Back to Journals » Drug Design, Development and Therapy » Volume 15
Dopamine-Mediated Vanillin Multicomponent Derivative Synthesis via Grindstone Method: Application of Antioxidant, Anti-Tyrosinase, and Cytotoxic Activities
Authors Mani A, Ahamed A, Ali D, Alarifi S , Akbar I
Received 23 October 2020
Accepted for publication 30 December 2020
Published 23 February 2021 Volume 2021:15 Pages 787—802
DOI https://doi.org/10.2147/DDDT.S288389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Arunadevi Mani,1 Anis Ahamed,2 Daoud Ali,3 Saud Alarifi,3 Idhayadhulla Akbar1
1Research Department of Chemistry, Nehru Memorial College (Affiliated to Bharathidasan University), Puthanampatti -621007, Tiruchirappalli District, Tamil Nadu, India; 2Department of Botany & Microbiology, College of Sciences, King Saud University (KSU), Riyadh, Saudi Arabia; 3Department of Zoology, College of Sciences, King Saud University (KSU), Riyadh, 11451, Saudi Arabia
Correspondence: Idhayadhulla Akbar Tel +91-9994265115
Email [email protected]
Purpose: This study aimed to determine the extent of contribution of dopamine to antioxidant and anti-tyrosinase activities, by dopamine addition to vanillin. This study achieved the synthesis of dopamine-associated vanillin Mannich base derivatives prepared via a one-step reaction involving a green chemistry approach, and investigation of antioxidant and anti-tyrosinase activities.
Methods: Novel one-pot synthesis of Mannich base dopamine-connected vanillin ( 1a-l) derivatives can be achieved via green chemistry without using a catalyst. Newly-prepared compounds were characterised with FTIR and NMR (1H and 13C) spectra, mass spectra, and elemental analyses. In total, 12 compounds ( 1a-l) were synthesised and their antioxidant and anti-tyrosinase activities evaluated. Antioxidant activities of 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitric oxide (NO), hydrogen peroxide (H2O2), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and diammonium assays, ABTS•+ radical scavenging, and linoleic acid peroxidation were used to screen all synthesised compounds ( 1a-l) for anti-tyrosinase activities and cytotoxicity against MCF-7 and Vero cell lines;.
Results: The compound 1k inhibited (IC50:11.02μg/mL) the DPPH-scavenging activity to a greater extent than the standard BHT (IC50:25.17μg/mL), and showed high activity in H2O2 and NO scavenging assays. Compound 1e was more potent (96.21%) against ABTS and compound 1k was more potent (95.28%) against 2,2ʹ-azobis(2-amidinopropane)dihydrochloride antioxidant than the standard trolox. All synthesised compounds were screened for anti-tyrosinase inhibitory activity. Compound 1e had higher activity against tyrosinase (IC50=10.63 μg/mL), than kojic acid (IC50=21.52μg/mL), and was more cytotoxic (GI50 0.01μM) against MCF-7 cell line than the doxorubicin standard and other tested compounds.
Conclusion: In this study, all compounds were found to possess significant antioxidant and anti-tyrosinase activities. Compounds 1e and 1k performed well, compared with other compounds, in all assays. In addition, this study successfully identified several promising molecules that exhibited antioxidant and anti-tyrosinase activities.
Keywords: Mannich base, grindstone chemistry, antioxidant, anti-tyrosinase activity, cytotoxicity
Introduction
Tyrosinase inhibitors have natural, synthetic, and semi-synthetic sources,1 such as tropolone, hydroquinone, kojic acid,2 arbutin, and bibenzyl glycosides.3 A drawback of these inhibitors is the low efficacy of designing the drug.4,5 For example, tyrosine and dopamine of phenol hydroxyl (OH) compounds can inhibit the activity of tyrosinase,6 whereas flavonoids of phenolic OH groups can have anti-tyrosinase and antioxidant activities.7 The present study focused on dopamine with vanillin-containing compounds. Vanillin has anti-apoptotic, neuroprotective, antioxidative, and anticancer activities,8,9 and mushroom tyrosinase active vanillin derivatives have been identified.10
The design and development of dopamine with vanillin derivatives via the Mannich condensation reaction based on Mannich base derivatives has been conducted previously using many different bioactive molecules, such as aminoalkyl derivatives,11 chiral types, β-amino-carbonyl compounds, peptides, alkaloids, antibiotics, and vitamins.12 Additionally, Mannich base bioactivity includes antioxidative,13 antifungal,14 anti-inflammatory,15 analgesic,16 anticancer,17 vasorelaxing,18 antimalarial,19 and antitubercular20 activities.
In particular, phenolic compounds of Mannich bases, such as chalcones, thymols, and flavanones, have antioxidant compounds.21,22 Tyrosinase active Mannich base kojic acid derivatives have also been identified.23 However, there have been no previous studies, to our knowledge, regarding biologically active dopamine connected to vanillin derivatives.
This study focused on the antioxidant activity of title compounds based on screening using various free-radical assays as oxidative stress may be the main cause of neurodegenerative diseases.24 The brain’s dependence on oxygen (O2) and high consumption of glucose makes it highly susceptible to oxidative stress, as leaked O2 has been implicated in the generation of free radicals, such as superoxide anions, hydrogen peroxide (H2O2), and OH.25–28 Some molecules have both active antioxidant and tyrosinase activities, such as isoeugenol29 (Figure 1). Designing antioxidant molecules using biosystems can protect inhibit tyrosinase enzymes and prevents related diseases. Flavonoids will consider the phenolic OH on the effetely antioxidant and tyrosinase activities.30,31
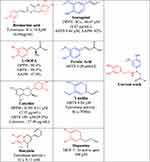 |
Figure 1 Deigning of target molecules. |
For example, phenolic hydroxyl on the ring catechins,32,33 baicalein,34 L-DOPA, and rosmarinic acid (Figure 1) can greatly enhance the tyrosinase activity. For the evidence, two phenolic hydroxyls can more effect the tyrosinase activity compared to one hydroxyl substitutions,35,36 and other example phenol hydroxyl-containing tyrosine and dopamine have inhibited the tyrosinase enzyme.6
Tyrosinase inhibitors are used for various applications in the food,37 cosmetics,38 and medicinal industries, and tyrosinase is responsible for melanogenesis in mammals.39,40
However, very few inhibitors have been approved for clinical use or for use as skin-whitening agents, and there are limited rapid assays for the in vitro screening of tyrosinase inhibitors41 hence, effective and low-cost methods need to be developed. Therefore, in this study, we selected the Grindstone method, which is a branch of green chemistry where solvent-free chemical reactions42,43 can take place to produce a high yield in an inexpensive.44 This method is used in the pharmaceutical industry with minimal environmental impact. The undertaking of reactions under solvent-free reaction conditions using a grinding technique is an alternative to other methods.43,45
Therefore, this study had two goals; to provide the best model of Mannich base vanillin-connected dopamine derivatives, and to test the obtained Mannich bases for possible anti-tyrosinase and antioxidative activities as well as provide a suitable mechanism. In addition, cytotoxic effects were investigated for the synthesis of Mannich bases against MCF-7 cancer cell lines.
Experimental
Synthesis
Spectrophotometer lambda 850 was used to check all bioactivities. FT-IR (4000–400 cm–1) was recorded by Shimadzu 8201PC analysis. Bruker DRX-300 MHz, 75 MHz was used for the analysis of 1H and13C NMR spectra. A Vario EL III organic element analyzer was used to analyze the percentage (%) elements (C, H, N and S) presents in synthesised compounds.
General Procedure for the Synthesis of Compound 1a-l
A reaction mixture consisting of dopamine (0.01 mol, 1.53 g), vanillin (0.01 mol, 1.52 g), and N-methylacetamide (0.01 mol, 0.730 g) was mixed in a mortar and ground for up to 15 min at 30°C. Subsequently, the powdered material was washed with water and filtered. The filtered final solid material was separated by column chromatography (ethyl acetate/hexane, 4:6) and recrystallized from suitable alcohol. The same method was followed for compounds 1b-l.
3-((3,4-Dihydroxyphenethyl)amino)-3-(4-Hydroxy-3-Methoxyphenyl)-N-Methylpropan Amide (1a)
A pale yellow solid, yield 92%; MF = C19H24N2O5; MW = 360.40; m.p. = 152–154°C; IR (KBr) νmax: 3415 (O-H, stretch), 3345, 2853, 1640, 1603, 1400, 1080, 1039 cm−1; 1H NMR (DMSO-d6) δ: 8.05 (s, 1H), 6.98 (s,1H), 6.80 (s, 1H), 6.78 (d, J = 11.0 Hz, 1H), 6.68 (d, J = 11.12Hz, 1H), 6.69 (d, 1H), 6.66 (d, 1H), 5.33 (s, 3H, OH), 4.13 (dd, J=11.0 Hz, J = 11.2 Hz, 1H, CH), 3.81 (s, 3H), 3.06 (s, 3H), 2.85 (s, 2H), 2.71 (d, J = 11.0 Hz, 1H), 2.68 (2H, s, CH2), 2.40 (1H, d, J=11.2 Hz), 2.08 (s, 1H); 13C NMR (DMSO-d6) δ: 172.1, 147.3, 147.0, 145.6, 144.5, 131.9, 131.6, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 57.0, 56.6, 45.2, 42.5, 36.5, 26.9; EI–MS: m/z 360 [M]+(20); HREIMS: m/z: calcd for C19H24N2O5: 360.17, found 360.21; Anal. calcd C19H24N2O5: C, 63.32; H, 6.71; N, 7.77; Found: C, 63.34; H, 6.74; N, 7.75.
3-((3,4-Dihydroxyphenethyl)amino)-N-Ethyl-3-(4-Hydroxy-3-Methoxyphenyl)propan Amide(1b)
Yellow solid, yield 90%; MF = C20H26N2O5; MW = 374.43; m.p. = 171–174°C; IR (KBr) νmax: 3423, 3349, 2835, 1631, 1592, 1406, 1083, 1036 (-O-CH3) cm−1; 1H NMR (DMSO-d6) δ: 8.05 (s, 1H), 6.92 (s, 1H), 6.84((s, 1H), 6.76 (d, 1H), 6.72 (d, 1H), 6.68 (d, 1H), 6.64 (d, J = 11.0 Hz, 1H,), 5.31 (3H, s, OH), 4.18 (1H, dd, CH), 3.84 (s, 3H), 3.10 (s, 2H), 2.87 (s, 2H), 2.69 (d, J = 11.0 Hz, 1H), 2.67 (s, 2H), 2.45 (d, J =11.0Hz, 1H), 1.14 (s, 3H), 2.10 (s, 1H); 13C NMR (DMSO-d6) δ: 175.1, 147.3, 146.8, 144.9, 143.8, 131.9, 131.6, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 57.5, 56.1, 45.2, 42.5, 36.5, 34.2, 15.0; EI–MS: m/z 374[M]+(24); HREIMS: m/z: calcd for C20H26N2O5: 374.43, found 374.40; Anal. calcd C20H26N2O5: C, 64.15; H, 7.00; N, 7.48; Found: C, 64.17; H, 7.02; N, 7.46.
4-((3,4-Dihydroxyphenethyl)amino)-4-(4-Hydroxy-3-Methoxyphenyl)butan-2-one (1c)
A pale yellow solid, yield 89%; MF = C19H23NO5; MW = 345.39; m.p. = 160–162°C; IR (KBr) νmax: 3441, 3332, 2831, 1630, 1595, 1404, 1080, 1021 cm−1; 1H NMR (DMSO-d6) δ: 6.98 (s, 1H), 6.80((s, 1H), 6.78 (d, J = 11.0 Hz, 1H), 6.70 (d, 1H, Ph-H), 6.69 (d, 1H, Ph-H), 6.66 (d, 1H), 5.30 (s, 3H, OH), 4.11 (dd, 1H), 3.81 (s, 3H), 2.95 (2H, d, J = 11.0 Hz), 2.85 (2H, s, CH2), 2.73 (d, J = 11.0 Hz, 2H), 2.68 (s, 2H, CH2), 2.31 (1H, s, -NH), 2.13 (3H, s); 13C NMR (DMSO-d6): δ: 210.6, 147.3, 146.0, 145.1, 143.8, 131.9, 131.6, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 56.0, 55.1, 45.5, 42.2, 36.5; EI–MS: m/z 345 [M]+(12); HREIMS: m/z: calcd for C19H23NO5: 345.30, found 345.38; Anal. calcd C19H23NO5: C, 66.07; H, 6.71; N, 4.06; Found: C, 66.09; H, 6.70; N, 4.04;.
3-((3,4-Dihydroxyphenethyl)amino)-3-(4-Hydroxy-3-Methoxyphenyl)propanamide (1d)
Yellow solid, yield 87%; MF = C18H22N2O5; MW = 346.38; m.p. = 149–151°C; IR (KBr) νmax: 3452, 3339, 2821, 1715, 1621, 1590, 1408, 1078, 1019 cm−1; 1H NMR (DMSO-d6)δ: 8.08 (s, 2H, NH2), 6.98 (s, 1H), 6.80 (s,1H), 6.70 (d, 1H), 6.69 (d, 1H), 6.78 (d, J = 11.3Hz, 1H), 6.63 (d, 1H), 5.37 (s, 3H, OH), 4.10 (dd, J= 11.3Hz, J= 11.12Hz, 1H, CH), 3.81 (s, 3H), 2.85 (s, 2H), 2.71 (d, 2H, J= 11.30Hz, CH2), 2.46 (d, J= 11.12Hz, 2H, CH2), 2.67 (s, 2H), 2.03 (s, 1H); 13C NMR (DMSO-d6) δ: 174.3, 147.6, 147.3, 144.3, 140.1, 131.9, 131.6, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 57.1, 53.3, 45.2, 44.5, 36.5; EI–MS: m/z 346 [M]+(31); HREIMS: m/z: calcd for C18H22N2O5: 346.38, found 346.10; Anal. calcd C18H22N2O5: C, 62.42; H, 6.40; N, 8.09; Found: C, 62.44; H, 6.38; N, 8.07;
3-((3,4-Dihydroxyphenethyl)amino)-3-(4-Hydroxy-3-Methoxyphenyl)-N-Phenylpropan Amide (1e)
A pale yellow solid, yield 93%; MF = C24H26N2O5; MW = 422.47; m.p.130–133°C; 3463, 3323, 2819, 1626, 1580, 1412, 1070, 1001cm−1, 1H NMR (DMSO-d6) δ: 8.0 (s, 1H), 7.61 (d, J = 10.1, 2H), 7.43 (d, J = 10.1, 2H), 7.19 (1H, d, J =10.1 Hz), 6.98 (s, 1H), 6.80((s, 1H), 6.70 (d, 1H), 6.78 (d, J = 11.0 Hz, 1H), 6.69 (d, 1H), 6.66 (d, 1H), 5.39 (s, 3H), 4.17 (dd, J=11.0Hz, J =11.2 Hz, 1H, CH), 3.81 (s, 3H, -CH3), 2.85 (m, 2H CH2), 2.71 (d, J =11.0Hz, 2H), 2.68 (d, 2H, CH2), 2.52 (d, J = 11.2 Hz, 2H), 2.12 (s, 1H); 13C NMR (DMSO-d6) δ: 173.9, 147.3, 147.0, 146.2, 144.7, 138.5, 131.9, 131.6, 128.9, 128.1, 122.9, 121.6, 120.3, 116.5, 115.8, 115.2, 111.2, 57.2, 56.2, 45.2, 41.5, 36.5; EI–MS: m/z 422 [M]+(08); HREIMS: m/z: calcd for C24H26N2O5: 422.17, found 422.19; Anal. Calcd C24H26N2O5: C, 68.23; H, 6.20; N, 6.63; Found: C, 68.25; H, 6.22; N, 6.66;
2-((3,4-Dihydroxyphenethyl)amino)-2-(4-Hydroxy-3-Methoxyphenyl)-1-(4-Methoxyphenyl) Ethanone (1f)
A pale yellow solid, yield 94%; MF = C24H25NO6; MW = 423.17; m.p. = 165–167°C; IR (KBr) νmax: 3512, 3312, 2889, 1621, 1582, 1410, 1121, 1012 cm−1; 1H NMR (DMSO-d6) δ: 6.98 (s, 1H), 6.80((1H, s), 6.69 (d, 1H), 6.70 (d, 1H), 6.66 (d, 1H), 6.78 (d, J = 11.0 Hz, 1H), 7.86 (d, J=10.23Hz, 2H), 7.06 (d, J=10.21Hz, 2H), 5.40 (s, 3H, OH), 4.43 (dd, J=10.34Hz, J=10.36Hz, 1H, CH), 3.81 (s, 6H, -CH3), 3.08 (d, J=10.34Hz, 2H, CH2), 2.85 (d, J=10.36Hz, 2H, CH2), 2.88 (s, 2H), 2.64 (s, 2H), 2.14 (s, 1H); 13C NMR (DMSO-d6) δ: 201.36, 185.2, 147.3, 147.2, 145.3, 143.9, 131.9, 131.6, 129.8, 129.1, 114.5, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 65.8, 56.2, 56.0, 45.2, 36.5, 32.8; EI–MS: m/z 360 [M]+(20), 189 (100); HREIMS: m/z: calcd for C24H25NO6: 423.17, found 423.10; Anal. calcd C19H24N2O5: C, 63.32; H, 6.71; N, 7.77; Found: C, 63.34; H, 6.74; N, 7.75.
1-(4-Bromophenyl)-3-((3,4-Dihydroxyphenethyl)amino)-3-(4-Hydroxy-3-Methoxyphenyl) Propan-1-one (1g)
A pale yellow solid, yield 89%; MF = C24H24BrNO5; MW = 486.36; m.p. = 149–151°C; IR (KBr) νmax: 3612, 3314, 2891, 1623, 1597, 1412, 1118, 1023 (-O-CH3), 758 (C-Br)cm−1; 1H NMR (DMSO-d6) δ: 6.98 (1H, s, Ph-H), 6.80((1H, s, Ph-H), 6.69 (1H, d, Ph-H), 6.70 (1H, d, Ph-H), 6.66 (1H, d, Ar–H), 6.78 (1H, d, J = 11.0 Hz, Ph-H), 7.98 (d, J = 10.6 Hz, 2H), 7.65 (d, J = 10.9 Hz, 2H), 5.42 (3H, s, OH), 4.13 (dd, J = 11.0 Hz, J = 11.2 Hz, 1H, CH), 3.81 (s, 3H, -CH3), 3.09 (2H, d, J = 11.0 Hz), 2.81 (d, J = 11.2 Hz, 2H), 2.85 (s, 2H), 2.68 (s, 2H), 2.05 (s, 1H); 13C NMR (DMSO-d6) δ: 200.65, 147.3, 146.8, 145.0, 144.1, 135.7, 131.5, 131.9, 131.6, 129.8, 127.6, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 72.9, 57.3, 56.9, 45.2, 36.5; 131.6, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 57.2, 45.2, 42.5, 36.5, 26.9; EI–MS: m/z 486 [M]+(35), 189 (100); HREIMS: m/z: calcd for C24H24BrNO5: 486.36, found 486.36; Anal. calcd C24H24BrNO5: C, 59.27; H, 4.97; N, 2.88; Found: C, 59.29; H, 4.95; N, 2.87;
3-((3,4-Dihydroxyphenethyl)amino)-3-(4-Hydroxy-3-Methoxyphenyl)-1-(4-Nitrophenyl) Propan-1-one (1h)
A pale yellow solid, yield 90%; MF = C24H24N2O7; MW = 452.46; m.p. = 132–35°C; IR (KBr) νmax: 3621, 3329, 2874, 1699, 1628, 1591, 1410, 1109, 1039 cm−1; 1H NMR (DMSO-d6) δ: 6.98 (1H, s, Ph-H), 6.80 (1H, s, Ph-H), 6.69 (1H, d, Ph-H), 6.70 (1H, d, Ph-H), 6.66 (d, 1H, Ar–H), 6.78 (1H, d, J = 11.0 Hz), 8.34 (d, J = 11.0 Hz, 4H), 5.44 (3H, s, OH), 4.15 (1H, dd, J = 11.0 Hz, J = 11.2 Hz, CH), 3.81 (3H, s, -CH3), 3.01 (d, J = 11.0 Hz, 2H), 2.80 (2H, d, J = 11.2 Hz), 2.85 (s, 2H), 2.68 (s, 2H), 2.07 (s, 1H); 13C NMR (DMSO-d6) δ: 202.11, 147.3, 147.9, 145.1, 144.6, 135.7, 131.5, 131.9, 131.6, 129.8, 127.6, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 72.9, 58.1, 56.2, 45.2, 36.5; EI–MS: m/z 452[M]+(18), 189 (100); HREIMS: m/z: calcd for C24H24N2O7: 360.17, found 249.02; Anal. calcd C24H24N2O7: C, 63.71; H, 5.35; N, 6.19; Found: C, 63.70; H, 5.37; N, 6.17;
1-(4-Chlorophenyl)-3-((3,4-Dihydroxyphenethyl)amino)-3-(4-Hydroxy-3-Methoxyphenyl) Propan-1-one (1i)
A pale yellow solid, yield 87%; MF=C24H24ClNO5; MW = 441.90; m.p.165–168 °C; IR (KBr) νmax: 3512, 3341, 2873, 1620, 1612, 1593, 1412, 1119, 1031, 745 cm−1; 1H NMR (DMSO-d6) δ: 6.98 (1H, s, Ph-H), 6.80((1H, s, Ph-H), 6.69 (1H, d, Ph-H), 6.70 (1H, d, Ph-H), 6.66 (1H, d, Ar–H), 6.78 (1H, d, J =11.0 Hz, Ph-H), 7.94 (d, J =10.2 Hz, 2H), 7.44 (d, J=10.4 Hz, 2H), 5.31 (3H, s, OH), 4.16 (1H, dd, J = 11.0 Hz, J =11.2 Hz, CH), 3.81 (3H, s, -CH3), 3.09 (2H, d, J=11.0 Hz), 2.85 (2H, s, CH2), 2.81 (2H, d, J=11.2 Hz), 2.68 (2H, s, CH2), 2.10 (1H, s, -NH); 13C NMR (DMSO-d6) δ: 201.07, 147.3, 146.9, 145.1, 144.8, 138.7, 134.8, 131.5, 131.9, 130.8, 128.6, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 72.9, 57.0, 56.1, 45.2, 36.5; EI–MS: m/z 441 [M]+(37); HREIMS: m/z: calcd for C24H24ClNO5: 441.90, found 441.87; Anal. calcd C24H24ClNO5: C, 65.23; H, 5.47; N, 3.17; Found: C, 65.25; H, 5.45; N, 3.16;.
3-((3,4-Dihydroxyphenethyl)amino)-3-(4-Hydroxy-3-Methoxyphenyl)-1-(p-Tolyl)propan-1-one (1j)
Yellow solid, yield 91%; MF =C25H27NO5; MW=421.49; m.p. = 165–167°C; IR (KBr) νmax: 3485, 3379, 2870, 1614, 1636, 1589, 1484, 1402, 1145, 1036 cm−1; 1H NMR (DMSO-d6) δ: 7.31 (2H,d, J=, Ph), 6.98 (1H, s, Ph-H), 6.80((1H, s, Ph-H), 6.78 (1H, d, J =11.0 Hz, Ph-H), 6.72 (d, J = 11.0 Hz, 2H), 6.69 (d, 1H), 6.70 (d, 1H), 6.66 (d, 1H), 5.40 (3H, s, OH), 4.10 (1H, dd, J = 11.0 Hz, J = 11.2 Hz, CH), 3.81 (s, 3H), 3.09 (d, J = 11.0 Hz, 2H), 2.86 (d, J = 11.2 Hz, 2H), 2.83 (2H, s, CH2), 2.68 (2H, s, CH2), 2.34 (3H, s, CH3), 2.09 (1H, s, -NH); 13C NMR (DMSO-d6) δ: 200.02, 147.9, 147.3, 145.0, 144.1, 138.7, 134.8, 131.9, 131.5, 130.8, 128.6, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 72.9, 57.8, 56.3, 45.2, 36.5, 21.3; EI–MS: m/z 421 [M]+(41), 189 (100); HREIMS: m/z: calcd for C25H27NO5: 421.49, found 421.37; Anal. calcd C25H27NO5: C, 71.24; H, 6.46; N, 3.32; Found: C, 71.26; H, 6.44; N, 3.30;
N-(4-Bromophenyl)-3-((3,4-Dihydroxyphenethyl)amino)-3-(4-Hydroxy-3-Methoxyphenyl) Propanamide (1k)
A pale yellow solid, yield 96%; MF = C24H25BrN2O5; MW = 501.37; m.p.141–143°C; IR (KBr) νmax: 3409, 3361, 2865, 1646, 1618, 1581, 1482, 1404, 1140, 1032, 702 cm−1; 1H NMR (DMSO-d6) δ: 7.71 (d, J = 11.0 Hz, 2H), 7.56 (d, J = 11.0 Hz, 2H), 7.25 (1H, s, NH), 6.98 (1H, s, Ph-H), 6.80 (s, 1H), 6.78 (d, J = 11.0 Hz, 1H), 6.70 (d, 1H), 6.69 (d, 1H), 6.66 (d, 1H), 5.45 (s, 3H, OH), 4.13 (dd, J = 11.0 Hz, J = 11.2 Hz, 1H), 3.81 (s, 3H, -CH3), 3.11 (d, J = 11.0 Hz, 2H), 2.85 (s, 2H, CH2), 2.80 (d, J = 11.2 Hz, 2H), 2.68 (s, 2H, CH2), 2.12 (s, 1H, -NH); 13C NMR (DMSO-d6) δ: 173.6, 147.3, 147.0, 145.6, 144.5, 137.6, 131.9, 131.8, 131.6, 122.9, 121.9, 121.5, 120.3, 116.5, 115.8, 115.2, 111.2, 57.2, 56.1, 45.2, 42.5, 36.5; EI–MS: m/z 360 [M]+(20), 189 (100); HREIMS: m/z: calcd for C24H25BrN2O5: 501.37, found 501.32; Anal. calcd C24H25BrN2O5: C, 57.49; H, 5.03; N, 5.59;; Found: C, 57.47; H, 5.05; N, 5.61;
1-(4-(Tert-Butyl)phenyl)-3-((3,4-Dihydroxyphenethyl)amino)-3-(4-Hydroxy-3-Methoxy Phenyl)propan-1-one (1l)
A pale yellow solid, yield 96%; MF = C28H33NO5; MW = 463.57; m.p. = 194–196°C; IR (KBr) νmax: 3512, 3360, 2861, 1654, 1612, 1583, 1481, 1410, 1138, 1030 cm−1; 1H NMR (DMSO-d6) δ: 7.37 (d, J=10.23Hz, 2H), 6.98 (s, 1H, Ph-H), 6.80 (s, 1H, Ph-H), 6.69 (d, 1H, Ph-H), 6.78 (d, J = 11.0 Hz, 1H), 6.70 (d, J=11.23Hz, 1H), 6.66 (d, Ar–H, 1H), 6.87 (d, J=11.23Hz, 2H), 5.35 (s, 3H, OH), 4.17 (1H, dd, J = 11.0 Hz, J = 11.2 Hz, CH), 3.81 (3H, s, -CH3), 3.05 (2H, d, J = 11.0 Hz), 2.84 (d, J = 11.2 Hz, 2H,), 2.85 (s, 2H), 2.68 (s, 2H), 2.15 (s, 1H, -NH), 1.36 (9H, s, CH3); 13C NMR (DMSO-d6) δ: 200.02, 155.6, 147.3, 147.0, 146.6, 144.5, 138.7, 131.5, 131.9, 130.8, 126.4, 124.9, 122.9, 120.3, 116.5, 115.8, 115.2, 111.2, 72.9, 57.2, 45.2, 36.5,34.3, 31.3; EI–MS: m/z 463 [M]+(24); HREIMS: m/z: calcd for C28H33NO5: 463.57, found 463.55; Anal. calcd C28H33NO5: C, 72.55; H, 7.18; N, 3.02;; Found: C, 72.52; H, 7.20; N, 3.04;.
Biological Activity
Antioxidant 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Scavenging Activity
DPPH antioxidant activity was screened for compounds (1a-l) following the methods of a previous study.46 The detailed method is provided in the supplementary information section.
H2O2 Scavenging Activity
H2O2 scavenging activity was screened for all compounds (1a-l) following the methods of a previous study.46 The detailed method is provided in the supplementary information section.
Nitric Oxide (NO) Scavenging Activity
The compounds (1a-l) were screened for NO scavenging activity following the methods of a previous study.46 The detailed method is provided as a supplementary file in the experimental section.
2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Antioxidant Activity
The compounds (1a-l) were screened for the ABTS assay. The antioxidant ABTS•+ scavenging activity was checked with all compounds via spectrophotometric analysis according to the method previously described by Surendra kumar et al47. The detailed method is provided in the supplementary information section.
Inhibition of 2,2ʹ-Azobis(2-Amidinopropane) Dihydrochloride (AAPH) Assay Free-Radical Analysis
A linoleic acid peroxidation assay was used to analyse all synthesised compounds (1a-l) following the methods of a previous study.47 The detailed method is provided in the supplementary information section.
Anti-Tyrosinase Activity
All compounds (1a-l) were screened for anti-tyrosinase activities. The mushroom tyrosinase
(powder, ≥1000 unit/mg solid, EC 1.14.18.1) inhibitory activities were measured spectrophotometrically via a previously reported method.48 The detailed method is provided in the supplementary information section.
Cell Lines and Cell Culture
The cell lines, MCF-7 and normal cell lines were obtained from the American Type Cell Collection (ATCC; Manassas, VA, USA). The cells were cultured at 37°C and 5% CO2 environment to get 70–80% confluence in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco®, Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (FBS) (Gibco®).
Cytotoxic Screening
The newly synthesised compounds (1a-l) were tested for cytotoxicity following the methods of a previous study.47 The detailed method is provided in the supplementary information section.
Statistical Analysis
The mean of the results was calculated based on at least three independent evaluations and the standard deviations (SD) were also calculated using Microsoft Excel.
Result and Discussion
Chemistry
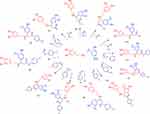
The one-pot dopamine-connected vanillin multicomponent derivatives were synthesised using the Mannich base method achieved via solvent-free green chemistry. The final solid material was recrystallised using a suitable alcohol to obtain a pure product, as shown in Scheme 1. The optimisation of the reaction conditions is presented in Scheme 2. Target compounds were analysed by FTIR, 1H, and 13C NMR spectra. The key assignments of the compounds showed significant bands at 3621–3409, 1039–1001, 3379–3312, and 1654–1620 cm−1 in the IR spectrum, conforming to the –OH, –O-CH3, NH, and –CH2-CO- groups, respectively. 1H NMR showed signals at δ 5.45–5.30, 4.10–4.43, 3.11–2.69, 2.85–2.40, and 2.15–2.03 ppm, indicating –C-OH, CH, -CH-CH2, -CH-CH2, and NH protons. The 13C NMR showed peaks at δ 210.6–200.2, 148.0–146.6, 146.2–144.3, 144.7–140.1, 58.1–56.0, and 56.9–53.3 ppm, which conformed to the –CH2-CO, –C-HO, –C-HO, –C-HO, -CH-, and –O-CH3 atoms. Mass spectroscopy and elemental analysis results were also consistent with the conformation of all compounds.
 |
Scheme 1 Synthesis of dopamine connected vanillin Mannich base derivatives (1a-1l). |
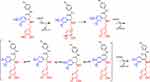
 |
Scheme 2 Optimization of reaction condition (1a-1l). |
Biological Activity
Antioxidant activity was tested using a UV-visible spectrophotometer for compounds (1a-l) via DPPH, H2O2, NO, ABTS, and AAPH assays. The compounds 1a-l were screened for cytotoxic activity against MCF-7 and Vero cell lines.
DPPH free-radical scavenging activity increased with an increase in concentration, with compound 1k showing a maximum of 100% activity at 50 µg/mL. The other compounds 1e, 1f, 1h, and 1i showed significant scavenging activity (IC50: 14.97, 19.23, 14.56, and 15.28 µg/mL) compared with standard BHT (IC50: 25.17 µg/mL). The DPPH free-radical scavenging activity results are presented in Table 1. Scheme 3 indicates that the mechanism of compound 1k against the DPPH assay, which is attributed to the highly significant contributions of dopamine and vanillin, plays a major role in activity compared to standard BHT.
 |
Table 1 DPPH-Scavenging Activity of Compounds (1a-1l) |
 |
Scheme 3 DPPH-scavenging mechanism of compound 1k. |
The dopamine-connected vanillin (1a-l) showed H2O2 scavenging activity between 10 and 100 μg/mL. Compounds 1e, 1h, 1i, 1j, 1k, and 1L showed high activity (100% activity at 100 µg/mL) compared with standard BHT (82.32%) at a concentration of 100µg/mL, with is IC50 values corresponding to 13.52, 11.82, 14.00, 13.27, 10.11, and 13.55 µg/mL. The values are shown in Table 2.
 |
Table 2 Hydrogen Peroxide (H2O2) Scavenging Activity of Compounds (1a-1l) |
The antioxidant mechanism could be explained based on its chemical structures, which comparison with isoeugenol derivatives.49 For example, compound 1k, which bears an ortho-dihydroxy, can donate an H atom from its phenol group to DPPH to form the resonance-stabilized free-radical intermediate (Scheme 3). Furthermore, intermediate could react with a second DPPH to form an inactive anion, which on cleavage by protonation would give again quinone structures. Therefore, ortho-dihydroxylated (ie catechol) benzene ring system is generally known to be very efficient systems to delocalized electrons, but not for metadihydroxylated system (ie resorcinol).50
The NO radical reacted with Griess reagent to give formazon, which was measured spectrophotometrically by all synthesised compounds (1a-l). Compounds 1g, 1h, 1i, 1k, and 1L were highly active (100% activity at 100 µg/mL) against standard (83.32% activity at 100 µg/mL) and other compounds. The IC50 values of 1g, 1h, 1i, 1k, and 1L were 11.00, 10.36, 14.15, 9.94, and 12.56 µg/mL, respectively. However, compound 1k was highly active, followed by standard compounds 1g, 1h, 1i, and 1L. The NO free-radical scavenging activity results are presented in Table 3.
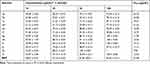 |
Table 3 NO Scavenging Activity of Compounds (1a-1l) |
Dopamine-connected vanillin (1a-l) was tested for the ABTS•+ assay. Compound 1e (96.21%) was highly active compared with trolox (85.2%). Compounds 1f, 1g, 1h, 1i, 1j, 1k, and 1L showed >90% more activity than trolox. The ABTS scavenging activity results are presented in Table 4.
 |
Table 4 ABTS•+ and AAPH Activities of Compounds (1a-1l) |
The ABTS radical assay is based on a decolourization, with the stable blue/green ABTS•+ directly generated before its reaction. All compounds are highly active compounds >80 to 94% activity compared with standard trolox. Mechanism ABTS of activity was represented in scheme 4.
 |
Scheme 4 Reaction mechanism of ABTS•+ radical. |
The compounds (1a-l) were screened using an AAPH assay for the conjugated diene hydroperoxide by the oxidation of linoleic acid at 234 nm, which was the formation of conjugated diene hydroperoxides caused by the hydrophilic AAPH initiator. The mechanism of activity is represented in Scheme 5. This assay was performed to characterise the antioxidant activity of the synthesised compounds, with 1k being highly active at 95.28% at a concentration of 100 µg/mL.
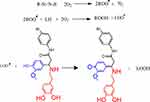 |
Scheme 5 Mechanism of lipid peroxidation and its inhibition 1k. |
The antioxidant action mechanism, mainly based on the inhibition of the formation of reactive O2 species (ROS), can chelate with metal ions, such as Cu(II) or Fe(II).51,52
The compound 1k was highly active against DPPH (IC50:11.02μg/mL), H2O2 (IC50: 10.11 μg/mL), and NO (IC50:9.94 μg/mL) assays whereas low active (IC50:12.11μg/mL) for anti-tyrosinase screening. The compound 1e (IC50:9.94 μg/mL) was highly active against anti-tyrosinase activity whereas low active against DPPH (IC50:14.97μg/mL), H2O2 (IC50:13.52 μg/mL) assays.
Cytotoxicity activity 1e (IC50:0.16 μg/mL) was highly toxic compared with 1k (IC50: 0.51 μg/mL), since these activities are only present in concentration greater than 9.94 μg/mL, a concentration that is 100% toxic to MCF-7 and Vero cell lines. Therefore, the compounds 1e and 1k were observed highly active against antioxidant and anti-tyrosinase activities in cytotoxic concentrations for both cell lines (MCF-7 and Vero cell lines).
Figure 2 indicates that structure–activity relationship, the compound 1k have acetamide with 4-bromophenyl group, which shows that it is high antioxidant activity than compound 1e and 1g, whereas the compound 1e has acetamide without halogen, which shows that it is highly anti-tyrosinase activity compared with compounds 1k and 1g. The compound 1g has acetophenone with halogens, which shows that high toxic (LC50:0.30μg/mL) in MCF-7 cell line and twice the concentrations (LC50:15.61 μg/mL) in Vero cell line, whereas it is low in active of antioxidant and anti-tyrosinase screening.
 |
Figure 2 Structure–activity relationship and comparison of highly active compounds. |
Compared with previous studies, rosmarinic acid was considered as competitive inhibitors53,54 by mushroom tyrosinase with the IC50 values of 16.8 µM, respectively, which is less active than compared with compound 1e. Another example, the ferulic acid55 was less active against AAPH antioxidant assay (82%) than compound 1k. The compound 1k was compared with L-DOPA,56 which is less active against DPPH (80.6%), ABTS (99.0%), and AAPH (67.9%) assays. Isoeugenol was also low active (82%) against AAPH assay49 than compound 1e. In comparison with vanillin, the vanillin is not active in the DPPH assay57 and also vanillin was low active for tyrosinase inhibitory58 than compound 1e.
Dopamine was compared with compounds 1k and 1e against the MCF-7 and Vero cell lines, however, the dopamine was absolutely inactive up to 100 μM for all cell lines tested.59
To estimate the anti-tyrosinase inhibitory activities, the synthesized dopamine-connected vanillin (1a-l) was exposed to a tyrosinase inhibitor using L-DOPA as a substrate. Kojic acid, which is used as a skin-whitening ingredient, was used as a reference. The inhibitory effects of the compounds (1a-l) are presented in Table 5. Compounds 1e and 1k bearing a dopamine-connected vanillin substituent showed better inhibitory activity with IC50 values of 10.63 and 12.11µg/mL, respectively, compared to other compounds and kojic acid with an IC50 value of 21.52 µg/mL.
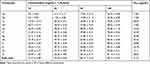 |
Table 5 The Compounds (1a-1l) Tyrosinase Screening |
Inhibition of dopamine-connected vanillin was tested using L-DOPA as a substrate. Kojic acid is used as a basic skin-whitening element, and was used as a reference compound in this study. The carboxyl and NH groups were present in compounds 1c-l and kojic acid, which play a major role in this mechanism.60 Compound 1e showed the highest inhibition, among them, the mechanism of inhibition was represented in Scheme 6.
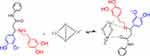 |
Scheme 6 The binuclear active site of tyrosinase with reversible competitive binding of compound 1e. |
The kinetic behavior of the most active compound 1e was studied with respect to the oxidation of L-DOPA by mushroom tyrosinase at different concentrations. As shown in Figure 3, Lineweaver -Burk plots of 1/V versus 1/[S] resulted in a family of straight lines with the same intercept on the vertical axis. The plots obtained indicated that compound 1e is a competitive inhibitor and that its inhibitory activity decreases with increasing substrate concentration.
 |
Figure 3 Inhibition of compound 1e – Lineweaver-Burk plot. |
Antioxidant agents can form free-radical scavengers and inhibit enzymes, which are related to the design of chemical structures.61–63 Target compounds (1a-l) can act as hydrogen donors, providing atoms directly to the radicals and preventing the formation of toxic OH radicals to the cell membrane peroxidation.64
The compounds 1f, 1g, and 1h were at close concentration range of (0.30 to 0.67 µm/mL) in MCF-7 cells with different activity such as DPPH (15.22 to 19.23, µm/mL), NO (10.36 to 12.75 µm/mL), H2O2 (11.82 to 14.40 µm/mL) assays, and anti-tyrosinase activity (25.47 to 36.59, µm/mL), whereas that are cytotoxic in VERO cells only at twice the concentrations.
The compound 1j, and 1l were equipotent activity against H2O2 antioxidant assay (13.27, and 13.55 µm/mL), closely related activity against NO antioxidant assay (11.13, and 12.56 µm/mL), and the closely related against anti-tyrosinase activity (24.76, and 28.63µm/mL), whereas that are cytotoxic in MCF-7 cell line (9.36, 4.49 µm/mL), and Vero cells (26.18, and 28.11µm/mL) concentrations, respectively.
Further the activity of all effective compounds were tested against the normal cell line (VERO cell line) and it was concluded that most of compounds were obtained cytotoxic at twice the concentrations to normal cell compared than MCF-7 cell line.
The cytotoxic results of each test are reported as the growth of treated cells in Table 6. As a result, among the synthesized compounds evaluated, compound 1e, 1f, and 1g were highest cytotoxic against MCF-7 cell line and low active against Vero cell line than that of doxorubicin. Moreover, the selectivity index (SI) of the compounds 1e, 1k, and 1g (Vero and MCF-7) was equipotent than that of doxorubicin with SI values. The IC50 values and selectivity index (SI) that obtained from the MTT assay are presented in Table 6.
 |
Table 6 Cytotoxicity Activity of Compounds (1a-j) |
Conclusion
New dopamine-connected vanillin multicomponent derivatives (1a-l) were synthesised via the grindstone method in high yields (85–92%) via a one-pot Mannich base without using catalysis. This method is inexpensive and produces a high yield. We synthesised 12 dopamine-connected vanillin derivatives and evaluated their anti-tyrosinase and antioxidant activities as well as their cytotoxicity. Compound 1k was highly active in DPPH, H2O2 scavenging, and NO scavenging. On the other hand, compounds 1e and 1k were highly active in ABTS•+ and AAPH assays compared with the trolox standard. Compounds 1e and 1k significantly inhibited tyrosinase activity compared with standard kojic acid, and compound 1e (GI50 = 0.01 µM) showed higher cytotoxicity in the MCF-7 cancer cell line. Therefore, lead compounds 1e and 1k are the new class of most effective antioxidant and anti-tyrosinase agents, and further development is required.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group project RGP-180.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Liu J, Cao R, Yi W, et al. A class of potent tyrosinase Inhibitors alkylidenethiosemicarbazide compounds. Eur J Med Chem. 2009;44(4):1773–1778. doi:10.1016/j.ejmech.2008.04.002
2. Garcia A, Fulton JE. The combination of glycolic acid and hydroquinone or kojic acid for the treatment of melasma and related conditions. Dermatol Surg. 1996;22(5):443–447. doi:10.1111/j.1524-4725.1996.tb00345.x.
3. Tajima R, Oozeki H, Muraoka S, et al. Synthesis and evaluation of bibenzyl glycosides as potent tyrosinase inhibitors. Eur J Med Chem. 2011;46(4):1374–1381. doi:10.1016/j.ejmech.2011.01.065.
4. Lee SY, Baek N, Nam TG. Natural semisynthetic and synthetic tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2016;31(1):1–13. doi:10.3109/14756366.2015.1004058.
5. Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10(6):2440–2475. doi:10.3390/ijms10062440.
6. Thome Andersson K, Sterner O, Hansson C. Tyrosinase mediated formation of a reactive quinone from the depigmenting agents 4-tertbutyphenol and 4-tert-butyl-catechol. Pigment Cell Res. 2000;13:33–38. doi:10.1034/j.1600-0749.2000.130107.x.
7. Zuo AIR, Dong HH, Yu YY, et al. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin Med. 2018;13:51. doi:10.1186/s13020-018-0206
8. Belaidi AA, Bush AI. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem. 2016;139:179–197. doi:10.1111/jnc.13425
9. Dhanalakshmi C, Manivasagam T, Nataraj J, Justin Thenmozhi A, Essa MM. Neurosupportive role of vanillin a natural phenolic compound on rotenone induced neurotoxicity in SHSY5Y neuroblastoma cells. Alternat Med. 2015;626028. doi:10.1155/2015/626028
10. Ashraf Z, Rafiq M, Seo SY, Babar MM, Zaidi NS. Synthesis kinetic mechanism and docking studies of vanillin derivatives as inhibitors of mushroom tyrosinase. Bioorg Med Chem. 2015;23(17):5780. doi:10.1016/j.bmc.2015.06.068.
11. Untung J, Iskandarsyah I, Hayun H. 2-[(2,6-Dimethylmorpholin-4-yl)methyl]-4-[(E)- 2-{3-[(E)-2-{3-[(2,6-dimethylmorpholin-4-yl)methyl]-4-hydroxy-5-methoxy phenyl} ethenyl]- 1H-pyrazol-5-yl}ethenyl]-6-methoxyphenol. Molbank. 2017;3:M949. doi:10.3390/M949
12. Toure BB, Hall DG. Natural product synthesis using multicomponent reaction strategies. Chem Rev. 2009;109:4439–4486. doi:10.1021/cr800296p.
13. Park DH, Venkatesan J, Kim SK, Ramkumar V, Parthiban P. Discovery of monoamine oxidase inhibitors by medicinal chemistry approaches. Bioorg Med Chem Lett. 2012;22:6362–6367. doi:10.1039/c8md00446c.
14. Gul HI, Ojanen T, Vepsalainen J, et al. Vanillic Mannich bases synthesis and screening of biological activity Mechanistic insight into the reaction with 4-chloroaniline. RSC Adv. 2001;51:72–75. doi:10.1039/c4ra03909b
15. Kouskoura M, Hadjipavlou Litina D, Giakoumakou M. Crystal structure of 3-(4-methoxy phenyl)-1-(4-methylphenyl)prop-2-en-1-one, C17H16O2. Med Chem. 2008;4:586–596. doi:10.1515/ncrs-2016-0357.
16. Malinka W, Swiatek P, Filipek B, Sapa J, Jerierska A, Koll A. 4-(4-Bromobenzylideneamino)- 3-{1-[4-(2-methylpropyl)phenyl]ethyl}-1-(morpholinomethyl)-1H-1,2,4-triazole-5(4H) -thione. Farmaco. 2005;60:961–968. doi:10.1107/S160053680802254
17. Holla BS, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1, 2, 4-triazoles. Eur J Med Chem. 2003;38:759–767. doi:10.1016/s0223-5234(03)00128-4.
18. Ferlin MG, Chiarelotto G, Antonucci F, Caparrotta L, Froldi G. 1-(2-Benzoyl-1-phenylethyl)-4-[(2-hydroxy-1-naphthyl)methylideneamino]-3-methyl-1H-1,2,4-triazole-5(4H)-thione. Eur J Med Chem. 2002;37:427–434. doi:10.1107/S1600536810052979.
19. Lopes F, Capela R, Goncaves JO, et al. Crystal structure spectroscopic and redox behaviour of novel imidazolidine ligand. Tetrahedron Lett. 2004;45:7663–7666. doi:10.1016/j.molstruc.2010.10.037
20. Joshi S, Khosla N, Tiwari P. In vitro study of some medicinally important Mannich bases derived from antitubercular agent. Bioorg Med Chem. 2004;12:571–576. doi:10.1016/j.bmc.2003.11.001
21. Roman G. Mannich bases in medicinal chemistry and drug design. Eur J Med Chem. 2015;89:743–816. doi:10.1016/j.ejmech.2014.10.076.
22. Bandgar BP, Patil SA, Gacche RN, et al. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorganic Med Chem Lett. 2010;20:730. doi:10.1016/j.bmcl.2009.11.068.
23. Karakayaa G, Tureb A, Ercanc A, Onculc S, Aytemira MD. Synthesis computational molecular docking analysis and effectiveness on tyrosinase inhibition of kojic acid derivatives. Bioorg Chem. 2019;88:102950. doi:10.1016/j.bioorg.2019.102950.
24. Kim GH, Kim JE, Rhie SJ, Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24:325–340. doi:10.5607/en.2015.24.4.325.
25. Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi:10.1016/j.redox.2018.01.008.
26. Patel M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci. 2016;37:768–778. doi:10.1016/j.tips.2016.06.007.
27. Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid β-peptide. Trends Mol Med. 2001;7:548. doi:10.1016/S1471-4914(01)02173-6
28. Huang X, Moir RD, Tanzi RE, Bush AI, Rogers JT. Redox-active metals, oxidative stress, Alzheimer’s disease pathology. Ann NY Acad Sci. 2014;1012:153–163. doi:10.1196/annals
29. Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H. Skin pharmacol physiol, antioxidant properties of ferulic acid. Skin Pharmacol Physiol. 2018;31:332–336. doi:10.1159/000491755.
30. Kim D, Park J, Kim J. Flavonoids as mushroom tyrosinase inhibitors: a fluorescence quenching study. J Agric Food Chem. 2006;54:935–941. doi:10.1021/jf0521855.
31. Khatib S, Nerya O, Musa R, Shmuel M, Tamir S, Vaya J. Chalcones as potent tyrosinase inhibitors: the importance of a 2,4-substituted resorcinol moiety. Bioorg Med Chem. 2005;13:433–441. doi:10.1016/j.bmc.2004.10.010
32. Grzesik M, Naparło K, Bartosz G, Sadowska-Bartosz I. Antioxidant properties of catechins: comparison with other antioxidants. Food Chem. 2018;241:480–492. doi:10.1016/j.foodchem.2017.08.117
33. Evacuasiany E, Ratnawati H, Liana LK, et al. Cytotoxic and antioxidant activities of catechins in inhibiting the malignancy of breast cancer. Oxid Antioxid Med Sci. 2014;3(2):141–146. doi:10.5455/oams.240614.or.066
34. Guo N, Wang C, Shang C, You X, Zhang L, Liu W. Integrated study of the mechanism of tyrosinase inhibition by baicalein using kinetic, multispectroscopic and computational simulation analyses. Int J Biol Micromol. 2018;118:57–68. doi:10.1016/j.ijbiomac.2018.06.055
35. Shimizu K, Yasutake S, Kondo R. A new stilbene with tyrosinase inhibitory activity from Chlorophora excelsa. Chem Pharm Bull. 2003;51:318–319. doi:10.1248/cpb.51.318.
36. Kenji O, Toshiyuki T, Tetsuro I. Inhibitory effects of resveratrol derivatives from dipterocarpaceae plants on tyrosinase activity. Biosci Biotechnol Biochem. 2003;67:1587–1589. doi:10.1271/bbb.67.1587
37. Halaouli S, Asther M, Kruus K. Characterization of a new tyrosinase from Pycnoporus species with high potential for food technological applications. J Appl Microbiol. 2005;98:332–343. doi:10.1111/j.1365-2672.2004.02481.x.
38. Sahu RK, Roy A, Dwivedi J, Jha AK. Promotion and computation of inhibitory effect on tyrosinase activity of herbal cream by incorporating indigenous medicinal plants. Pak J Biol Sci. 2014;17:146–150. doi:10.3923/pjbs.2014.146.150.
39. Dembitsky VM, Kilimnik A. Anti-melanoma agents derived from fungal species. M J Pharma Sci. 2016;1:1–16.
40. Maghsoudi S, Adibi H, Hamzeh M, et al. Kinetic of mushroom tyrosinase inhibition by benzaldehyde derivatives. J Rep Pharma Sci. 2013;2:156–164.
41. Jeon SH, Jong HK, Kwang Hoon K. Inhibitory effects on L-dopa oxidation of tyrosinase by skin-whitening agents. Bull Korean Chem Soc. 2005;26:1135–1137. doi:10.5012/bkcs.2005.26.7.1135
42. Fenandez-Bertran JF. Mechanochemistry: an overview. Pure Appl Chem. 1999;71:581–586. doi:10.1351/pac19997104058
43. Walsh PJ, Li H, Parrodi CA. A green chemistry approach to asymmetric catalysis: solvent-free and highly concentrated reactions. Chem Rev. 2007;107:2503. doi:10.1021/cr0509556.
44. Bose AK, Pednekar S, Ganguly SN, Chakraborty G, Manhas MS. 1,5-Bis(4-chlorophenyl)-3-(2-thienyl)-pentane-1,5-dione. Tetrahedron Lett. 2004;45:8351–8353. doi:10.1107/S1600536808038993.
45. Garay AL, Pichon A, James SL. Solvent free synthesis of metal complexes. Chem Soc Rev. 2007;36:846–855. doi:10.1039/B600363J.
46. Alaklabi A, Arif IA, Ahamed A, Radhakrishnan Surendra Kumar RS, Idhayadhulla A. Evaluation of antioxidant and anticancer activities of chemical constituents of the Saururus chinensis root extracts. Saudi J Biol Sci. 2018;25:1387–1392. doi:10.1016/j.sjbs.2016.12.021
47. Surendra Kumar R, Moydeen M, Al-Deyab SS, Aseer M, Idhayadhulla A. Synthesis of new morpholine-connected pyrazolidine derivatives and their antimicrobial, antioxidant, and cytotoxic activities. Bioorg Med Chem Lett. 2017;27:66–71. doi:10.1016/j.bmcl.2016.11.032
48. Xia L, Idhayadhulla A, Rok Lee YR, Wee YJ, Kim SH. Anti- tyrosinase, antioxidant, and antibacterial activities of novel 5- hydroxy-4-acetyl-2,3-dihydronaphtho[1,2-b]furans. Eur J Med Chem. 2014;86:605–612. doi:10.1021/co500002s
49. Fındık E, Ceylan M, Elmastas M. Isoeugenol-based novel potent antioxidants: synthesis and reactivity. Eur J Med Chem. 2011;46:4618–4624. doi:10.1016/j.ejmech.2011.07.041
50. Arora A, Nair MG, Strasburg GM. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radical Biol Med. 1998;24(9):1355–1363. doi:10.1016/s0891-5849(97)00458-9.
51. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med. 1996;20:933–956. doi:10.1016/0891-5849(95)02227-9
52. Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi:10.1016/S1360-1385(97)01018-2
53. Lin L, Dong Y, Zhao H, Wena L, Yang B, Zhao M. Comparative evaluation of rosmarinic acid, methyl rosmarinate and pedalitin isolated from Rabdosia serra (MAXIM.) HARA as inhibitors of tyrosinase and a-glucosidase. Food Chem. 2011;129:884–889. doi:10.1016/j.foodchem.2011.05.039.
54. Kang HS, Park HJ, Kim HR, Byun DS, Choi JS. Rosmarinic acid as a tyrosinase inhibitors from salvia miltiorrhiza. Nat Prod Sci. 2004;10:80–84.
55. Piazzon A, Vrhovsek U, Masuero D, Mattivi F, Mandoj F, Nardini M. Antioxidant activity of phenolic acids and their metabolites: synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic Acids and of the acyl glucuronide of ferulic Acid. J Agric Food Chem. 2012;60:12312–12323. doi:10.1021/jf304076z
56. Gulcin I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids. 2007;32:431–438. doi:10.1007/s00726-006-0379-x.
57. Tai A, Sawano T, Yazama F, Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim Biophys Acta. 2011;1810:170–177. doi:10.1016/j.bbagen.2010.11.004.
58. Ashraf Z, Rafiq M, Seo SY, et al. Synthesis, kinetic mechanism and docking studies of vanillin derivatives as inhibitors of mushroom tyrosinase. Bioorg Med Chem. 2015;23(17):5870–5880. doi:10.1016/j.bmc.2015.06.068
59. Akimov MG, Gretskaya NM, Zinchenko GN, Bezuglov VV. Cytotoxicity of endogenous lipids N-acyl dopamines and their possible metabolic derivatives for human cancer cell lines of different histological origin. Anticancer Res. 2015;35:2657–2662.
60. Battaini G, Monzani E, Casella L, Santagostini L, Pagliarin R. Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid. J Biol Inorg Chem. 2000;5:262–268. doi:10.1007/s007750050370.
61. Bezerra GSN, Pereira MAV, Ostrosky EA, et al. Compatibility study between ferulic acid and excipients used in cosmetic formulations by TG/DTG, DSC and FTIR. J Therm Anal Calorim. 2017;127:1683–1691. doi:10.1007/s10973-016-5654-9
62. Lodovici M, Guglielmi F, Meoni M, Dolara P. Effect of natural phenolic acids on DNA oxidation in vitro. Food Chem Toxicol. 2001;39:1205–1210. doi:10.1016/s0278-6915(01)00067-9.
63. Masella R, Vari R, d’Archivio M, et al. Extra virgin olive oil biophenols inhibit cell-mediated oxidation of LD by increasing the mRNA transcription of glutathione-related enzymes. J Nutr. 2004;134:785–791. doi:10.1093/jn/134.4.785.
64. Scharffetter-Kochanek K, Brenneisen P, Wenk J, Herrmann G, Ma W, Kuhr L. Photoaging of the skin from phenotype to mechanisms. Exp Gerontol. 2000;35:307–316. doi:10.1016/S0531-5565(00)00098-X
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
