Back to Journals » Journal of Pain Research » Volume 16
Effect of High-Definition Transcranial Direct Current Stimulation on Headache Severity and Central μ-Opioid Receptor Availability in Episodic Migraine
Authors DaSilva AF, Kim DJ , Lim M , Nascimento TD , Scott PJH , Smith YR, Koeppe RA, Zubieta JK, Kaciroti N
Received 23 February 2023
Accepted for publication 27 June 2023
Published 21 July 2023 Volume 2023:16 Pages 2509—2523
DOI https://doi.org/10.2147/JPR.S407738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Andrea Tinnirello
Alexandre F DaSilva,1,2,* Dajung J Kim,1,2,* Manyoel Lim,1,2 Thiago D Nascimento,1,2 Peter JH Scott,3 Yolanda R Smith,4 Robert A Koeppe,3 Jon-Kar Zubieta,5 Niko Kaciroti6
1Headache and Orofacial Pain Effort (H.O.P.E.) Laboratory, Department of Biologic and Materials Sciences & Prosthodontics, University of Michigan School of Dentistry, Ann Arbor, MI, USA; 2Michigan Neuroscience Institute, University of Michigan, Ann Arbor, MI, USA; 3Department of Radiology, University of Michigan, Ann Arbor, MI, USA; 4Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA; 5Department of Psychiatry, Mass General Brigham, Newton-Wellesley Hospital, Newton, MA, USA; 6Department of Biostatistics, University of Michigan, Ann Arbor, MI, USA
*These authors contributed equally to this work
Correspondence: Alexandre F DaSilva, Headache and Orofacial Pain Effort (H.O.P.E.) Laboratory, Michigan Neuroscience Institute, 205 Zina Pitcher PI, Room 1021, Ann Arbor, MI, 48109-5720, USA, Email [email protected]
Objective: The current understanding of utilizing HD-tDCS as a targeted approach to improve headache attacks and modulate endogenous opioid systems in episodic migraine is relatively limited. This study aimed to determine whether high-definition transcranial direct current stimulation (HD-tDCS) over the primary motor cortex (M1) can improve clinical outcomes and endogenous μ-opioid receptor (μOR) availability for episodic migraineurs.
Methods: In a randomized, double-blind, and sham-controlled trial, 25 patients completed 10-daily 20-min M1 HD-tDCS, repeated Positron Emission Tomography (PET) scans with a selective agonist for μOR. Twelve age- and sex-matched healthy controls participated in the baseline PET/MRI scan without neuromodulation. The primary endpoints were moderate-to-severe (M/S) headache days and responder rate (≥ 50% reduction on M/S headache days from baseline), and secondary endpoints included the presence of M/S headache intensity and the use of rescue medication over 1-month after treatment.
Results: In a one-month follow-up, at initial analysis, both the active and sham groups exhibited no significant differences in their primary outcomes (M/S headache days and responder rates). Similarly, secondary outcomes (M/S headache intensity and the usage of rescue medication) also revealed no significant differences between the two groups. However, subsequent analyses showed that active M1 HD-tDCS, compared to sham, resulted in a more beneficial response predominantly in higher-frequency individuals (> 3 attacks/month), as demonstrated by the interaction between treatment indicator and baseline frequency of migraine attacks on the primary outcomes. These favorable outcomes were also confirmed for the secondary endpoints in higher-frequency patients. Active treatment also resulted in increased μOR concentration compared to sham in the limbic and descending pain modulatory pathway. Our exploratory mediation analysis suggests that the observed clinical efficacy of HD-tDCS in patients with higher-frequency conditions might be potentially mediated through an increase in μOR availability.
Conclusion: The 10-daily M1 HD-tDCS can improve clinical outcomes in episodic migraineurs with a higher baseline frequency of migraine attacks (> 3 attacks/month). This improvement may be, in part, facilitated by the increase in the endogenous μOR availability.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier - NCT02964741.
Keywords: HD-tDCS, neuromodulation, migraine, headache, pain, mu-opioid receptor
Introduction
Migraine is a highly disabling primary headache disorder featuring recurrent headache attacks associated with throbbing pain, vomiting, nausea, and photophobia/phonophobia,1,2 with a 1-year prevalence of nearly 15% in the general population.3 Although our understanding of the pathogenesis of migraine has progressed much, currently available therapeutics to reduce the severity or prevent attacks are still unsatisfactory to many migraine sufferers.4 Moreover, inadequate management of pain and overuse or misuse of medications such as opioids could exacerbate the disease process (eg, increased headache frequency), which deteriorates the quality of life of affected individuals.5 In this context, non-invasive brain stimulation techniques such as transcranial direct current stimulation (tDCS) have showed promise as a potential non-pharmacological treatment approaches for those who are ill-suited to existing treatment options.6
tDCS is designed to shift the excitability of the brain, either hyperpolarizing (inhibitory) or depolarizing (excitatory), by focalizing current flow using two types of electrodes consisting of an anode and cathode; thereby, tDCS modulates the neural network by promoting network synchronization and plasticity.7 To this end, the primary motor cortex (M1) has been studied extensively as a target for alleviating pain symptoms.8 Indeed, M1-tDCS applied to episodic migraineurs has produced promising results in reducing headache days and pain intensity in a safe and well-tolerated way.9,10 However, we are aware that there are remaining challenges to be addressed to optimize outcomes.11,12 One key challenge identified is to increase the number of sessions at a clinical setting to optimize and extend the therapeutic effect of neuromodulation on a patient’s outcome. To address this challenge, we have been developing a new protocol that incorporates home-based HD-tDCS to increase the dose of treatment and patients’ adherence to the treatment regimen.8
Accumulating evidence has shown that tDCS with novel high-definition (HD) montages can promote the focality of current distribution compared to conventional tDCS, which activates the brain far beyond the targeted region, for example, through widespread structural and functional neural connection.13,14 Moreover, HD-tDCS outperformed the conventional tDCS in terms of inducing neuroplasticity which lasted longer15,16 and was validated for its potential in managing chronic pain such as temporomandibular disorder17 and fibromyalgia.18 However, no studies have been conducted to establish the efficacy of M1 HD-tDCS in episodic migraineurs and, most importantly, the neuro-mechanism by which it relieves migraine symptoms.
It has been revealed that µOR concentration would be compromised as acute pain develops in a chronic form,19 which can be observed by a higher release of µOR activating peptides (eg, beta-endorphin, enkephalin). These observations hold for various chronic pain disorders, including migraine,20 fibromyalgia,21 orofacial pain,22 and neuropathic pain.23 We showed that μOR availability was lower in patients with chronic migraine compared to episodic migraine in the right amygdala and left parahippocampal gyrus implicated in pain chronification24 and hypersensitivity.25 Moreover, reduced μOR availability was correlated with a lower pain threshold and headache severity,20 indicating that μOR characteristics are possibly associated with ineffective pain processing, which might be the neural correlate of migraine severity.
Herein, we assessed whether M1-tDCS with a highly focused neuromodulation montage would reduce headache days and pain severity in episodic migraineurs. We further evaluated the hypothesis that the therapeutic effect of HD-tDCS would be mediated by restoring μOR availability in the limbic regions.
Materials and Methods
Study Design
This was a single-center, randomized, double-blind, and sham-controlled study including five phases of evaluation following: baseline screening, pre-treatment Positron Emission Tomography (PET)/Magnetic Resonance Imaging (MRI) 10-daily M1 HD-tDCS either active or sham over 2 weeks, post-treatment PET/MRI, and 1-week, 1-, and 2-month follow-up assessments between February 2017 and April 2020 (Figure 1A). Of note, according to the guidelines of the International Headache Society for controlled trials of preventive treatment of migraine attacks in migraine,26,27 our pre-specified outcomes in the original protocol were switched from a change in pain intensity to moderate-to-severe (M/S) headache days (primary) and headache intensity and rescue medication use (secondary). This switch in clinical outcomes was also in agreement with the National Institute of Health.
 |
Figure 1 Trial design and CONSORT study flow. (A) This study included five phases of evaluation: screening; baseline PET/MRI session; 10 daily M1 HD-tDCS, either active or sham, over two weeks; follow-up PET/MRI session; and follow-up assessments at 1-week, 1-month, and 2-month after the end of the treatment. (B) CONSORT flow diagram includes screening, allocation to treatment, follow-ups, and analyses. Adapted from Schulz KF, Altman DG, Moher D, CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3):e1000251. Copyright: © 2010 Schulz et al. Creative Commons Attribution License.28 Abbreviations: HD-tDCS, high-definition transcranial direct current; MRI, magnetic resonance imaging; PET, positron emission tomography. |
Patient Recruitment
Study participants were recruited from the University of Michigan Hospital clinics or the local community via advertisements posted on bulletin boards. Episodic migraine with or without aura was defined by the International Classification of Headache Disorders (ICHD), 3rd edition (beta version) (ICHD-3.1/2),1 experiencing 2 to 14 headache days per month and at least one year of history. The exclusion criteria for this study were 1) a history of other chronic pain disorders and other types of migraine such as hemiplegic migraine or cluster headache, 2) medication overuse or opiate intake for over 6 months, 3) hormonal contraceptives, 4) a history of concurrent psychiatric disorders or neurological disorders, 5) ongoing and unresolved disability litigation, 6) preventive medication and use of an investigational drug or device within 30 days of study entry, 7) pregnant or planning to become pregnant, or 8) contraindication to MRI such as implanted pacemaker or claustrophobia. The patients agreed to take abortive rescue medicine for managing headaches but not for preventive purposes.
Randomization and HD-tDCS Intervention
Among 293 patients assessed for eligibility, we enrolled 28 patients and randomized them into active or sham treatment in a 1:1 ratio using the Taves covariate adaptive randomization method balancing sex and age.29 We reported the study flow under the Consolidated Standards of Reporting Trials (CONSORT) guideline (Figure 1B).28 A sample size of twenty-five who completed the study would have 80% power to detect an effect size of 1.2 SD with α = 0.05 two-sided Type I error. Twelve age- and sex-matched healthy control participants consisting of 1 male and 11 females (mean age, 32.4, SD 15.3 years) were also included for the baseline PET/MRI scan without neuromodulation. The principal investigator, research staff, and patients were blinded to the treatment allocation during and until the end of the data analyses, except for the research staff responsible for operating HD-tDCS.
We used M1 HD-tDCS stimulator (Soterix Medical Inc., NY, USA) to promote spatial focality of stimulation, thereby increasing current intensity on the targeted region.14 A 2 × 2 ring configuration with 12-mm diameter disk electrodes, developed in-house, consisting of two anodes and two cathodes, was placed posterior to the anterior direction across the face/head homuncular M1 region contralateral to the worst headache side. In the case of bilateral pain, we placed electrodes on the left M1. Explicit 10–10 system locations for anodes were C3 and C5, and cathodes were FC3 and FC5. An active M1 HD-tDCS with 2 mA was applied for 20 minutes/day over 2 weeks. The same montages were used for a sham session; however, the current was applied for only 30 seconds at the beginning and end of the session, delivering a tingling sensation to the scalp,30 believed to be reliable for a sham experiment.
PET/MRI Acquisition
We acquired the pre-treatment and post-treatment PET with [11C]CFN data several days before and after M1 HD-tDCS treatment (4.7 and 6.2 days on average, respectively). [11C]CFN was synthesized as previously described.31 Each PET session consisted of a 40-min early resting state after [11C]CFN injection followed by a 50-min late phase, including the Sustained Thermal Pain Threshold Stress (STPTS) challenge. The PET/MRIs were performed outside attacks (interictal period). Detailed procedures for PET and T1-weighted MRI acquisition, pre-processing, and analyses are described in Supplementary Data. Of the 25 patients who participated in active or sham HD-tDCS treatment, 13 and 11 patients for each active and sham group were included in the PET analyses due to a technical failure during pre-treatment PET scanning for one sham patient.
Clinical Outcomes
The two primary endpoints were the number of moderate-to-severe (M/S) headache days and the proportion of participants with ≥50% reduction in the M/S headache days 1-month following the end of the two-week tDCS session. The M/S headache days were defined as days with moderate or severe pain intensity with a minimum value of 4 based on a 0–10 numeric rating scale (NRS) and at least 4 hours of untreated headache. However, a minimum time was not specified if patients had rescue medication (migraine-specific, non-steroidal anti-inflammatory drug, or non-aspirin pain reliever). The secondary endpoints were the headache intensity that classified none-to-mild pain (NRS: 0–3) or M/S pain (NRS: 4–10) and any rescue medication intake over a 1-month post-intervention (yes/no). At each visit, headache frequency, intensity, and medication usage were assessed by patients’ recall and recorded using PainTrek technology (University of Michigan, MI), a 3D-pain tracking mobile application. Following data collection over a one-month period, we computed the average headache intensity within that timeframe. As an additional exploratory outcome, we also recorded the maximum pain intensity and location (eg, body subregion, side) using the PainTrek. We utilized the PainTrek-technology to enable patients to record not only their pain intensity but also the specific area and location of their headaches, allowing for a comprehensive analysis of the spatial aspects of their pain and enhancing the overall understanding of their migraine episodes.32
Statistical Analysis
Demographic and clinical characteristics between diagnostic and treatment groups were compared using an independent sample t-test and chi-square test for continuous variables and categorical variables, respectively. We used Poisson regression, suitable for count type measures, on the number of days with M/S headache over 1-month post-intervention as the outcome variable and treatment indicator (active vs sham) as a predictor. We performed logistic regression for the other primary outcome – a responder rate (achieving 50% or more reduction in M/S headache days compared to baseline), and for secondary outcome measures, including headache intensity (none-to-mild or M/S) and rescue medication use (yes/no) over 1-month post-intervention as the outcome variable and treatment indicator as a predictor. We sought to assess if the intervention effect varied by baseline migraine severity as measured by the migraine attack frequency. Therefore, we included the monthly migraine attacks at baseline (during the last 30 days) and their interaction with treatment in the model for each primary and secondary outcome. A significant interaction term would indicate a differential treatment effect based on the baseline frequency of migraine attacks. We displayed predicted values and 95% confidence interval (CI) of clinical outcomes by treatment group and pre-treatment frequency of migraine attacks. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC), and statistical significance was set as p < 0.05 (2-sided).
PET Data Analysis
Linear mixed-model analysis was applied to measure longitudinal changes of BPND during the resting state and during the STPTS challenge phase including treatment group (active vs sham), time, and a group-by-time interaction term as factors of interest (fixed effect) and subject as a random effect while adding age as a covariate of no interest using Statistical parametric Mapping (SPM) 12 (Wellcome Department of Cognitive Neurology, University College London, England; http://www.fil.ion.ucl.ac.uk/spm/) implanted in MATLAB (version R2016b, MathWorks, Inc). Only voxels with values over 0.1 (BPND) were included in the analysis. We utilized the small-volume correction (SVC) approach to focus on our a priori hypothesized regions for both basal resting and STPTS phase including the left and right amygdala, parahippocampal gyrus, temporal pole, hypothalamus, thalamus, and rostral anterior cingulate cortex (rACC) linked to opioidergic pain modulatory function, migraine pathophysiology, and tDCS effects as identified in previous studies.33–36 We used the Harvard-Oxford cortical and subcortical structural atlas to define a priori mask regions. For the hypothalamus, we used Neurosynth meta-analytic maps (http://neurosynth.org) with the term “hypothalamus” (Figure S1). The significance was set to voxel-level p < 0.005 (uncorrected), combined with a cluster-level family-wise error (FWE)-corrected p < 0.05. Additionally, we assessed the ventrolateral periaqueductal gray (vlPAG) as a region of interest due to its small size and tested changes of vlPAG µOR BPND only during the STPTS challenge phase, considering its essential role in µOR-related anti-nociception.37–39 The coordinates and size for right and left vlPAG were MNI (Montreal Neurological Institute) xyz = 4, −30, −8 and xyz = −4, −30, −8 surrounded by 3-mm radius sphere, respectively.38,39
Acknowledging that M1 HD-tDCS efficacy on clinical outcomes was varied by baseline frequency of migraine attack, we assessed if regional changes in µOR BPND (showing significant interaction between time and treatment group) would differ between active and sham treatment in each stratified group into higher (>3 attacks/month) and lower (≤3 attacks/month) using the Mann–Whitney U-test. Bonferroni correction was applied to correct multiple comparison (α = 0.006; 0.05/8 comparisons).
Moreover, we conducted an exploratory mediation analysis to examine and corroborate, in higher-frequency patients, whether the treatment effect (predictor) on M/S headache days (outcome) was mediated through the voxel-wise changes in µOR BPND (mediator) within our a priori masks including temporal pole, hippocampus, parahippocampal gyrus, hypothalamus, and amygdala. As shown in Figure S2, path a denotes the association between predictor and mediator, and path b the association between mediator and outcome controlling for predictor. Path c represents the overall relationship between predictor and outcome, whereas path c’ is the direct effect controlling for the mediator. The mediating effect (a*b) was tested based on the significance of c-c’ determined with a bootstrap resampling (N = 10,000 iterations) using the Mediation Toolbox for Matlab (https://github.com/canlab/MediationToolbox).40,41
Patient-Reported Side Effects
We used a questionnaire administered every tDCS session to assess side effects, including headache, neck pain, scalp pain, scalp burns, tingling, skin redness, sleepiness, trouble concentrating, acute mood change, and others. Symptom severity was classified as absent, mild, moderate, and severe. In each side effect, we calculated the percentage of patients by dividing the number of patients who reported complaints at least once out of ten sessions by the total number of patients in each treatment group.
Results
Baseline Characteristics
Among those screened for eligibility (n = 293), 28 episodic migraineurs were enrolled, and a total of 13 active and 12 sham participants completed 10 daily HD-tDCS and follow-ups. The two treatment groups were similar in terms of baseline demographic and migraine characteristics, including M/S headache days, pain intensity, the proportion of patients taking rescue medication, frequency, chronicity, and presence of an aura (Table 1).
 |
Table 1 Baseline Characteristics of Episodic Migraineursa |
M1 HD-tDCS Effect on Primary Outcomes
M1 HD-tDCS was well tolerated without any serious adverse effects in either group, consistent with the known low risk of tDCS.42,43 The M/S headache days over 1-month following HD-tDCS treatment were not different between the active and sham-treated groups; 2.9 days (95% confidence interval [CI], 2.1 to 3.9) and 3.3 days (95% CI 2.4 to 4.4), respectively (p = 0.64).
In addition, a responder rate (≥50% reduction of M/S headache days compared to baseline) was greater for active (76.9%, 95% CI 55.1 to 99.2) than the sham group (50.0%, 95% CI 22.6 to 76.5) but not significant (p = 0.15). However, M1 HD-tDCS efficacy on M/S headache days over 1-month after treatment varied between the two groups by baseline frequency of migraine attacks (β = −0.35, 95% CI −0.53 to −0.18, p < 0.001 for interaction) (Figure 2A).
M1 HD-tDCS Effect on Secondary Outcomes
The secondary outcome assessed the presence of M/S headache pain and the use of rescue medication over 1-month follow-up. Our findings indicate that the occurrence of M/S headache for the active group was 53.8% (95% CI, 28.2 to 77.6) and the sham group was 66.7% (95% CI 37.6 to 86.9). The difference between the groups, however, was not statistically significant (p = 0.52). Similarly, the use of rescue medication was 38.5% (95% CI 17.0 to 0.65.6) in the active group and 66.7% (95% CI 37.6 to 86.9) in the sham group. Again, the difference between the groups was not statistically significant (p = 0.16).
Subgroup Analysis
Following a significant interaction term between baseline frequency and M/S headache days at 1-month follow-up, we stratified the patients into higher (n = 14; 8 for active and 6 for sham) and lower frequency groups (n = 11; 5 for active and 6 for sham). Among higher-frequency patients (>3 attacks/month), the active group had fewer M/S headache days (2.6, 95% CI 1.8 to 4.2) compared with sham group (5.5, 95% CI 3.9 to 7.7; p = 0.012), whereas this therapeutic effect was opposite in direction in the lower-frequency patients (≤3 attacks) (active: 3.4, 95% CI 2.1 to 5.5; sham: 1.2, 95% CI 0.6 to 2.4; p = 0.017). Logistic regression analysis revealed that the treatment effect on responder rate was changed by baseline frequency of migraine attacks (β = 2.37, 95% CI 0.74–4.62, p = 0.017 for interaction) (Figure 2B), such that higher-frequency patients of the active group showed a better response rate than the peers of the sham group (active: 87.5%, 95% CI 46.3 to 98.3; sham: 6.7%, 95% CI 2.3 to 63.1; p < 0.018), whereas this effect was not observed in lower-frequency patients (p = 0.36).
As to the secondary outcomes, the effect of treatment on the probability of having an M/S headache and the use of rescue medication over 1-month post-intervention differed by monthly migraine attack frequency (p < 0.001 for interaction) (Figure 2C and D). We then determined the predicted probabilities of secondary endpoints in each frequency group. We found that the active treatment reduced the likelihood of having M/S headache pain compared with the sham group (62.5% vs 100%; p < 0.001), only for higher-frequency patients. Similarly, the active treatment for higher-frequency patients reduced the likelihood of abortive medication intake compared to the sham counterpart (37.5% vs 100%; p < 0.001). However, we did not observe treatment effects of HD-tDCS neither on the headache intensity (active vs sham, 40.0% vs 33.3%; p = 0.82) nor on the abortive medication intake (40.0% vs 33.3%; p = 0.82) in lower-frequency patients.
Additional Secondary Outcomes Specific to the Side of the M1 HD-tDCS
To investigate whether the HD-tDCS specifically modulates pain across ipsilateral or contralateral side regions to the stimulation, we additionally compared the maximum pain intensity subdivided into 4 categories (0, none; 1, mild; 2, moderate; 3, severe). Briefly, active HD-tDCS, compared with the sham control, lowered the probability of having M/S headache pain contralateral to the stimulation for 1-month after treatment as the migraine attack frequency at baseline increased (p < 0.001 for interaction) (Figure S3A). However, this treatment effect was not observed on the ipsilateral side of the stimulation (p = 0.35 for interaction) (Figure S3B). Similarly, higher-frequency patients treated with active HD-tDCS were also less likely to have M/S intensity pain on the contralateral side of HD-tDCS compared to those with sham HD-tDCS (active vs sham, 75.0% vs 100%; p < 0.001). This effect was absent in the lower-frequency patients (40.0% vs 33.3%, p = 0.82). In contrast, we did not find any beneficial effect in the ipsilateral side to HD-tDCS, on either higher-frequency group (50.0% vs 66.7%, p = 0.54) or lower-frequency group (40.0% vs 16.7%, p = 0.40).
M1 HD-tDCS Effect on μOR Availability
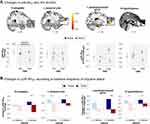
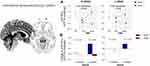
Notably, there was an interaction effect between time and treatment on voxel-wise µOR BPND in a priori hypothesized regions, including the right amygdala (peak MNI xyz = 28, 4, -28; k = 14) and left temporal pole (xyz = −28, 8, -46; k = 50) during the resting state and right hypothalamus (xyz = 2.4,-18; k = 8) and left parahippocampal gyrus (xyz = −18, -2, -32, k = 54) during the late phase, driven mainly by increased μOR BPND in the active group or decrease in the sham group (Figure 3A). Further, the μOR BPND increases were primarily observed in higher-frequency patients treated with active HD-tDCS compared to sham counterparts specifically in the amygdala (during resting) and the parahippocampal gyrus (during STPTS challenge), with a significant change found in the parahippocampal gyrus after a Bonferroni correction (Figure 3B).
As to the ROI analysis of vlPAG, we found a similar pattern, such that active treatment increased right vlPAG μOR BPND compared to sham during STPTS challenge (Figure 4A). Furthermore, we observed a marked increase only in patients with higher-frequency condition, although the significance was marginal (Figure 4B). When we re-analyzed the interaction effects between treatment group and time while only including higher-frequency patients with the same statistical threshold, we additionally found increased μOR BPND in the right rostral anterior cingulate cortex (rACC) after active treatment compared to sham (Figure S4).
Increased μOR Availability as a Potential Mediator of HD-tDCS Efficacy
We conducted the mediation analysis to identify voxels of mediators within the amygdala and temporal pole (resting-state) and hypothalamus and parahippocampal gyrus (STPTS phase) with a treatment indicator as the predictor and M/S headache days over the 1-month follow-up as the outcome in higher-frequency patients. The result revealed that a portion of the left parahippocampal gyrus mediated the relationship between treatment and M/S headache days over the 1-month follow-up (Figure S2). Specifically, the active HD-tDCS over M1, compared to sham, increased the µOR BPND in the anterior portion of the left parahippocampal gyrus (path a: peak voxel xyz coordinates: −22, 0, −36; Z = 3.83, p = 0.0001), which predicted a fewer M/S headache days (path b: Z = −4.03; p = 0.0001; path a*b: Z = −4.67; p < 0.0001), controlling for treatment indicator (q < 0.05 false discovery rate-corrected).
Patient-Reported Side Effects
As to side effects reported by participants after each HD-tDCS session, the tingling sensation, primarily mild, was the most frequently reported; 53.8% for the active and 91.7% for the sham group (Figure 5). We confirmed discomfort on the head and neck from the treatment soothed shortly after each session. In addition, the patients acknowledged that they could withdraw their participation at any point during the study. There were no treatment-related serious adverse events. No patients decided to stop treatment due to adverse events.
Discussion
Overall Observations from the Study Participants
The primary outcomes, specifically the number of moderate-to-severe (M/S) headache days and 50% responder rate, and the secondary outcomes (M/S headache intensity and use of rescue medication) were not significantly different between the active and sham groups following the 2-week session of HD-tDCS treatment. However, in subsequent analyses, we discovered that the efficacy of M1 HD-tDCS on M/S headache days over 1-month period after treatment varied between the two groups depending on the baseline frequency of migraine attacks. Specifically, the active HD-tDCS was more effective than the sham in both the primary and secondary outcomes in those with higher baseline frequency (>3 attacks/month). These favorable outcomes in high-frequency patients were potentially mediated by an increase in the concentration of µOR in the brain regions involved in pain modulation,44 stress response,45 and possible origin of a migraine attack.46
Efficacy of tDCS in Comparisons with Previous Data
Although still debatable as to tDCS efficacy in migraine,12,47 combined efforts over the past years have demonstrated that enhancing the focality of the stimulation target15 or increasing the number of treatment sessions9 can overcome prior mixed results. We first applied M1 HD-tDCS with molecular neuroimaging arms to episodic migraine, and this neuromodulation technique was well tolerated in patients, with few adverse events aside from tingling and sleepiness. The impact of M1 HD-tDCS seemed to take effect shortly after treatment since we found reduced M/S headache days over 1-month after the end of treatment. However, the therapeutic benefit for active M1 HD-tDCS compared to sham was only seen in patients with higher frequency migraine attacks (>3 attacks/month), while no significant differences were observed in patients with lower frequency (≤3 attacks/month) before treatment. These trends were also noted in the secondary outcomes, including pain intensity and abortive medication, such that higher-frequency patients treated with active HD-tDCS were less likely to exhibit M/S pain or use of rescue medication over 1-month post-treatment compared with their sham counterparts. The present result partly aligns with previous investigations showing excitatory M1 stimulation reduced headache intensity and attack frequency.10 However, it is worth noting that the effectiveness of excitatory conventional M1 tDCS in reducing headache intensity and attack frequency remains still inconsistent. Hence, further research is still needed to better understand the precise mechanisms through which tDCS exerts its effects on episodic migraines.
Evidence of Contralateral Pain Management Induced by HD-tDCS
On a further note, maximum pain intensity (pain edge) measured by PainTrek over a 1-month follow-up according to the baseline attack frequency was only observed on the contralateral side of the stimulation but not on the ipsilateral one, suggesting that focused M1 HD-tDCS modulates migraine pain in a highly controlled way as demonstrated in our previous M1 HD-tDCS study involving patients with temporomandibular disorder.17,32 It is well known that the primary sensorimotor cortices (S1/M1) mainly control pain and movement in/from the opposite body side. Thus, this study extends the idea that bilateral excitatory-focused M1 stimulation could be more effective in treating chronic pain disorders, given that many chronic pain patients suffer from bilateral pain symptoms.8
Clinical Implication of µOR Function in Migraine
Migraine attacks typically appear in adolescents or young adults (eg, 17.5 yr on average in the current study).3 Thus, migraineurs’ brains likely have a high μ-opioid peptide drive to manage long-term attacks, pain, and stress, which might translate into μOR downregulation or internalization.19 This phenomenon can significantly impact the effectiveness of exogenous opioid in pain relief during severe migraine attacks. As opioids primarily bind to the μORs, and any alterations or decreased availability of these receptors may result in limited efficacy of opioids in managing migraine pain. Here, our repetitive active M1 HD-tDCS enhances baseline µOR concentration, compared to the sham. The observed changes occurred in the limbic and paralimbic regions; specifically, we found sustained or increased µOR BPND in the right amygdala and left temporal pole during the resting state in the active group, whereas the sham group showed only decreased patterns. Likewise, we found an increased µOR BPND in the parahippocampal gyrus and hypothalamus during the thermal pain threshold challenge. Notably, aligned with the clinical findings, higher-frequency patients treated with active M1 HD-tDCS displayed a marked increase in µOR BPND. This was particularly evident when compared to the sham counterpart, especially in areas such as the right amygdala and left parahippocampal gyrus, which is responsible for emotional pain processing48 and stress response to aversive stimuli.49
These results align with the studies supporting the involvement of limbic systems in pain relief50 and even a migraine attack.51,52 Indeed, recent research showed that the hypothalamus plays a pivotal role in generating migraine attacks supposedly through inter-relationship with limbic regions.51
Changes of µOR Availability as a Potential Mechanism of Brain Stimulation
It is also known that the µ-opioid system plays an essential role in the regulation of hedonic homeostasis, such as promoting stress responses.53 Moreover, µOR acts as an interface between emotional and physical stress regulation.54 Thus, it is reasonable to assume that an increased µOR concentration might enable migraineurs to cope better with internal/external stressors, which might protect migraineurs from having another attack and related pain, given that stress is one of the most probable trigger factors in migraine symptoms.55 The exploratory mediation analysis supported the hypothesis mentioned above that the µOR BPND changes in the parahippocampal region potentially facilitated the therapeutic efficacy of the M1 HD-tDCS on M/S headache days. The possibility that tDCS could potentially elevate μOR availability within the regions crucial for pain perception and modulation gives it a distinct advantage over prescribed opioids. Traditional opioid medications for pain relief are notorious for their significant risk of adverse side effects, including tolerance, addiction, and deleterious physical and psychological consequences. In contrast, tDCS appears to enhance our natural pain-modulating systems, potentially offering a therapeutic benefit while avoiding the risks linked with opioid usage. However, given the small sample size in our study, we caution against drawing definitive conclusions from our exploratory mediation analysis. Instead, these findings should be viewed as initial observations, requiring further investigation in larger-scale studies.
Previous preclinical and clinical studies have demonstrated that M1 stimulation facilitates descending pain modulatory pathways in concert with the opioid receptor system.56 In an invasive M1 stimulation combined with longitudinal PET with non-selective opioid receptor agonist [11C]diprenorphine applied to neuropathic pain, there was significant pain reduction associated with an endogenous opioid release in the anterior middle cingulate cortex and PAG.57 In our previous single M1 tDCS study with a specific µOR radiotracer [11C]CFN PET, we observed stimulation-induced µ-opioid peptide release in pain modulatory regions in chronic pain and healthy participants.58,59 Conversely, opioid antagonist naloxone, exhibiting the greatest affinity to µOR, abolished the analgesic effect of M1 stimulation.60 These findings collectively suggest that the therapeutic effects of tDCS might rely on the µOR function.
Notably, M1 HD-tDCS treatments conducted over a 2-week period increased µOR BPND in the right vlPAG, a region involved in descending pain modulation, during STPTS challenge, compared to sham, with a more marked increase in the higher-frequency migraineurs. Thus, these findings align with previous reports that suggest an impact of M1 stimulation on the opioid system, albeit with a slight deviation. Specifically, we observed an increase in µOR availability, also suggesting less release of µOR-activating peptides. We speculate that this increase might reflect to enhanced efficiency of the µOR system, which potentially facilitates better pain modulation or stress responses.
Limitations
Regarding other factors potentially contributing to the treatment effect, it can be argued that non-therapeutic factors, such as regression to the mean, could be associated with clinical improvement.61 Also, treatment expectancy is a known factor contributing to pain relief, possibly by activating endogenous opioid circuits; however, using a sham control group reduces the likelihood that placebo-related mechanisms underlie the observed effects of M1 HD-tDCS. While a 10-10 method is a widely adopted method for tDCS target placement especially for the motor cortex, we acknowledge the potential limitation of this method. To address this limitation, future studies could consider incorporating additional techniques, such as neuroimaging or neuro-navigation, to improve the precision and reliability of electrode placement.
Additionally, most of our subjects were female; therefore, the current results may not be entirely generalizable to male migraineurs. Moreover, although we used M/S headache days as a primary outcome and clinical correlation, patients were monitored in our frequent in-person lab sessions, not at home, in the first 1-week and 1- and 2-month follow-ups using the PainTrek. At this time, we acknowledge that our findings are primarily applicable to the specific population studied here; larger studies are warranted to extend these findings to a broader population of migraine with potentially varying characteristics and more treatment days to extend the current findings as well as maximize the HD-tDCS efficacy on migraine symptoms.
Conclusion
Altogether, our results highlighted that M1 HD-tDCS could be beneficial in reducing M/S headache days, pain intensity, and the use of rescue medication in high-frequency patients with episodic migraine along with increased µOR availability in the brain in a manner proportional to the improvement in migraine severity, representing a potential therapeutic mechanism of M1 HD-tDCS actions on clinical outcomes. Lastly, our results highlight that the indirect modulation of µORs by M1 HD-tDCS – as opposed to direct by externally administered opioid medications – may be an effective non-pharmacological treatment option to explore in high-frequent migraine patients. However, a study with a larger sample size is needed to confirm the robustness of our findings.
Data Sharing Statement
The data supporting this study’s findings are available from the corresponding author ([email protected]) upon reasonable request.
Ethics Approval and Informed Consent
The study protocol was approved by the University of Michigan Institutional Review Boards (HUM00107286) and registered at ClinicalTrials.gov (NCT02964741). We conducted the study under the Declaration of Helsinki. All study participants provided written informed consent.
Acknowledgments
We thank the patients who participated in this randomized trial, previous research coordinators (Ifeyinwa Arinze and Dalya Saleem) who performed administrative, technical, or material support, and staff at the PET Center of the University of Michigan hospital and fMRI Lab for providing core facilities and services throughout the study.
Funding
This research was supported by grant 1-R01-NS094413 from the National Institute of Neurological Disorders and Stroke (Dr. DaSilva). The funding agency had no role in the design and conduct of the study.
Disclosure
The contents described within this study, PainTrek and H.O.P.E. M1 HD-tDCS, have been developed at the University of Michigan and disclosed to the University of Michigan Office of Technology Transfer. All intellectual property rights, including but not limited to patents/patent applications, trademarks, and copyright of software, algorithms, reports, displays, and visualizations, are owned by the Regents of the University of Michigan. Dr. DaSilva is the main inventor of the mobile technology now called PainTrek (previously GeoPain, MoxyTech), which is owned by the University of Michigan. Other authors declare no conflict of interest in this work. Present address for Manyoel Lim: Food Convergence Research Division, Korea Food Research Institute, Republic of Korea.
References
1. Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808. doi:10.1177/0333102413485658
2. Ashina M, Ropper AH. Migraine. N Engl J Med. 2020;383:1866–1876. doi:10.1056/NEJMRA1915327
3. Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–976. doi:10.1016/S1474-4422(18)30322-3
4. Pike J, Mutebi A, Shah N, et al. Factors associated with a history of failure and switching migraine prophylaxis treatment: an analysis of clinical practice data from the United States, Germany, France, and Japan. Value Health. 2016;19:A68. doi:10.1016/j.jval.2016.03.213
5. May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12:455–464. doi:10.1038/nrneurol.2016.93
6. Moisset X, Lefaucheur JP. Non pharmacological treatment for neuropathic pain: invasive and non-invasive cortical stimulation. Rev Neurol (Paris). 2019;175:51–58. doi:10.1016/j.neurol.2018.09.014
7. Knotkova H, Borckardt JJ, Riggs A, DaSilva AF. Transcranial Direct Current Stimulation Potential for Pain Management. Springer International Publishing; 2019.doi:10.1007/978-3-319-95948-1_18
8. DaSilva AF, Datta A, Swami J, Kim DJ, Patil PG, Bikson M. The concept, development, and application of a home-based high-definition tDCS for bilateral motor cortex modulation in migraine and pain. Front Pain Res. 2022;3:7.
9. Cai G, Xia Z, Charvet L, Xiao F, Datta A, Androulakis XM. A systematic review and meta-analysis on the efficacy of repeated transcranial direct current stimulation for migraine. J Pain Res. 2021;14:1171–1183. doi:10.2147/JPR.S295704
10. Feng Y, Zhang B, Zhang J, Yin Y. Effects of non-invasive brain stimulation on headache intensity and frequency of headache attacks in patients with migraine: a systematic review and meta-analysis. Headache. 2019;59:1436–1447. doi:10.1111/head.13645
11. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128:56–92. doi:10.1016/j.clinph.2016.10.087
12. O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2018;4:CD008208. doi:10.1002/14651858.CD008208.pub5
13. DaSilva AF, Truong DQ, DosSantos MF, Toback RL, Datta A, Bikson M. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front Neuroanat. 2015;9:89. doi:10.3389/fnana.2015.00089
14. Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–207.e1. doi:10.1016/j.brs.2009.03.005
15. Kuo HI, Bikson M, Datta A, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul. 2013;6:644–648. doi:10.1016/j.brs.2012.09.010
16. Agboada D, Mosayebi-Samani M, Kuo MF, Nitsche MA. Induction of long-term potentiation-like plasticity in the primary motor cortex with repeated anodal transcranial direct current stimulation – better effects with intensified protocols? Brain Stimul. 2020;13:987–997. doi:10.1016/j.brs.2020.04.009
17. Donnell A, Nascimento TD, Lawrence M, et al. High-definition and non-invasive brain modulation of pain and motor dysfunction in chronic TMD. Brain Stimul. 2015;8:1085–1092. doi:10.1016/j.brs.2015.06.008
18. Castillo-Saavedra L, Gebodh N, Bikson M, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain. 2016;17:14–26. doi:10.1016/j.jpain.2015.09.009
19. Gouty S, Silveira JT, Cote TE, Cox BM. Aversive stress reduces mu opioid receptor expression in the intercalated nuclei of the rat amygdala. Cell Mol Neurobiol. 2021;41:1119–1129. doi:10.1007/S10571-020-01026-7/FIGURES/5
20. Jassar H, Nascimento TD, Kaciroti N, et al. Impact of chronic migraine attacks and their severity on the endogenous μ-opioid neurotransmission in the limbic system. Neuro Image Clin. 2019;23:101905. doi:10.1016/j.nicl.2019.101905
21. Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta J-K. Decreased central μ-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi:10.1523/JNEUROSCI.2849-07.2007
22. Nascimento TD, Yang N, Salman D, et al. µ-opioid activity in chronic TMD pain is associated with COMT polymorphism. J Dent Res. 2019;98:1324–1331. doi:10.1177/0022034519871938
23. Zhou X-L, Yu LN, Wang Y, et al. Increased methylation of the MOR gene proximal promoter in primary sensory neurons plays a crucial role in the decreased analgesic effect of opioids in neuropathic pain. Mol Pain. 2014;10:51. doi:10.1186/1744-8069-10-51
24. Kato F, Sugimura YK, Takahashi Y. Pain-associated neural plasticity in the parabrachial to central amygdala circuit: pain changes the brain, and the brain changes the pain. Adv Exp Med Biol. 2018;1099:157–166. doi:10.1007/978-981-13-1756-9_14/FIGURES/2
25. Moulton EA, Becerra L, Maleki N. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine states. Cereb Cortex. 2011;21:435–448. doi:10.1093/cercor/bhq109
26. Tassorelli C, Diener H-C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38:815–832. doi:10.1177/0333102418758283
27. Diener H-C, Tassorelli C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of migraine attacks in episodic migraine in adults. Cephalalgia. 2020;40:1026–1044. doi:10.1177/0333102420941839
28. Schulz KF, Altman DG, Moher D, CONSORT Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3):e1000251.
29. Kang M, Ragan BG, Park J-H. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43:215–221. doi:10.4085/1062-6050-43.2.215
30. Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi:10.1016/j.clinph.2005.12.003
31. Blecha JE, Henderson BD, Hockley BG, et al. An updated synthesis of [11C]carfentanil for positron emission tomography (PET) imaging of the μ-opioid receptor. J Label Compd Radiopharm. 2017;60:375–380. doi:10.1002/JLCR.3513
32. Kaciroti N, DosSantos MF, Moura B, et al. Sensory-discriminative three-dimensional body pain mobile app measures versus traditional pain measurement with a visual analog scale: validation study. JMIR mHealth uHealth. 2020;8:e17754. doi:10.2196/17754
33. Schwedt TJ, Chiang -C-C, Chong CD, Dodick DW. Functional MRI of migraine. Lancet Neurol. 2015;14:81–91. doi:10.1016/S1474-4422(14)70193-0
34. Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi:10.1126/science.1060952
35. Meeker TJ, Keaser ML, Khan SA, Gullapalli RP, Seminowicz DA, Greenspan JD. Non-invasive motor cortex neuromodulation reduces secondary hyperalgesia and enhances activation of the descending pain modulatory network. Front Neurosci. 2019;13. doi:10.3389/fnins.2019.00467
36. Lim M, Kim DJ, Nascimento TD, et al. Functional magnetic resonance imaging signal variability is associated with neuromodulation in fibromyalgia. Neuromodul Technol Neural Interface. 2021;
37. Hemington KS, Coulombe M-A. The periaqueductal gray and descending pain modulation: why should we study them and what role do they play in chronic pain? J Neurophysiol. 2015;114:2080–2083. doi:10.1152/jn.00998.2014.-In
38. Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage. 2012;60:505–522. doi:10.1016/j.neuroimage.2011.11.095
39. Mills EP, Alshelh Z, Kosanovic D, et al. Altered brainstem pain-modulation circuitry connectivity during spontaneous pain intensity fluctuations. J Pain Res. 2020;13:2223–2235. doi:10.2147/JPR.S252594
40. Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi:10.1016/J.NEURON.2008.09.006
41. Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat. Neuroimage. 2009;47:821–835. doi:10.1016/j.neuroimage.2009.05.043
42. Matsumoto H, Ugawa Y. Adverse events of tDCS and tACS: a review. Clin Neurophysiol Pract. 2017;2:19–25. doi:10.1016/j.cnp.2016.12.003
43. Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9:641–661. doi:10.1016/j.brs.2016.06.004
44. Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8:143–151. doi:10.1097/SPC.0000000000000055
45. Valentino RJ, Van Bockstaele E. Endogenous opioids: the downside of opposing stress. Neurobiol Stress. 2015;1:23–32. doi:10.1016/j.ynstr.2014.09.006
46. Schulte LH, Menz MM, Haaker J, May A. The migraineur’s brain networks: continuous resting state fMRI over 30 days. Cephalalgia. 2020;40:1614–1621. doi:10.1177/0333102420951465
47. Ornello R, Caponnetto V, Ratti S, et al. Which is the best transcranial direct current stimulation protocol for migraine prevention? A systematic review and critical appraisal of randomized controlled trials. J Headache Pain. 2021;22:1–13. doi:10.1186/S10194-021-01361-0/FIGURES/7
48. Ploghaus A, Narain C, Beckmann CF, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi:10.1523/JNEUROSCI.21-24-09896.2001
49. Vachon-Presseau E, Martel MO, Roy M, et al. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci. 2013;33:6826–6833. doi:10.1523/JNEUROSCI.4584-12.2013
50. Porreca F, Navratilova E. Reward, motivation, and emotion of pain and its relief. Pain. 2017;158:S43–S49. doi:10.1097/j.pain.0000000000000798
51. Stankewitz A, Keidel L, Rehm M, et al. Migraine attacks as a result of hypothalamic loss of control. Neuro Image Clin. 2021;32:102784. doi:10.1016/j.nicl.2021.102784
52. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. 2016;139:1987–1993. doi:10.1093/brain/aww097
53. Valentino RJ, Volkow ND. Untangling the complexity of opioid receptor function. Neuropsychopharmacol. 2018;43:2514–2520. doi:10.1038/s41386-018-0225-3
54. Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and μ-opioid receptors. Prog Neuro Psychopharmacol Biol Psychiatry. 2005;29:1264–1280.
55. Avona A, Mason BN, Lackovic J, et al. Repetitive stress in mice causes migraine-like behaviors and calcitonin gene-related peptide-dependent hyperalgesic priming to a migraine trigger. Pain. 2020;161:2539–2550. doi:10.1097/j.pain.0000000000001953
56. DosSantos MF, Oliveira AT, Ferreira NR, Carvalho ACP, Rosado de Castro PH. The contribution of endogenous modulatory systems to TMS- and tDCS-induced analgesia: evidence from PET studies. Pain Res Manag. 2018;2018:1–14. doi:10.1155/2018/2368386
57. Maarrawi J, Peyron R, Mertens P, et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology. 2007;69:827–834. doi:10.1212/01.wnl.0000269783.86997.37
58. DosSantos MF, Love TM, Martikainen IK, et al. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front Psychiatry. 2012;3:93. doi:10.3389/fpsyt.2012.00093
59. DosSantos MF, Martikainen IK, Nascimento TD, et al. Building up analgesia in humans via the endogenous μ-opioid system by combining placebo and active tDCS: a preliminary report. PLoS One. 2014;9:e102350. doi:10.1371/journal.pone.0102350
60. Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183–186. doi:10.1126/science.301658
61. Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220. doi:10.1093/IJE/DYH299
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.