Back to Journals » International Journal of Women's Health » Volume 14
Dynamic Nomogram Based on the Metastatic Number and Sites and Therapy Strategies Predicting the Prognosis of Patients with Metastatic Cervical Cancer
Authors Ma Y, Li J , Tan X, Cai M, Zhang X, Ma J
Received 18 August 2022
Accepted for publication 6 December 2022
Published 22 December 2022 Volume 2022:14 Pages 1807—1819
DOI https://doi.org/10.2147/IJWH.S386689
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Yuan Ma, Jing Li, Xinyue Tan, Mengjiao Cai, Xiaozhi Zhang,* Jinlu Ma*
Department of Radiation Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinlu Ma; Xiaozhi Zhang, Email [email protected]; [email protected]
Background: Individual survival prediction is of vital importance to optimize the individualized treatment of metastatic cervical cancer (mCC) patients. The goal of this study was to identify the potential risk factors for the survival of mCC patients and construct a nomogram for their prognosis.
Methods: Medical records of patients with newly diagnosed mCC at the First Affiliated Hospital of Xi’an Jiaotong University were reviewed retrospectively. Risk factors were identified using Cox proportional hazards analysis and Kaplan–Meier curves. Random forest was used to identify factors associated with therapy strategy. Nomogram and dynamic nomogram were established using ‘rms’ and “DynNom” R package.
Results: A total of 98 patients with mCC were finally identified. In Cox analyses, multiple metastases and concurrent chemoradiotherapy (CCRT) were identified as independent predictors for overall survival (OS). We further explored the prognostic value of metastatic number and sites and therapy strategies for mCC patients by Kaplan–Meier curves. A dynamic nomogram including metastases number and sites (multiple metastases, liver and lymph node (LN) above diaphragm metastases) and chemoradiotherapy strategies (CCRT, postradiotherapy chemotherapy, and radiotherapy to metastatic sites) was constructed for predicting the prognosis of mCC patients. For newly diagnosed patients, we strongly recommended the combination of chemotherapy and definitive pelvic radiotherapy and, if possible, radiation to metastatic site, but CCRT should be implemented with caution. We constructed a dynamic nomogram indicating that patients with younger age, shorter symptom duration, and better laboratory test results are suitable for CCRT.
Conclusion: Survival analyses showed that the metastatic number and sites and therapy strategies are associated with the prognosis of mCC patients. The CCRT and prognostic nomograms may help clinicians to make better clinical decisions and effectively predict the prognosis for newly diagnosed mCC patients.
Keywords: metastatic cervical cancer, nomogram, multiple metastases, concurrent chemoradiotherapy, real world
Introduction
Cervical cancer is the fourth most common cancer in women with an estimated 604,000 new cases and 342,000 deaths worldwide in 2020.1 Although precancerous lesions screening and vaccination against human papilloma virus are becoming common in developed areas, cervical cancer still is the most commonly diagnosed cancer in 23 countries and is the leading cause of cancer death in 36 countries.1,2 Despite much cervical cancer being localized due to vagina bleeding or abnormal leucorrhea, approximately 2–7.6% of patients are diagnosed with synchronous distant metastases with a 5-year prognosis of 16.5% compared to 91.5% for localized cervical cancer.3,4 Due to the overall rarity and heterogeneity of metastatic cervical cancer (mCC), accurate survival prediction is crucial for risk stratification and individualized precision therapy strategy for the advanced tumor. Therefore, a new prognostic tool is needed in clinical practice.
In recent years, nomogram has been a frequently used and feasible tool that can incorporate multiple reliable factors and provide accurate and individualized prognostic information for clinicians in clinical practice.5,6 Previous studies have reported that factors such as neutrophil-lymphocyte ratio, location of metastases, histological subtype and radiotherapy are associated with the prognosis of mCC.7–9 Nevertheless, little attention has been focused on the concrete location and number of metastatic lesions of mCC patients at the initial diagnosis. A retrospective study demonstrated that patients with multiple organ metastases had a poorer prognosis compared to patients with single organ metastasis.10 Since knowledge of the prognosis of metastatic number and sites is crucial for pre-treatment evaluation, our study aimed to analyze the impact of metastases in different systems, such as lymph node (LN) and hematogenous (including lung, liver, bone and other organs) systems, and in specific locations on the prognosis of mCC patients. In addition, previous studies found that definitive pelvic radiotherapy and chemotherapy were associated with significantly longer survival compared with chemotherapy alone for cervical cancer patients,8,11,12 but the effect of the appropriate timing of chemoradiotherapy (CRT) in patients with mCC has not been well studied. Until now, many studies related to mCC have contained patients with both synchronous and recurrent metastatic diseases.7,13–15 But the reality is they have completely different treatment patterns that may affect the prognosis of patients.16,17
Therefore, this retrospective study aimed to explore the risk factors associated with the prognosis of newly diagnosis mCC patients and to construct nomograms to facilitate clinical practice.
Materials and Methods
Ethics Statement
Patient consent was waived due to the retrospective nature of this study. We confirm that the privacy of participants will be kept in confidence. And this study was approved by the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University (approval No. NCT03257475).
Patients
A retrospective chart review was conducted on patients diagnosed with histopathology-confirmed cervical cancer from January 2008 to December 2018 at the First Affiliated Hospital of Xi’an Jiaotong University. Patients newly diagnosed with International Federation of Gynecology and Obstetrics (FIGO) 20184 stage IVB were enrolled in this study. The distant metastases were confirmed through computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-CT (PET-CT), or biopsy of metastatic sites if possible. The LN metastasis was defined based on the short axis >10 mm in CT. Exclusion criteria were as follows: cervical cancer was not the first primary malignancy, patients diagnosed with mCC without any treatment, recurrent distant metastases disease diagnosed with local cervical cancer at first, and patients with incomplete records or surveillance/follow-up.
Clinical Variables
Clinical variables captured from the electronic medical records encompassed age at first diagnosis, year of diagnosis, metabolic disease (hypertension, diabetes, and cardiovascular disease), symptom duration before diagnosis, pathological type, tumor differentiation, tumor maximum size, the presence of rectal/bladder invasion, pelvic LN metastases, pelvic effusion, and hydronephrosis/ureteral hydrops, laboratory results (the level of squamous cell carcinoma antigen (SCCAg), hemoglobin, albumin, the count of platelet and leukocyte), distant metastatic site (hematogenous: lung, bone, liver, and other (spleen and adrenal gland); LN: intraperitoneal, inguinal, and LN above diaphragm (mediastinal, axillary, and supraclavicular LN)), number of metastatic site, treatment strategy, vital status, and survival time. Definitive radiotherapy was administrated with external beam radiotherapy (EBRT) of 45–50 Gy in whole-pelvic and para-aortic (if they have para-aortic LN metastases) and brachytherapy of 20–30 Gy, and non-definitive radiotherapy was palliative therapy to relieve vaginal bleeding or other symptoms and could not be achieved at above doses. First-line chemotherapy regimens mainly include TP (Paclitaxel + Platinum), DP (Docetaxel + Platinum), and PF (Platinum + 5-Fluorouracil). The primary endpoint of the survival analysis was overall survival (OS), which was defined as the date from cancer diagnosis to death or last follow-up. The follow-up was terminated on 31 December 2021.
Statistical Analysis
The distribution of demographic and clinicopathological variables was summarized with descriptive statistics. The categorical for different subgroups were compared using the chi-squared (χ2) test or Fisher’s exact test where appropriate, and continuous variables were compared using the Kruskal–Wallis test. The Cox proportional hazards regression analysis with hazard ratio (HR) and associated 95% confidence interval (CI) were utilized to investigate the effect of specific factors on the survival of mCC patients. Kaplan-Meier (KM) method was conducted to generate the survival curves, and the log-rank test was performed to investigate the differences between the curves based on the “survival” R package. And “randomForest” R package was used to evaluate the importance of clinicopathological parameters to therapy decision.
Nomogram and dynamic nomogram were established using the ‘rms’ and “DynNom” R package. Calibration curves were generated to verify the agreement between nomogram predicted survival and actual survival probabilities.18 Decision curve analysis (DCA) was further used to evaluate the applicability of the nomogram to clinical practice through the “rmda” R package.19 The multi-index receiver operating characteristic (ROC) curve was used to compare the predictive accuracy of the nomogram and other risk factors with the “survivalROC” R package.20
The R software version 4.1.1 (The R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org) and the IBM SPSS statistical software version 23.0 (SPSS, Armonk, New York, USA) were utilized for all data analyses. All statistical tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Clinicopathological Characteristics
Table 1 summarizes the clinicopathological characteristics of 98 mCC patients enrolled in this study. Histologically, 90 (91.8%) patients were diagnosed with squamous cell carcinoma, and 8 (8.2%) had adenocarcinoma. And 28 (28.6%) patients were diagnosed with single metastasis (SM), while 70 (71.4%) patients with multiple metastases (MM). In addition, 87 (88.8%) patients received chemotherapy, while 83 (84.7%) patients received definitive pelvic radiotherapy, with the median dose being 70.4 (10–75) Gy, and 55 (56.1%) and 23 (23.5%) patients received concurrent and sequential chemoradiotherapy, respectively (Table S1). And 25 (25.5%) patients received radiotherapy in metastatic lesions, mainly in bone and LN (inguinal and supraclavicular LN).
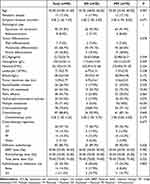 |
Table 1 The Clinicopathological Characteristics of mCC Patients |
Exploration of Prognostic Factors of OS
By using univariate Cox regression analysis in the mCC cohort, the results indicated that characteristics including symptom duration before diagnosis, multiple metastases, external-beam radiation therapy (EBRT) dose, concurrent chemoradiotherapy (CCRT), postradiotherapy chemotherapy were all significantly associated with the OS of mCC patients (Figure 1A). Subsequently, multivariate Cox regression analysis indicated multiple metastases (p = 0.040) and CCRT (p = 0.002) as independent risk factors for OS (Figure 1B).
Characteristics of Metastases
Table 2 summarizes the distribution of metastatic sites of mCC patients. Amongst patients with distant metastases, we found that retroperitoneal LN was the most common site of distant metastases, followed by inguinal LN, lung, LN above diaphragm, bone, and liver. Subsequently, Kaplan–Meier survival analyses were used to evaluate the effects of metastases number and sites on the OS of mCC patients. Patients with single metastasis had better survival compared to patients with multiple metastases (median OS: less than 50% with death events vs 24 months, p = 0.019, Figure 2A). And for specific sites, metastases in liver (with vs without: 14 months vs 46 months, p = 0.029, Figure 2B) and LN above diaphragm (with vs without: 17 months vs 48 months, p = 0.016, Figure 2C) had significant impacts on OS. However, metastases in lymphatic and hematogenous systems, bone, lung, inguinal LN, and retroperitoneal LN were all not associated with the outcome of patients (p > 0.05, Figure 2D and Figure S1). Table 3 exhibits the metastatic sites and median OS of 10 metastatic forms involving the liver and above-diaphragm LN metastases (There are overlaps between these groups bacause too much metastatic patterns).
 |
Table 2 Distribution of Metastases Sites for mCC Patients |
 |
Table 3 Distribution and Overall Survival of Patients with Different Metastases Patterns |
Prognostic Effects of Chemoradiotherapy
As shown in Table S1, 55 (56.1%) patients received CCRT. Of patients without CCRT, 32 (74.4%) patients received chemotherapy, and 28 (63.6%) received pelvic radiotherapy. Besides, patients with CCRT had relatively higher hemoglobin (p = 0.065) and albumin levels (p = 0.032). For newly diagnosed patients, it is essential to find out which patients are suitable for CCRT. The random forest algorithm was used to screen for factors associated with the administration of CCRT. Seven clinicopathological factors with importance greater than 2 were then included in the nomogram to predict the probability of a patient being suitable for CCRT (Figure S3A, B). Moreover, we deployed the nomogram on the shinyapps website (https://metastaticcervicalcancer.shinyapps.io/DynNomapp_CCRT/), which allows clinicians to conveniently utilize this model (Figure S3C).
Figure S4 and Table S2 exhibited the distribution and mOS of patients with different therapeutic strategies and revealed that patients with CCRT plus postradiotherapy chemotherapy have the longest OS. In further analyses, both CCRT (57 months vs 17 months, p = 0.0043, Figure 3A) and postradiotherapy chemotherapy (47 months vs 23 months, p = 0.037, Figure 3B) were predictors of better prognosis for mCC patients. And patients with CCRT had better survival in both the multiple metastases (46 months vs 12 months, p = 0.0026, Figure S2) and overall cohort. Although radiotherapy to metastatic sites had no prognostic impact on the overall cohort (Figure 1A), in subgroup analyses we found that radiotherapy to lymph node and bone prolonged the survival of mCC patients with LN and bone metastases, respectively (Figure 3C, D). However, year of diagnosis and first-line chemotherapy regimens have no effects on patient survival (Figure 3E, F).
Construction and Verification of the Prognostic Nomogram
Kaplan–Meier curves revealed that metastases number and sites (liver and LN above diaphragm) and therapy modifies (CCRT, postradiotherapy chemotherapy, and radiotherapy to metastatic site) were associated with the OS of mCC patients (Figures 2 and 3). Then, the above-mentioned 6 prognostic parameters were incorporated to develop the nomogram for predicting 1-, 2-, and 3-year OS (Figure 4A). The dynamic nomogram can be accessed via https://metastaticcervicalcancer.shinyapps.io/DynNomapp_OS_predict/ (Figure 4B). The calibration plots of the nomogram showed satisfactory agreement between the nomogram predicted and actual survival (Figure 4C). As shown in Figure 5A, the DCA curves indicated that the nomogram has gained significant benefits in predicting 1-, 2-, and 3-year OS. In the ROC curve analysis, the 1-, 2-, and 3-year AUC values of the nomogram were 0.796, 0.9, and 0.896, respectively, which were superior to other individual clinicopathological parameters (Figure 5B).
Discussion
Since distant metastasis remains a major cause of cervical-cancer-related death in women, in which complex biological profiles posed a huge challenge for clinical treatment, there is a need to identify clinical risk and prognostic factors of newly diagnosed mCC patients to improve their survival condition.16,21 In this population-based study, we retrospectively evaluated the clinical characteristics and survival of mCC patients and constructed a nomogram to predict the prognosis of these patients. Several studies have constructed nomograms to predict the prognosis of cervical cancer patients,22–24 but few studies built nomogram for stage IVB cervical cancer patients. A SEER-based nomogram can predict the survival of mCC patients, but lack more detailed information such as treatment strategies and specific metastasis number and locations.9 In the current study, Cox regression analysis showed significant impacts of multiple metastases and CCRT on prognosis. We further explored the prognostic value of metastatic sites and chemoradiotherapy strategies. Ultimately, six parameters (multiple metastases, liver, and LN above diaphragm metastases, concurrent chemoradiotherapy, postradiotherapy chemotherapy, and radiotherapy to metastatic site) were included in the nomogram with excellent predictive ability for the prognosis of mCC patients.
Cervical cancer metastasis to multiple distant sites is not rare, previous studies have reported that nearly one in four cases of distant organ metastasis had two or more site involvements, which is consistent with our study.25 In addition, some studies have shown that cancer survival may be associated with different metastatic locations.26–29 Previous studies have reported that cervical cancer patients with single metastasis have longer survival than those with multiple metastases, but these findings are based on the fact that metastases only involved organs.10,25,30 In this study, we also found that multiple metastases in both the lymphatic and hematological systems can affect the prognosis of mCC patients. And lymphatic metastases are not rare, 62.3% (61/98) and 28.6% (28/98) of mCC patients in this study had LN and disseminated metastases, respectively.
Given the significant impact of metastases sites on survival, we further investigated the relationship between specific metastatic locations and cervical cancer survival in the current study. Kaplan–Meier plots revealed that metastases in liver and LN above diaphragm have significant impacts on the OS of mCC patients. However, there were no significant differences in survival for other metastatic sites. It has been reported that patients with hematogenous metastases had a 5.3-fold higher risk of death compared to those with lymphatic metastases,31 but there was no survival difference between those two groups in our findings, which might be due to the exclusion of patients with only retroperitoneal LN metastases in the 2018 FIGO stage IVB and the current study.4 Liver metastases are associated with a poorer prognosis of breast cancer, rectal cancer, and lung cancer.32–34 In cervical cancer, the median OS for patients with liver metastases was 6.8–10.9 months.10,35 Besides, we found that liver metastases patients with or without other metastatic lesions had similar prognoses (Table 3), indicating that liver metastasis is an independent prognostic risk factor for mCC patients. Patients with LN above diaphragm metastases are more likely to accompany other metastatic lesions, and these patients resulted in poorer survival rates than those with LN above diaphragm metastases only. Several studies have reported that 52–74% of patients with left supraclavicular LN metastases experienced pathological responses after chemoradiotherapy with acceptable toxicity.36,37 But mCC patients with LN above diaphragm and organ metastases had dismal outcomes, and how to control these organ metastases may be the key to prolonging survival.
Growing studies have demonstrated that radiotherapy and chemotherapy are administrated for survival improvement in cervical cancer patients with metastatic disease.8,38–41 Besides, our results depicted longer survival in patients developing metastases after CCRT, which was compatible with the results in the previous study.42 Furthermore, the effects remained significant and strong in the multiple metastases subgroup. This finding is in line with previous studies that demonstrated an increased sensitivity of radiotherapy when combined with concurrent chemotherapy.16 Up till now, CCRT was generally considered the primary treatment choice for stages IB2, IIA2, and IIB to IVA cervical cancer.43,44 Kim et al reported that 52% patients with left supraclavicular and paraaortic LN metastases could achieve complete response from CCRT.36 A prospective observational study revealed that CCRT induces increased infiltration of CD8+ T cells and activation of IFN signaling, indicating great promise for the combination of immunotherapy and radiotherapy.45 A multi-institutional retrospective study showed that adjuvant chemotherapy after CCRT has a potential role in improving disease control for locally advanced cervical cancer patients.46 In Table S2, patients receiving CCRT plus postradiotherapy chemotherapy had the longest OS, but there was overlap between patients treated with CCRT and postradiotherapy chemotherapy, reminding that postradiotherapy chemotherapy may be a confounder for CCRT, resulting in postradiotherapy chemotherapy not being statistically significant in multiple Cox regression analysis. In addition, there were survival benefits for patients receiving sequential chemoradiotherapy. Radiotherapy in metastatic lesions (bone and LN) prolonged the OS of patients in subgroup analysis, which is consistent with previous research.37 There is a consensus that chemoradiotherapy has significant effect on the survival of cervical cancer patients.4,16 The ROC curves showed that the nomogram without chemoradiotherapy factors had relative lower AUC values compared to the prognostic nomogram. As shown in Table S2, patients with different therapeutic regimens had completely different survival. Collectively, we included these three therapy-related factors into the nomogram for better predictive ability.
Due to the poor prognosis of patients receiving only chemotherapy or radiotherapy, we strongly recommend the combination of chemotherapy and definitive radiotherapy for newly diagnosed mCC patients, but the sequence of these treatments should be prudently decided to prevent intolerable toxic effects and treatment interruptions. A randomized clinical trial suggested sequential chemoradiation, rather than CCRT, improved disease-free survival and reduced the risk of cancer death in women with early-stage cervical cancer.47 Many previous studies and our results indicated that patients with CCRT have the best prognosis.44,48 However, in clinical practice, it remains a huge challenge for oncologists to find the optimal treatment for individual patients to prevent catastrophic toxic effects. Because patients in this study had positive therapy with manageable side effects, we further analyzed the factors associated with the implementation of CCRT. Random forest and nomogram revealed that patients with younger age, shorter symptom duration, and better laboratory test results were intended to receive CCRT. Then, a dynamic nomogram was built that can be used as a support system for clinical decision. There are studies reporting that pretreatment hemoglobin level, leukocyte, and platelet counts are associated with the prognosis of locally advanced cervical cancer patients undergoing CCRT.48,49 After using the CCRT-nomogram to predict the probability that individual patient is suitable to receive CCRT, the prognostic nomogram could effectively predict the prognosis of newly diagnosed mCC patients. However, the administration of CCRT in this study was affected by human subjective factors, and the model should be validated with larger samples and in prospective study.
In summary, previous studies support that these metastatic sites and therapy regimens are prognostic risk factors for mCC patients. To our knowledge, there are some SEER-based nomograms for cervical cancer, but few studies focused on stage IVB disease. Compared to one nomogram constructed for mCC patients,9 our prognostic model consists of more detailed metastatic and therapeutic information and presents high predictive ability, which can be proven by the relative higher AUCs value of ROC curves. Besides, previous studies related to mCC have mainly focused on the effect of singular factors on patient survival, the current study analyzed factors including metastatic status and therapy strategies and put forward a nomogram that can predict individual survival. Besides, previous study for metastatic patients including synchronous and recurrent metastatic diseases.13–15 The survival and subsequent therapy of recurrent mCC patients may be influenced by previous treatment, so we only included newly diagnosed mCC patients to prevent the impact of other confounders. This model also gives us a clue that mCC patients can benefit from chemoradiotherapy, which can be administrated for newly diagnosed mCC patients. The sequence of definitive radiotherapy and chemotherapy can be decided using the CCRT nomogram.
Despite the valuable findings above, we have to admit there are several limitations in our study due to its retrospective nature and long duration. The first is the uncontrolled, non-randomized retrospective design. Secondly, the clinical target volumes of radiotherapy in this study are potential confounders. However, all treatments implemented according to standard treatment guidelines of cervical cancer. Thirdly, not all prognostic factors were identified due to the limited sample size in the study, and more studies with larger sample sizes were needed in the future.
Conclusions
We constructed a prognostic nomogram including the number and sites of metastases (multiple metastases, liver and LN above diaphragm metastases) and chemoradiotherapy strategies (concurrent chemoradiotherapy, postradiotherapy chemotherapy, and radiotherapy to metastatic sites) for predicting the prognosis of mCC patients. Newly diagnosed patients with younger age, shorter symptom duration, and better laboratory test results are suitable for CCRT according to the CCRT-nomogram, which can facilitate treatment decisions and help the use of prognostic nomogram for untreated mCC patients.
Data Sharing Statement
The data for this study can be found in the Jinlu Ma (E-mail: [email protected]).
Funding
The present study was supported by the National Natural Scientific Foundation of China (Grant No.81972861).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Mattern J, Letendre I, Sibiude J, et al. Diagnosis of advanced cervical cancer, missed opportunities? BMC Womens Health. 2022;22(1):97. doi:10.1186/s12905-022-01668-3
3. Bi Y, Li L. Pathologically confirmed brain metastases from primary uterine cervical tumors: two cases and a literature review. World J Surg Oncol. 2019;17(1):174. doi:10.1186/s12957-019-1720-7
4. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36. doi:10.1002/ijgo.12611
5. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–180. doi:10.1016/S1470-2045(14)71116-7
6. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi:10.1200/JCO.2007.12.9791
7. Ittiamornlert P, Ruengkhachorn I. Neutrophil-lymphocyte ratio as a predictor of oncologic outcomes in stage IVB, persistent, or recurrent cervical cancer patients treated by chemotherapy. BMC Cancer. 2019;19(1):51. doi:10.1186/s12885-019-5269-1
8. Wang Y, Farmer M, Izaguirre EW, et al. Association of definitive pelvic radiation therapy with survival among patients with newly diagnosed metastatic cervical cancer. JAMA Oncol. 2018;4(9):1288–1291. doi:10.1001/jamaoncol.2018.2677
9. Yu W, Huang L, Zhong Z, et al. A nomogram-based risk classification system predicting the overall survival of patients with newly diagnosed stage IVB cervix uteri carcinoma. Front Med. 2021;8:693567. doi:10.3389/fmed.2021.693567
10. Yin Z, Tang H, Li L, et al. Impact of sites versus number of metastases on survival of patients with organ metastasis from newly diagnosed cervical cancer. Cancer Manag Res. 2019;11:7759–7766. doi:10.2147/CMAR.S203037
11. Zhang H, Yu R, Zhang L, Wang R, Xiao L. Chemotherapy versus chemoradiotherapy for FIGO stages IB1 and IIA1 cervical squamous cancer patients with lymphovascular space invasion: a retrospective study. BMC Cancer. 2022;22(1):202. doi:10.1186/s12885-022-09309-6
12. Kim HS, Kim T, Lee ES, et al. Impact of chemoradiation on prognosis in stage IVB cervical cancer with distant lymphatic metastasis. Cancer Res Treat. 2013;45(3):193–201. doi:10.4143/crt.2013.45.3.193
13. Ruengkhachorn I, Leelaphatanadit C, Therasakvichya S, Hunnangkul S. Oncologic outcomes of stage IVB or persistent or recurrent cervical carcinoma patients treated with chemotherapy at Siriraj Hospital: Thailand’s largest tertiary referral center. Int J Gynecol Cancer. 2016;26(6):1154–1161. doi:10.1097/IGC.0000000000000712
14. Saito I, Kitagawa R, Fukuda H, et al. A Phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB, persistent or recurrent cervical cancer: gynecologic cancer study group/japan clinical oncology group study (JCOG0505). Jpn J Clin Oncol. 2010;40(1):90–93. doi:10.1093/jjco/hyp117
15. Ishikawa M, Nakamura K, Shibata T, et al. A randomized phase II/III trial of conventional paclitaxel and carboplatin with or without bevacizumab vs dose-dense paclitaxel and carboplatin with or without bevacizumab, in stage IVB, recurrent or persistent cervical carcinoma: Japan clinical oncology group study (JCOG1311). Jpn J Clin Oncol. 2018;48(12):1096–1100. doi:10.1093/jjco/hyy137
16. Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016;27(4):e43. doi:10.3802/jgo.2016.27.e43
17. Moore KN, Rowland MR. Treatment advances in locoregionally advanced and stage IVB/recurrent cervical cancer: can we agree that more is not always better? J Clin Oncol. 2015;33(19):2125–2128. doi:10.1200/JCO.2015.61.0998
18. Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: from utopia to empirical data. J Clin Epidemiol. 2016;74:167–176. doi:10.1016/j.jclinepi.2015.12.005
19. Van Calster B, Wynants L, Verbeek JFM, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74(6):796–804. doi:10.1016/j.eururo.2018.08.038
20. Obuchowski NA, Bullen JA. Receiver operating characteristic (ROC) curves: review of methods with applications in diagnostic medicine. Phys Med Biol. 2018;63(7):07tr01. doi:10.1088/1361-6560/aab4b1
21. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169–182. doi:10.1016/S0140-6736(18)32470-X
22. Polterauer S, Grimm C, Hofstetter G, et al. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br J Cancer. 2012;107(6):918–924. doi:10.1038/bjc.2012.340
23. Feng Y, Wang Y, Xie Y, Wu S, Li Y, Li M. Nomograms predicting the overall survival and cancer-specific survival of patients with stage IIIC1 cervical cancer. BMC Cancer. 2021;21(1):450. doi:10.1186/s12885-021-08209-5
24. Jiang K, Ai Y, Li Y, Jia L. Nomogram models for the prognosis of cervical cancer: a SEER-based study. Front Oncol. 2022;12:961678. doi:10.3389/fonc.2022.961678
25. Joh S, Violette CJ, Khetan VU, et al. Metastatic extent-specific prognosis of women with stage IVB cervical cancer: multiple versus single distant organ involvement. Arch Gynecol Obstet. 2022. doi:10.1007/s00404-022-06611-3
26. Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019;19(1):1091. doi:10.1186/s12885-019-6311-z
27. Merkel S, Weber K, Croner RS, et al. Distant metastases in colorectal carcinoma: a proposal for a new M1 subclassification. Eur J Surg Oncol. 2016;42(9):1337–1342. doi:10.1016/j.ejso.2016.03.034
28. Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: a population-based analysis. Cancer Med. 2020;9(1):361–373. doi:10.1002/cam4.2673
29. Liu Y, Chi S, Zhou X, Zhao R, Xiao C, Wang H. Prognostic value of distant metastatic sites in stage IV endometrial cancer: a SEER database study of 2948 women. Int J Gynaecol Obstet. 2020;149(1):16–23. doi:10.1002/ijgo.13084
30. Zhang Y, Guo Y, Zhou X, Wang X, Wang X. Prognosis for different patterns of distant metastases in patients with uterine cervical cancer: a population-based analysis. J Cancer. 2020;11(6):1532–1541. doi:10.7150/jca.37390
31. Kim K, Cho SY, Kim BJ, Kim MH, Choi SC, Ryu SY. The type of metastasis is a prognostic factor in disseminated cervical cancer. J Gynecol Oncol. 2010;21(3):186–190. doi:10.3802/jgo.2010.21.3.186
32. Huang Z, Hu C, Liu K, et al. Risk factors, prognostic factors, and nomograms for bone metastasis in patients with newly diagnosed infiltrating duct carcinoma of the breast: a population-based study. BMC Cancer. 2020;20(1):1145. doi:10.1186/s12885-020-07635-1
33. Gaitanidis A, Alevizakos M, Tsaroucha A, Tsalikidis C, Pitiakoudis M. Predictive nomograms for synchronous distant metastasis in rectal cancer. J Gastrointest Surg. 2018;22(7):1268–1276. doi:10.1007/s11605-018-3767-0
34. Wu KL, Tsai MJ, Yang CJ, et al. Liver metastasis predicts poorer prognosis in stage IV lung adenocarcinoma patients receiving first-line gefitinib. Lung Cancer. 2015;88(2):187–194. doi:10.1016/j.lungcan.2015.02.012
35. Gardner AB, Charo LM, Mann AK, Kapp DS, Eskander RN, Chan JK. Ovarian, uterine, and cervical cancer patients with distant metastases at diagnosis: most common locations and outcomes. Clin Exp Metastasis. 2020;37(1):107–113. doi:10.1007/s10585-019-10007-0
36. Kim JY, Kim JY, Kim JH, Yoon MS, Kim J, Kim YS. Curative chemoradiotherapy in patients with stage IVB cervical cancer presenting with paraortic and left supraclavicular lymph node metastases. Int J Radiat Oncol Biol Phys. 2012;84(3):741–747. doi:10.1016/j.ijrobp.2012.01.070
37. Mukai Y, Yokota NR, Sugiura M, et al. Outcome of radiation therapy for stage IVB uterine cervical cancer with distant lymph nodes metastases; sequential irradiation for distant lymph nodes metastases. In Vivo. 2021;35(2):1169–1176. doi:10.21873/invivo.12365
38. Yin Z, Lou H, Tang H, Ni J, Zhou Q, Chen M. Efficacy of radical doses of pelvic radiotherapy for primary tumor treatment in patients with newly diagnosed organ metastatic cervical cancer. Radiat Oncol. 2019;14(1):82. doi:10.1186/s13014-019-1297-x
39. Li H, Pang Y, Cheng X. Surgery of primary sites for stage IVB cervical cancer patients receiving chemoradiotherapy: a population-based study. J Gynecol Oncol. 2020;31(1):e8. doi:10.3802/jgo.2020.31.e8
40. Huang K, Jia M, Li P, et al. Radiotherapy improves the survival of patients with metastatic cervical cancer: a propensity-matched analysis of SEER database. Int J Gynecol Cancer. 2018;28(7):1360–1368. doi:10.1097/IGC.0000000000001313
41. Xu JY, Chen JN, Lei J, Hu M, Wu SG, Zhou J. Local treatment improves survival in patients with stage IVB cervical cancer. Gynecol Oncol. 2022;165(3):538–545. doi:10.1016/j.ygyno.2022.04.013
42. Hattori S, Yoshikawa N, Mogi K, et al. Significance of concurrent chemoradiotherapy as primary treatment in patients with metastatic cervical cancer. Curr Oncol. 2021;28(3):1663–1672. doi:10.3390/curroncol28030155
43. Qin F, Pang H, Yu T, Luo Y, Dong Y. Treatment strategies and prognostic factors of 2018 FIGO stage IIIC cervical cancer: a review. Technol Cancer Res Treat. 2022;21:15330338221086403. doi:10.1177/15330338221086403
44. Qiao Y, Li H, Peng B. Neoadjuvant and adjuvant treatments compared to concurrent chemoradiotherapy for patients with locally advanced cervical cancer: a bayesian network meta-analysis. Front Oncol. 2022;12:745522. doi:10.3389/fonc.2022.745522
45. Chen J, Chen C, Zhan Y, et al. Heterogeneity of IFN-mediated responses and tumor immunogenicity in patients with cervical cancer receiving concurrent chemoradiotherapy. Clin Cancer Res. 2021;27(14):3990–4002. doi:10.1158/1078-0432.CCR-20-4521
46. Wu N, Su X, Song H, et al. A multi-institutional retrospective analysis of oncologic outcomes for patients with locally advanced cervical cancer undergoing platinum-based adjuvant chemotherapy after concurrent chemoradiotherapy. Cancer Control. 2021;28:1073274821989307. doi:10.1177/1073274821989307
47. Huang H, Feng YL, Wan T, et al. Effectiveness of sequential chemoradiation vs concurrent chemoradiation or radiation alone in adjuvant treatment after hysterectomy for cervical cancer: the STARS phase 3 randomized clinical trial. JAMA Oncol. 2021;7(3):361–369. doi:10.1001/jamaoncol.2020.7168
48. Liu J, Tang G, Zhou Q, Kuang W. Outcomes and prognostic factors in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy. Radiat Oncol. 2022;17(1):142. doi:10.1186/s13014-022-02115-1
49. Thongkhao P, Janmunee N, Tangkananan A. Prognostic factors for post-recurrence survival among patients with locally advanced cervical cancer who underwent definitive concurrent chemoradiation. Rep Pract Oncol Radiother. 2022;27(4):615–623. doi:10.5603/RPOR.a2022.0078
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





