The SAPB can block not only the lateral cutaneous branch of the intercostal nerve and the long thoracic nerve but also the thoracic dorsal nerve.20 The block area of SAPB ranged from T2 to T6 on the anterior chest wall and T2 to T8 on the lateral chest wall, which can successfully cover the surgical incisions and chest tube sites of VATS.13 It should be noted that catheterization in the serratus anterior plane layer is also simpler than that in TEA and TPVB. The current study on cSAPB found that continuous SAPB had an advantage in reducing postoperative opioid use, was hemodynamically stable, and could provide effective analgesia in breast surgery, rib fracture, minimally invasive heart surgery (MIHS) and VATS.21–23
Except for catheter-based techniques, which can sustain pain management during the perioperative period, adjuvants are also effective medications that work synergistically with LAs. In this study, we added dexamethasone to ropivacaine for cSAPB as an adjuvant to explore the analgesic effect in VATS. Dexamethasone is a long-acting synthetic corticosteroid that has multiple administered routes: intravenously, intrathecally, epidurally and locally, as an adjuvant.24 The precise mechanism by which dexamethasone prolongs the duration of sensory block is not completely understood. Studies have demonstrated that dexamethasone weakened the activity of unmyelinated C fibers after binding to glucocorticoid receptors by inhibiting the exchange of potassium ions. C fibers are associated with pain transmission.25 Dexamethasone can also enhance the sensitivity of catecholamine and delay the absorption of LAs by constricting the blood vessels and reducing capillary permeability.26 The existence of a dose-dependent effect of dexamethasone added to peripheral nerve block is controversial. Many studies have confirmed that dexamethasone, as an adjuvant, for regional anesthesia is relatively safe and does not increase the risk of complications.27,28 The study by Bravo et al showed that 2 mg, 5 mg and 8 mg dexamethasone provided similar effects of motor and sensory block in brachial plexus blocks.29 Furthermore, a meta-analysis of Kirkham et al27 showed that the addition of 4–10 mg of dexamethasone to ropivacaine can provide an effective role in prolonging the action time. Therefore, 0.1 mg/kg dexamethasone was used in this study according to the weight range of most patients.
In the current study, after a bolus injection and continuous administration with a pump, the VAS scores in Group RD from 12 to 48 hours postoperatively were statistically lower than those in Group R, whether at rest or on coughing. It has been reported that plasma ropivacaine concentrations peaked at 30 mins and remained high for approximately 6 hours after transversus abdominis plane (TAP) block.30 As dexamethasone was added to Group RD, it may affect the absorption and metabolism of ropivacaine, and our data also support the use of adjuvant medications for prolonged cSAPB duration. Although there was no clinical difference in VAS scores between the two groups from 2 to 48 hours postoperatively, fewer pressing numbers and longer times of the first patient-controlled bolus were observed in Group RD.
Intravenous (i.v.) dexamethasone has been shown to reduce postoperative pain in a meta-analysis.31 It is different that we used perineural dexamethasone with ropivacaine in our study. Considering the anatomical basis, cSAPB is unlikely to affect the pleurae, which are richly innervated and likely provide significant pain when coughing or forced expiration. Therefore, 50 mg of tramadol, as a rescue medication, was used when the VAS score was ≥4. The result of rescue analgesia showed no significant difference during the PCA pump infusion between the two groups. From 60 h to 120 h postoperatively, we found that the VAS scores first increased and then declined in Group R. This fluctuation may be caused by the recommended early postoperative exercise, which led to increased pain scores and more analgesia requirements after the end of PCA infusion. However, the declining VAS scores in Group RD may be related with the biologic half-life of dexamethasone, which is between 36 and 54 hours. It could still provide a lasting analgesic effect due to the prolonged analgesic duration. In a study, Barry et al32 demonstrated that perioperative i.v. dexamethasone use may also have an effect on the reduction of rebound pain incidence. In this paper, the VAS scores in Group RD did not increase after the end of PCA infusion. However, we did not design more details about rebound pain, and further research elucidating the use of perineural dexamethasone on reducing rebound pain is still warranted based on this study.
The use of systemic steroids as an antiemetic has been well confirmed in the literature and is often used in routine anesthesia practice. Meanwhile, a recent meta-analysis, which added dexamethasone to LAs in TAP for perineural administration, also found decreased postoperative nausea and vomiting (PONV).33 Although this finding is contrary to our study, it may be a byproduct of better analgesia effect, resulting in the reduction of opioid use. The potential adverse effects of dexamethasone include immune suppression, increased blood glucose, the risk of infection and impaired wound healing.24 In this study, the increase in postoperative blood glucose may be caused by the continuous use of dexamethasone. Since we excluded patients with diabetes before surgery, the glucose metabolism function of both groups was normal. This blood glucose fluctuation may return to normal after the withdrawal of dexamethasone. Besides, no incision infection or other adverse events were observed in any patient, despite the rise of postoperative blood glucose.
The present study has several limitations. First, the optimal dosage of dexamethasone was not compared experimentally. Further studies are recommended to improve our understanding of the dose and concentration of other LAs and adjuvants. Second, there was no sensory testing during the operation, while the patients were extubated and unconscious. Instead, we confirmed the adequate distribution of LAs in the target layer by ultrasound. Finally, this was a single-center study. However, the same team of performing surgeons and anesthetists ensured the standardization and consistency of the work. To add more evidence, multicenter studies are warranted to test the results and conclusions of our trial.
Conclusion
We conclude that the addition of perineural dexamethasone to ropivacaine for cSAPB significantly prolonged the duration of analgesia and motor blockade, reduced pain intensity and rescued analgesia requirements after the end of PCA infusion for patients undergoing VATS. Although there was no report of adverse events directly related to our trial, we are unable to comment on the safety of this therapeutic approach due to the lack of the blood concentration test of LAs and adjuvants. In conclusion, adding dexamethasone to ropivacaine in cSAPB improved the analgesia effect and reduced rescue analgesia requirements after the end of PCA infusion for VATS, which may provide further supplementary and improvement to the continuous perineural block.
Abbreviations
cSAPB, continuous serratus anterior plane block; VATS, video-assisted thoracoscopic surgery; PCA, patient-controlled analgesia; VAS, visual analog scale; PPCs, postoperative pulmonary complications; CPSP, chronic postsurgical pain; TEA, thoracic epidural analgesia; TPVB thoracic paravertebral block; LA, local anesthetic; SAPB, serratus anterior plane block; ASA, American Society of Anesthesiologists; BMI, body mass index; BIS, bispectral index; ETCO2, end-tidal carbon dioxide pressure; LD, latissimus dorsi; SA, serratus anterior; IM, intercostal muscle; MAP, mean arterial pressure; IQR, interquartile range.
Data Sharing Statement
The authors state that all data in the manuscript are accessible if requested (contact e-mail address di.wang@ustc. edu.cn). The authors verify that all data intended for sharing are deidentified.
Ethics Approval and Informed Consent
The Ethics Committee at the First Affiliated Hospital of USTC approved this prospective trial. Written informed consent was obtained from all patients recruited to the study, in accordance with the code of the Declaration of Helsinki. The trial was registered at the Chinese Clinical Trial Registry (ChiCTR2100053518) on November 24, 2021. This manuscript adheres to the applicable CONSORT guidelines.
Acknowledgments
The Key Research and Development Project Foundation of Anhui Province (No. 1804h08020286) provided funding for the study. Jia-qi Chen and Jie-ru Chen are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Petersen RH, Holbek BL, Hansen HJ, Kehlet H. Video-assisted thoracoscopic surgery-taking a step into the future. Eur J Cardiothorac Surg. 2017;51(4):694–695. doi:10.1093/ejcts/ezw381
2. Bai Y, Sun K, Xing X, et al. Postoperative analgesic effect of hydromorphone in patients undergoing single-port video-assisted thoracoscopic surgery: a randomized controlled trial. J Pain Res. 2019;12:1091–1101. doi:10.2147/JPR.S194541
3. Yoon S, Hong WP, Joo H, et al. Long-term incidence of chronic postsurgical pain after thoracic surgery for lung cancer: a 10-year single-center retrospective study. Reg Anesth Pain Med. 2020;45(5):331–336. doi:10.1136/rapm-2020-101292
4. Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ, Prospective A. Study of chronic pain after thoracic surgery. Anesthesiology. 2017;126(5):938–951. doi:10.1097/ALN.0000000000001576
5. Horlocker TT, Vandermeuelen E, Kopp SL, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (fourth edition). Reg Anesth Pain Med. 2018;43(3):263–309.
6. Rosero EB, Joshi GP. Nationwide incidence of serious complications of epidural analgesia in the United States. Acta Anaesthesiol Scand. 2016;60(6):810–820.
7. Hanley C, Wall T, Bukowska I, et al. Ultrasound guided continuous deep serratus anterior plane block versus continuous thoracic paravertebral block for perioperative analgesia in videoscopic-assisted thoracic surgery. Eur J Pain. 2020;24(4):828–838. doi:10.1002/ejp.1533
8. Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: causes and management. Br J Anaesth. 2012;109(2):144–154. doi:10.1093/bja/aes214
9. D’Ercole F, Arora H, Kumar PA. Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth. 2018;32(2):915–927. doi:10.1053/j.jvca.2017.10.003
10. Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68(11):1107–1113. doi:10.1111/anae.12344
11. Zhang X, Zhang C, Zhou X, et al. Analgesic effectiveness of perioperative ultrasound-guided serratus anterior plane block combined with general anesthesia in patients undergoing video-assisted thoracoscopic surgery: a systematic review and metaanalysis. Pain Med. 2020;21(10):2412–2422. doi:10.1093/pm/pnaa125
12. Vorobeichik L, Brull R, Bowry R, Laffey JG, Abdallah FW. Should continuous rather than single-injection interscalene block be routinely offered for major shoulder surgery? A meta-analysis of the analgesic and side-effects profiles. Br J Anaesth. 2018;120(4):679–692. doi:10.1016/j.bja.2017.11.104
13. Chen JQ, Yang XL, Gu H, Chai XQ, Wang D. The role of serratus anterior plane block during in video-assisted thoracoscopic surgery. Pain Ther. 2021;10(2):1051–1066. doi:10.1007/s40122-021-00322-4
14. Gao W, Yang XL, Hu JC, et al. Continuous serratus anterior plane block improved early pulmonary function after lung cancer surgical procedure [published online ahead of print, 2021 Mar 2]. Ann Thorac Surg. 2021;113(2):436–443. doi:10.1016/j.athoracsur.2021.02.032
15. Prabhakar A, Lambert T, Kaye RJ, et al. Adjuvants in clinical regional anesthesia practice: a comprehensive review [published correction appears in Best Pract Res Clin Anaesthesiol. 2021 Dec;35(4):E3-E4]. Best Pract Res Clin Anaesthesiol. 2019;33(4):415–423. doi:10.1016/j.bpa.2019.06.001
16. Huynh TM, Marret E, Bonnet F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: a meta-analysis of randomized controlled trials. Eur J Anaesthesiol. 2015;32(11):751–758. doi:10.1097/EJA.0000000000000248
17. Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017;11(11):CD011770. doi:10.1002/14651858.CD011770.pub2
18. Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105(1–2):151–157. doi:10.1016/S0304-3959(03)00176-3
19. Yamamoto LG, Nomura JT, Sato RL, Ahern RM, Snow JL, Kuwaye TT. Minimum clinically significant VAS differences for simultaneous (paired) interval serial pain assessments. Am J Emerg Med. 2003;21(3):176–179. doi:10.1016/S0735-6757(02)42255-3
20. Yang XL, Gu H, Hu JC, et al. Operation, effectiveness, and limitations of continuous serratus anterior plane blocks for thoracoscopic surgery in adults. J Pain Res. 2020;13:2401–2410. doi:10.2147/JPR.S264139
21. Hards M, Harada A, Neville I, et al. The effect of serratus plane block performed under direct vision on postoperative pain in breast surgery. J Clin Anesth. 2016;34:427–431. doi:10.1016/j.jclinane.2016.05.029
22. Kunhabdulla NP, Agarwal A, Gaur A, Gautam SK, Gupta R, Agarwal A. Serratus anterior plane block for multiple rib fractures. Pain Physician. 2014;17(5):E651–E653. doi:10.36076/ppj.2014/17/E651
23. Berthoud V, Ellouze O, Nguyen M, et al. Serratus anterior plane block for minimal invasive heart surgery. BMC Anesthesiol. 2018;18(1):144. doi:10.1186/s12871-018-0614-5
24. Tolska HK, Hamunen K, Takala A, Kontinen VK. Systematic review of analgesics and dexamethasone for post-tonsillectomy pain in adults. Br J Anaesth. 2019;123(2):e397–e411. doi:10.1016/j.bja.2019.04.063
25. Abdallah FW, Johnson J, Chan V, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomized, triple-arm, double-blind, placebo-controlled trial [published correction appears in Reg Anesth Pain Med. 2015 Jul-Aug;40(4):398]. Reg Anesth Pain Med. 2015;40(2):125–132. doi:10.1097/AAP.0000000000000210
26. Rwei AY, Sherburne RT, Zurakowski D, et al. Prolonged duration local anesthesia using liposomal bupivacaine combined with liposomal dexamethasone and dexmedetomidine. Anesth Analg. 2018;126(4):1170–1175. doi:10.1213/ANE.0000000000002719
27. Kirkham KR, Jacot-Guillarmod A, Albrecht E. Optimal dose of perineural dexamethasone to prolong analgesia after brachial plexus blockade: a systematic review and meta-analysis. Anesth Analg. 2018;126(1):270–279. doi:10.1213/ANE.0000000000002488
28. Woo JH, Kim YJ, Kim DY, et al. Dose-dependency of dexamethasone on the analgesic effect of interscalene block for arthroscopic shoulder surgery using ropivacaine 0.5%: a randomised controlled trial. Eur J Anaesthesiol. 2015;32(9):650–655. doi:10.1097/EJA.0000000000000213
29. Bravo D, Aliste J, Layera S, et al. A multicenter, randomized comparison between 2, 5, and 8 mg of perineural dexamethasone for ultrasound-guided infraclavicular block. Reg Anesth Pain Med. 2019;44(1):46–51. doi:10.1136/rapm-2018-000032
30. Griffiths JD, Barron FA, Grant S, Bjorksten AR, Hebbard P, Royse CF. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105(6):853–856. doi:10.1093/bja/aeq255
31. De Oliveira GS, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588. doi:10.1097/ALN.0b013e31822a24c2
32. Barry GS, Bailey JG, Sardinha J, Brousseau P, Uppal V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth. 2021;126(4):862–871. doi:10.1016/j.bja.2020.10.035
33. Zhang D, Zhou C, Wei D, et al. Dexamethasone added to local anesthetics in ultrasound-guided transversus abdominis plain (TAP) block for analgesia after abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2019;14(1):e0209646. doi:10.1371/journal.pone.0209646
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.