Back to Journals » Patient Related Outcome Measures » Volume 14
Disutilities Associated with Intravenous Iron Infusions: Results from a Time Trade-off Survey and Diminishing Marginal Utility Model for Treatment Attributes in China
Authors Hu S, Wu D, Wu J, Zhang Y, Bøgelund M , Pöhlmann J , Pollock RF
Received 9 December 2022
Accepted for publication 15 September 2023
Published 26 September 2023 Volume 2023:14 Pages 253—267
DOI https://doi.org/10.2147/PROM.S400389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Howland
Shanlian Hu,1 Depei Wu,2 Jing Wu,3 Yabing Zhang,4 Mette Bøgelund,5 Johannes Pöhlmann,6 Richard F Pollock6
1School of Public Health, Fudan University, Shanghai, People’s Republic of China; 2First Affiliated Hospital of Soochow University, Soochow University, Suzhou, People’s Republic of China; 3School of Pharmaceutical Science and Technology, Tianjin University, Tianjin, People’s Republic of China; 4Shanghai Institute of Technology, Shanghai, People’s Republic of China; 5Incentive Partners ApS, Holte, Denmark; 6Covalence Research Ltd, Harpenden, United Kingdom
Correspondence: Richard F Pollock, Covalence Research Ltd, Rivers Lodge, West Common, Harpenden, AL5 2JD, United Kingdom, Tel +44 20 8638 6525, Email [email protected]
Purpose: Treatment process attributes can affect health state utilities associated with therapy. For intravenous iron, used to treat iron deficiency and iron deficiency anemia, research into process attributes is still lacking. This study estimated utilities associated with process attributes for intravenous iron infusions.
Methods: An online survey including seven health state vignettes and time trade-off tasks was administered to participants, who were not patients living with iron deficiency or iron deficiency anemia, from a Chinese online panel. Vignettes used an identical description of iron deficiency and iron deficiency anemia but differed in the annual number of infusions, infusion duration, and infusion-associated risk of hypophosphatemic osteomalacia. Disutilities and their rate of change as the number of infusions increased were examined using a power model.
Results: The survey was completed by 1091 participants. The highest utilities were observed for one annual infusion of 15– 30 minutes or 30– 60 minutes, without risk of hypophosphatemic osteomalacia (0.754 and 0.746, respectively). In comparison, more infusions and infusions with a risk of hypophosphatemic osteomalacia were associated with lower utilities. Utility continued to decrease, but at a diminishing rate, as the annual number of infusions increased, with utility decrements of 0.006 and 0.002, respectively, when going from zero to one and from four to five infusions per year. All marginal disutilities were small (values < 0.01).
Conclusion: This study suggested that treatment attributes of intravenous iron infusions affect health state utilities. Using intravenous iron formulations that allow for fewer and shorter infusions without the risk of hypophosphatemic osteomalacia can reduce the number of visits required and increase patients’ quality of life.
Plain Language Summary: A lack of iron in the body can be treated successfully using intravenous infusions of iron, but it is not yet known how different intravenous iron formulations affect patients’ quality of life.
Intravenous iron formulations differ in how often and for how long (per infusion) they need to be administered; for example, while one available intravenous iron formulation allows most iron deficits to be corrected in a single visit, others can require several visits. Formulations also seem to differ in the risk of complications relating to low phosphate and bone softening.
This study presented descriptions of treatment options, differing in the number and duration of infusions as well as in risk, to more than 1000 Chinese respondents. Based on their answers, the differences between intravenous iron administration regimes could be valued and linked to quality of life. Intravenous iron treatment with fewer, shorter infusions and without risk of bone softening was found to come with a better quality of life. Each additional infusion reduced quality of life, but less so if treatment already involved several infusions. These findings can help patients and physicians to make clinical decisions that focus not only on efficacy and safety but also on the quality of life.
Keywords: China, disutility, health-related quality of life, intravenous iron, iron deficiency, time trade-off
Introduction
Iron deficiency (ID) is defined as either insufficient total body iron or the inability to sufficiently mobilize existing stores and negatively affects many iron-dependent functions in the body, potentially resulting in iron deficiency anemia (IDA).1,2 Both ID and IDA are frequently observed in patients with chronic kidney disease or cancer. They are associated with reduced physical and mental functioning, which results in lethargy, fatigue, reduced exercise capacity, dizziness, dyspnea, headache, and difficulty staying focused.3–5 These symptoms not only impose a clinical burden but also reduce patients’ quality of life (QoL) and work productivity.6–8
Treatment of ID and IDA is centered on iron supplementation. Oral iron supplementation is currently the first-line therapy in many settings, as it is easy to administer and available at low cost. Parenteral iron therapy is an alternative to oral therapy recommended mainly if oral therapy is poorly tolerated or adhered to or if the response to oral iron is insufficient, particularly in the case of severe ID and IDA and chronic inflammation, which blocks iron absorption and bioavailability.9,10
Total iron deficits vary between patients but can frequently reach 1500 mg.11 Addressing such deficits with oral iron supplementation may take a considerable amount of time. High oral iron doses are associated with an increased risk of gastrointestinal side effects and low absorbed iron fractions, while lower doses naturally take longer to replenish a deficit.12 Treatment with intravenous (IV) iron, in contrast, can deliver higher doses of iron more rapidly.
Modern IV iron formulations include ferric derisomaltose (FDI), ferric carboxymaltose (FCM), and iron sucrose (IS).13–15 All modern IV iron formulations are generally safe and associated with few complications and adverse reactions.16,17 However, significant differences exist between IV iron formulations, including in the formulation-specific risk of hypophosphatemia leading to osteomalacia and fractures, which is elevated for FCM relative to FDI.9,17,18 Another difference concerns dosing. While FDI can be dosed up to 20 mg of iron per kilogram of body weight, maximum single doses of FCM and IS are limited to 1000 mg and 200 mg of iron, respectively.19 The potential to administer higher iron doses with FDI implies that, once a treatment plan has been developed based on the patient’s iron deficit, fewer infusions – indeed frequently only a single infusion – are needed to correct the iron deficit. This is relative to IV treatments with absolute dosing restrictions that are typically lower than average iron deficits, which necessitates multiple infusions, on separate days, and therefore multiple visits to healthcare providers.19,20 Administration frequency is one of many treatment-associated “process attributes”, ie, features of healthcare that reflect a broader concept of health than direct health gains.21–24 Other treatment process attributes include administration route, dose timing, or treatment location. In several therapy areas, these attributes have been shown to influence patient preferences and utilities associated with treatments; simpler, less frequent, and shorter treatments are generally preferred by patients.22,25–27 While usually less important to patients than efficacy and safety, process attributes often have a non-negligible effect on quality of life (QoL) and treatment adherence, so should be considered in clinical decisions and health economic evaluations.22,24,28
Process attributes that distinguish the various IV iron formulations, namely the number and duration of infusions required to correct iron deficits and the risk of side effects such as hypophosphatemia leading to osteomalacia, have not yet been investigated. The present study aimed to examine how differences in process attributes would be valued in China. Health-state utilities derived from valuations for each state were then used to develop a diminishing marginal disutility (DMD) model to estimate disutility reductions associated with each additional iron infusion.
Materials and Methods
Study Design
This vignette study was designed to elicit preferences regarding the frequency and duration of IV iron infusions as well as the associated risk and to estimate health state utilities associated with specific combinations of infusion frequency and duration.
To ensure that vignettes were accurate and reflected patient experience, the disease description and health state vignettes were developed from the literature, summaries of product characteristics, and patient leaflets.13–15,29–31 Development took place in collaboration with a hematologist and two health economists based in China. The seven vignettes all referred to the same disease description, treatment location, and treatment success. Vignettes differed only in the number of infusions required in a yearly treatment course, duration per infusion, and risk of hypophosphatemic osteomalacia as a long-term side effect. Any difference in health state valuation could therefore be attributed to differences in the process attributes and the risk of hypophosphatemic osteomalacia.
The health states were presented in an online survey to Chinese participants between December 2020 and January 2021. Health state utilities were elicited in a time trade-off (TTO) survey, for annual treatment over a lifetime horizon.
The study was conducted in line with the Market Research Society’s Code of Conduct and the ESOMAR/Global Research Business Network guidance on online research.32,33 Ethical review board approval in China was not required as valuation tasks are not considered to be medical interventions and, as the study was not a clinical trial, it neither included patients nor made use of human or biological samples, or personally identifiable information, in line with the “Measures for Ethical Review of Biomedical Research Involving Humans” policy issued by the National Health and Family Planning Commission.34,35 All participants had previously agreed to take part in research of the type presented here and had given explicit consent to participate in the present study at the start of the survey. Participants were free to terminate the survey at any point if they wished to do so, remained anonymous throughout the study, and could neither be identified nor linked to answers.
Development of Disease Description and Health State Vignettes
The survey began with a general disease description in textual form that presented the symptoms associated with ID and IDA, followed by the description of treatment benefits and modalities, including the infusion procedure and possible needle insertion pain and infusion site reactions (see Table S1). The disease description also outlined the need to travel to the hospital for every infusion and the time needed for preparation, waiting, and post-infusion monitoring.
This disease description was followed by health state vignettes. All vignettes referred to the disease description, stated that treatment for the disease was required every year, and that treatment was able to control the disease. Beyond these identical elements, health states were varied along three dimensions: the number of infusions required, the duration of an infusion (presented as a minimum-maximum range in minutes), and the risk of hypophosphatemic osteomalacia, described in vignettes as a “serious long-term side effect (softening of/fractured bones)” (see Table S2).
Health state (HS) 1 described treatment as one infusion lasting 30–60 minutes, with no risk of hypophosphatemic osteomalacia (Table 1). HS 2–4 differed from HS 1 in specifying two, five, and seven infusions, respectively, with each infusion lasting 30–60 minutes, again without risk of hypophosphatemic osteomalacia. HS 5 specified one infusion but of only 15–30 minutes, while HS 6 specified two infusions of 15–30 minutes, both without risk of hypophosphatemic osteomalacia. HS 7 differed from HS 6 in specifying that there was a risk of experiencing hypophosphatemic osteomalacia as a long-term serious side effect. As the FCM summary product characteristics reported that the frequency of hypophosphatemic osteomalacia as an adverse drug reaction is unknown, and no studies quantifying the risk of hypophosphatemic osteomalacia following IV iron treatment were identified, the osteomalacia risk was presented in vignettes as one in a thousand patients (considered a relatable but still small risk).
 |
Table 1 Overview of Health States and Differences in Process Attributes |
Health states 1–5 were pilot-tested in an online survey with 176 participants. Results suggested that participants were able to differentiate and trade between health states, with no indication that revisions were needed.
In the main survey, after a control question to familiarize participants with the TTO format, vignettes were presented in random order. While HS 1, 3, and 7 were shown to all participants, HS 2 and 4 were shown to only half of the participants and HS 5 and 6 were shown to the other half. This was to ensure that respondents had to consider at most five health states, to avoid respondent fatigue.
Participants
Participants were recruited from a Chinese online panel provided by a market research analysis company (Kantar, London, UK). Potential participants were required to be at least 18 years old and were quota-sampled to be representative of the 2020 age and sex distributions in China. Participants were not required to have ID or IDA.
Eligible panel members received a one-time link that preserved their anonymity, ie, answers could not be linked to individuals. Participants completing the survey were remunerated for their time with an online shopping token with a value equivalent to EUR 2–4.
Translation
The disease description and vignettes were developed in English. After finalization, they were translated into Standard Chinese by a certified translation agency. The translation was verified by an independent translator.
Statistical Analysis
Utility values were calculated for each health state, with standard errors and 95% confidence intervals (CIs) obtained from non-parametric bootstrapping based on 10,000 iterations. Disutilities (also referred to as “utility decrements” and “decreases in utility”) between states were calculated as the difference between utility values. The calculation of disutilities was performed after removing outliers (2.5% at the bottom and top of the utility distribution, respectively) to increase the reliability of the results.36 Utilities and disutilities were also reported and analyzed by sex. The differences between sex for each health state and each comparison were assessed for statistical significance using Welch’s t-test.37 The significance level was specified post hoc as α=0.05. These analyses were performed using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA).
In a survey, the number of vignettes that can be presented must be limited to avoid overburdening respondents. For the present survey, this meant that only one, two, five, and seven annual administrations could be investigated, for infusions with a duration of 30–60 minutes and no risk of hypophosphatemic osteomalacia (HS1–4). For cost-utility analyses, however, additional flexibility to capture a variable number of infusions might be desirable.38 This flexibility was achieved by implementing a DMD model, based on work by Lauridsen et al for hypoglycemia.39 Using this model rested on the assumption that, as for hypoglycemic events, the “first is the worst” for IV iron infusions – each subsequent infusion will reduce utility but by a smaller amount than the preceding infusion.
The model was implemented based on the three estimated disutility values obtained for the first four health states (HS 2 vs 1, HS 3 vs 1, HS 4 vs 1). A power function of the form 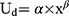 was estimated in a linear regression model with the log-transformed number of infusions and log-transformed disutilities as predictor and outcome, respectively.40 Uncertainty was captured by power functions applied to the lower and upper CI limits of the estimated disutility values. The DMD model was implemented using R version 4.1.1.41,42
was estimated in a linear regression model with the log-transformed number of infusions and log-transformed disutilities as predictor and outcome, respectively.40 Uncertainty was captured by power functions applied to the lower and upper CI limits of the estimated disutility values. The DMD model was implemented using R version 4.1.1.41,42
Results
Participant Characteristics
The link to the survey was shared with 1354 online panel members, of whom 1270 (93.8%) started the survey. Of the initial participants, 110 (8.7%) failed the TTO control question or did not trade between health states within the premise of the TTO design, 63 (5.0%) did not complete all TTO tasks, and 6 (0.47%) did not provide information on age and gender. This left 1091 respondents (80.6% of contacted panel members and 85.9% of those who started the survey) for analysis. Women accounted for 52% of respondents (Table 2). The mean age was 37.8 years, with nearly two-thirds of participants aged <40 years.
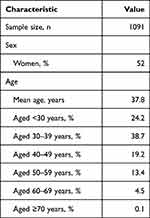 |
Table 2 Demographic Characteristics of Participants |
Health State Utilities
HS 5, with one 15–30 minutes infusion per year and no hypophosphatemic osteomalacia risk, had the highest mean utility, at 0.754 (95% CI: 0.730 to 0.776), followed by HS 1, with one 30–60 minutes infusion per year and no hypophosphatemic osteomalacia risk (Figure 1). In contrast, HS 4, with seven annual infusions of 30–60 minutes and no hypophosphatemic osteomalacia risk, corresponding to treatment with iron sucrose, had the lowest mean utility (0.701, 95% CI: 0.681 to 0.723). Health state 7, which specified a risk of hypophosphatemic osteomalacia, was associated with a utility of 0.715 (95% CI: 0.699 to 0.731).
Comparing pairs of health states showed a preference for fewer and shorter treatments and lower risk in most comparisons (Figure 2). Relative to one infusion of 30–60 minutes with no hypophosphatemic osteomalacia risk, seven such infusions were associated with a utility decrement of 0.039 (95% CI: 0.030 to 0.048), while five such infusions were associated with a utility decrement of 0.022 (95% CI: 0.016 to 0.029) and two infusions with a utility decrement of 0.017 (95% CI: 0.010 to 0.025). Similarly, comparing seven versus two such infusions directly yielded a utility decrement of 0.020 (95% CI: 0.011 to 0.029). The same pattern was again observed when comparing two infusions of 15–30 minutes, with no hypophosphatemic osteomalacia risk, with one such infusion, which yielded a small utility decrement of 0.008 (95% CI: −0.002 to 0.018). A reduction in duration from 30–60 minutes to 15–30 minutes for one infusion each, with no risk of hypophosphatemic osteomalacia, was also associated with a small reduction in the utility of 0.008 (95% CI: 0 to 0.017).
In all comparisons, the health state with a risk of hypophosphatemic osteomalacia was associated with reduced utility relative to states without a risk of hypophosphatemic osteomalacia. This reduction in utility was observed if states differed only in the risk of hypophosphatemic osteomalacia, but also if the risk-associated treatment required the same number but shorter infusions and if the risk-associated treatment required fewer and shorter infusions.
Subgroup Analyses
Men reported health state valuations that were consistently 0.02–0.03 higher than those reported by women (Figure 3A). These differences were not statistically significant for a significance level of 0.05, except for health state 6 (p = 0.05). The disutilities between health states reported by women were consistently smaller than those reported by men, but for no comparison was the difference by sex statistically significant at a significance level of 0.05 (Figure 3B).
Diminishing Marginal Disutility Model
The power model developed for disutilities associated with infusions with a duration of 30–60 minutes and no risk of hypophosphatemic osteomalacia had the functional form Ud=0.161×x0.39. This function allowed deriving disutilities for administration frequencies not directly covered in the TTO survey (Figure 4; for detailed values, see Table S3). For example, three IV iron infusions per year, each with a duration of 30–60 minutes and without risk of hypophosphatemic osteomalacia, would be associated with an annual utility decrement of 0.025 (95% CI: 0.017 to 0.033) relative to no such infusion in a year. Fifteen such infusions would be associated with an annual disutility of 0.047 (95% CI: 0.041 to 0.053) relative to no such infusion in a year.
Marginal disutilities derived from the power model indicated that, as the number of additional infusions increased, utility continuously decreased but at a diminishing rate (Figure 5; for detailed values, see Table S3). While receiving one annual 30–60 minutes infusion, without risk of hypophosphatemic osteomalacia, relative to receiving no such infusion reduced utility by 0.006 (95% CI: 0.005 to 0.007), going from four to five infusions reduced utility by 0.0024 (95% CI: 0.0023 to 0.0025) and going from fourteen to fifteen infusions by 0.0012 (95% CI: 0.0011 to 0.0015). It should be noted that the magnitude of marginal disutilities was small.
Discussion
This is the first study to assess how attributes and risks of IV iron infusions affect health state utilities associated with IV iron treatment. Results from a vignette-based TTO survey in Chinese study participants showed that treatment options with improved process attributes – fewer and shorter infusions, and no risk of hypophosphatemic osteomalacia – were associated with higher utility values than treatment options with more and longer infusions and those with a higher risk of hypophosphatemic osteomalacia. The disutility associated with each additional annual infusion diminished as the total number of annual infusions increased, indicating that the negative impact of additional infusions was highest at the lower end of the infusion frequency distribution.
The highest utility was observed for an annual treatment of one infusion of 15–30 minutes, followed by an annual treatment of one infusion of 30–60 minutes, both without risk of hypophosphatemic osteomalacia (Figure 1). These were the only regimens with just one annual infusion without hypophosphatemic osteomalacia risk, so arguably had the most favorable process attributes of the states presented to study participants. No statistically significant differences in health state valuations and disutilities were observed between women and men, except for health state 6, which women had valued worse than men. It remains unclear from the available data if this finding reflects a true difference in how women and men evaluate this state or if this finding of statistical significance is a false positive. Further research, including using patient data, will help illuminate this issue further. Women valued health states consistently lower than men, however, which is in line with published data on women self-reporting their health and functioning as lower than men across countries and measurement instruments.43,44
The finding that these states were associated with the smallest utility reductions was consistent with previous studies in different therapeutic areas. A review of the published hemophilia literature and US Food and Drug Administration drug development meeting documents for bleeding disorders showed that infusion frequency was the most important treatment attribute, ahead even of efficacy, in patients with hemophilia.45 Findings were also consistent with the literature investigating treatment frequency and risk in diabetes. Examples include a standard gamble interview study of patients with type 2 diabetes mellitus (T2DM), in whom daily relative to weekly injections were associated with a disutility of 0.023 and for whom frequency was the most important process attribute.46 A discrete choice experiment in patients with T2DM showed that mode and frequency of administration as well as the risk of side effects were the most important treatment attributes, ahead of clinical outcomes such as changes in weight and HbA1c.47 Matza et al reported that nearly half of patients with T2DM prefer injectable to oral treatment if the former can be administered weekly instead of daily with oral treatment.27 In oncology, Mansfield et al demonstrated that patients may prefer an IV schedule to oral treatment if the latter requires several pills a day or a complicated dosing schedule.48
While it should be acknowledged that results of treatment attribute studies are difficult to compare directly across treatments in different therapeutic areas, both the present and earlier studies showed that treatment process attributes, including treatment/administration frequency and associated risk, influence patient preferences for treatments and patients’ QoL.22,26,27 Process attributes may also affect treatment adherence.22,49 Intravenous treatments, for example, are associated with better adherence than oral treatments in anemia, inflammatory bowel disease, and osteoporosis.50–52 Non-adherence has been linked to forgetfulness, eg, if multiple tablets had to be taken daily or multiple appointments to be kept, and to more frequent side effects of therapy.9,53–55 These findings suggest that less frequent IV iron treatment, with fewer side effects, may translate to improved adherence and clinical outcomes. In addition, the increase in drug costs often associated with improving adherence may be offset partly by reduced costs associated with infusions and visits.56,57
The analysis of disutilities associated with 30–60 minutes infusions without hypophosphatemic osteomalacia risk indicated that disutilities increased at a diminishing rate with the number of infusions. This finding suggested that the first infusions of IV iron are the “worst” while additional infusions have a smaller albeit still detrimental effect on health state utilities. Diminishing marginal disutilities may result from adaptation to IV infusions, possibly including getting used to the logistics of traveling to/from the treatment location and waiting time during appointments, although the extent to which such adaptations factor into treatment attribute evaluation will require additional studies in patient samples. Viable alternative explanations include those by Lauridsen et al, namely unwillingness to trade-off remaining lifetime for fewer infusions beyond a certain point or consideration of health state descriptions while disregarding infusion frequencies.39
The vignette-based approach facilitated the isolation of process attributes of IV iron treatment and the estimation of utility differences arising from differences in process attributes. However, this approach has several limitations. Preferences, and consequently utilities, are ultimately expressed for vignettes and their content, not for actual health states as experienced by patients, in particular as vignettes were developed from the literature and with physician but not patient input.58 Nor is it possible to present the entire patient experience in vignettes, possibly resulting in the omission of important disease and treatment aspects and in underestimation of how differently patients may experience a condition. In the present study, detailed descriptions of ID and IDA, including their symptoms, and of the IV iron treatment process provided some context to respondents regarding disease and treatment attributes. The extent to which respondents’ understanding was aligned with patient experience ultimately remains uncertain, however.
Using TTO to value health states may also be considered a limitation. The method has been criticized as inconsistent with random utility and measurement theory, including confounding by respondents’ life expectancies and the cognitive challenges associated with trading between hypothetical health states.59,60 While these limitations were acknowledged before embarking on the present study, two key factors led to the decision to use TTO: the lack of sensitivity associated with generic preference-based measurements (designed to assess broader aspects of functioning and health domains rather than treatment attributes), and the need for a pragmatic assessment tool that could be presented online to many respondents. Despite the limitations of TTO and the continuing lack of methodological standardization, TTO is widely used, including in valuation protocols for the EQ-5D-5L.61,62
An additional limitation was the use of an online survey. Relative to face-to-face interviews, online surveys may yield data of lower quality as participants tend to be more likely to satisfice, ie, use cognitive shortcuts, which can result in distorted or nonsensical responses.63 Unusual responses or responses violating trading principles cannot be followed up, nor can missing responses be elicited if, as in the present study, participants remain anonymous. An initial test of the survey was conducted to assess if vignettes and TTO tasks were easy to understand, and there was no indication to the contrary. While satisficing or misunderstandings cannot be excluded, the online implementation allowed for recruiting more than 1000 participants – larger than most comparable studies conducted face-to-face, which usually included between 100 and 200 participants.27,46,49,64–66 It should be noted, however, that, as part of efforts to reduce respondent fatigue, not all patients responded to all questions in the online survey. As a result, some health states in the analysis were based on responses from different subsets of the overall population. Consequently, there were some limits to the comparability of absolute valuations and of health state and disutility estimates.
Another limitation, also resulting from the use of an online survey, was that the final sample population did not fully reflect the Chinese population. While the initial sampling of panel members contacted for participation accounted for the age and sex structure of the Chinese population, women and younger people were overrepresented in the final sample relative to the Seventh National Population Census.67 Reasons for age and sex differences in survey completion may have included differences in the attractiveness of the remuneration token, affinity to and time spent on the internet, or interest in the study topic, which may have been more relevant to younger women in whom anemia prevalence is higher. In light of this limitation, future treatment attribute studies in China should preferentially be conducted in a broad sample of patients and would ideally also include elderly patients.
The model used to derive disutilities was limited by being based on only three distinct values. The resulting uncertainty was quantified by also applying power functions to confidence intervals, but future users of these disutilities should be conscious of this uncertainty. The use of a diminishing marginal utility value also allowed deriving estimates for health states that could not be presented to avoid overburdening participants.
Despite these limitations, the utility values for IV iron infusions should prove useful for inclusion in cost-utility analyses.68 As in other studies, the differences in utilities attributable to treatment process attributes were small.27,46,48,49 Still, process attributes represent a treatment dimension in addition to efficacy and safety that is relevant to patients and should be considered in health economic analyses.21,24,27
Process attributes such as dosing and, subsequently, the number of visits required for treatment, could be considered more widely by clinicians and patients as a distinguishing feature of IV iron formulations. Such distinguishing features are currently mostly related to safety. They include the incidence of hypersensitivity reactions, which are more frequent, albeit still rare, with FCM and IS relative to FDI, as well as hypophosphatemia risk, which is significantly higher with FCM than other iron formulations.17,18,69,70 Using treatment process attributes as an additional distinguishing feature might allow for further optimization of treatment and should be of interest to healthcare payers as it reduces the need for, and thereby the costs associated with, repeated office or hospitals visits.20,71,72
In addition, results from the present study point to the likely benefit of considering treatment attribute preferences for IV iron treatment experiences. Manufacturers should strive to reduce the necessary number of infusions and infusion times while improving risk profiles as much as possible, which can be speculated to lead to better patient-reported outcomes with IV iron treatment. Similarly, healthcare professionals would ideally increase the use of available and future treatment options that are both clinically effective and meet preferred treatment attributes, again in an effort to reduce the treatment burden on patients.
Conclusions
To our knowledge, this study reports the first set of health state utilities associated with IV iron infusions in China. These utilities, obtained from a large sample of Chinese respondents in an online survey using a TTO framework, showed that more frequent IV infusions and even a very low risk of osteomalacia were associated with significant disutilities and that marginal disutilities decreased as the number of infusions increased. The study may inform future health economic evaluations, treatment choices, and reimbursement decisions for IV iron by patients, clinicians, and healthcare payers, particularly in the Chinese setting.
Data and Code Availability
Aggregate data derived from the time trade-off survey and the diminishing marginal disutility model are presented in this manuscript and the Supplementary Information. Participant-level data will not be made publicly available and will not be shared.
The R code for the marginal utility model can be obtained from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
Ethical review board approval in China was not required as valuation tasks are not considered to be medical interventions and, as the study was not a clinical trial, it neither included patients nor made use of human or biological samples, or personally identifiable information, as per the “Measures for Ethical Review of Biomedical Research Involving Human Beings” adopted by the National Health and Family Planning Commission of the People’s Republic of China and in effect since December 2016.
All participants had previously agreed to take part in research of the type presented here and had given explicit consent to participate in the present study at the start of the survey. Participants were free to terminate the survey at any point if they wished to do so, remained anonymous throughout the study, and could neither be identified nor linked to answers.
Acknowledgments
The authors would like to thank Henrik Holm Jensen and Cecilie Yssing at Incentive Partners ApS, Holte, Denmark, for their assistance with survey implementation and data analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The development and implementation of the survey were funded by consultancy fees paid from Pharmacosmos A/S to Incentive Partners ApS. The development of the marginal disutility model and the preparation of this manuscript were funded by consultancy fees paid from Pharmacosmos A/S to Covalence Research Ltd.
Disclosure
SH and DW have received honoraria from Pharmacosmos China for participation in advisory board meetings conducted by Pharmacosmos China. JW and YZ have nothing to disclose. MB is a Managing Partner at Incentive Partners ApS, which received consultancy fees from Pharmacosmos for the development and implementation of the survey. JP is an employee of, and RFP is a director at Covalence Research Ltd., which received consultancy fees from Pharmacosmos for the development of the marginal disutility model and the preparation of this manuscript. The authors report no other conflicts of interest in this work.
References
1. Cappellini MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med. 2020;287(2):153–170. doi:10.1111/joim.13004
2. Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. 2021;397(10270):233–248. doi:10.1016/S0140-6736(20)32594-0
3. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832–1843. doi:10.1056/NEJMra1401038
4. Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387(10021):907–916. doi:10.1016/S0140-6736(15)60865-0
5. Stugiewicz M, Tkaczyszyn M, Kasztura M, Banasiak W, Ponikowski P, Jankowska EA. The influence of iron deficiency on the functioning of skeletal muscles: experimental evidence and clinical implications. Eur J Heart Fail. 2016;18(7):762–773. doi:10.1002/ejhf.467
6. Strauss WE, Auerbach M. Health-related quality of life in patients with iron deficiency anemia: impact of treatment with intravenous iron. Patient Relat Outcome Meas. 2018;9:285–298. doi:10.2147/PROM.S169653
7. Shen Y, Wang J, Yuan J, et al. Anemia among Chinese patients with chronic kidney disease and its association with quality of life - results from the Chinese cohort study of chronic kidney disease (C-STRIDE). BMC Nephrol. 2021;22(1):64. doi:10.1186/s12882-021-02247-8
8. van Haalen H, Jackson J, Spinowitz B, Milligan G, Moon R. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: analysis of multinational real-world data. BMC Nephrol. 2020;21(1):88. doi:10.1186/s12882-020-01746-4
9. DeLoughery TG. Safety of oral and intravenous iron. Acta Haematol. 2019;142(1):8–12. doi:10.1159/000496966
10. Numan S, Kaluza K. Systematic review of guidelines for the diagnosis and treatment of iron deficiency anemia using intravenous iron across multiple indications. Curr Med Res Opin. 2020;36(11):1769–1782. doi:10.1080/03007995.2020.1824898
11. Koch TA, Myers J, Goodnough LT. Intravenous iron therapy in patients with iron deficiency anemia: dosing considerations. Anemia. 2015;2015:763576. doi:10.1155/2015/763576
12. Stoffel NU, von Siebenthal HK, Moretti D, Zimmermann MB. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865. doi:10.1016/j.mam.2020.100865
13. Datapharm Ltd. Monofer 100mg/mL solution for injection/infusion: summary of product characteristics (SmPC). emc; 2020. Available from: https://www.medicines.org.uk/emc/product/5676/smpc.
14. Datapharm Ltd. Venofer (iron sucrose) 20 mg iron/mL, solution for injection or concentrate for solution for infusion: summary of product characteristics (SmPC). emc; 2021. Available from: https://www.medicines.org.uk/emc/product/5911.
15. Datapharm Ltd. Ferinject (ferric carboxymaltose): summary of product characteristics (SmPC). emc; 2022. Available from: https://www.medicines.org.uk/emc/product/5910/smpc.
16. Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter-Gvili A. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc. 2015;90(1):12–23. doi:10.1016/j.mayocp.2014.10.007
17. Blumenstein I, Shanbhag S, Langguth P, Kalra PA, Zoller H, Lim W. Newer formulations of intravenous iron: a review of their chemistry and key safety aspects - hypersensitivity, hypophosphatemia, and cardiovascular safety. Expert Opin Drug Saf. 2021;20(7):757–769. doi:10.1080/14740338.2021.1912010
18. Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323(5):432–443. doi:10.1001/jama.2019.22450
19. Auerbach M, Henry D, DeLoughery TG. Intravenous ferric derisomaltose for the treatment of iron deficiency anemia. Am J Hematol. 2021;96(6):727–734. doi:10.1002/ajh.26124
20. Kalra PA, Bhandari S, Spyridon M, et al. NIMO-CKD-UK: a real-world, observational study of iron isomaltoside in patients with iron deficiency anaemia and chronic kidney disease. BMC Nephrol. 2020;21(1):539. doi:10.1186/s12882-020-02180-2
21. Brennan VK, Dixon S. Incorporating process utility into quality adjusted life years: a systematic review of empirical studies. PharmacoEconomics. 2013;31(8):677–691. doi:10.1007/s40273-013-0066-1
22. Higgins A, Barnett J, Meads C, Singh J, Longworth L. Does convenience matter in health care delivery? A systematic review of convenience-based aspects of process utility. Value Health. 2014;17(8):877–887. doi:10.1016/j.jval.2014.08.2670
23. Mooney G. Beyond health outcomes: the benefits of health care. Health Care Anal. 1998;6(2):99–105. doi:10.1007/BF02678115
24. NICE. Guide to the methods of technology appraisal 2013: process and Methods [PMG9]. London and Manchester: National Institute for Health and Care Excellence; 2013. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
25. Bien DR, Danner M, Vennedey V, Civello D, Evers SM, Hiligsmann M. Patients’ preferences for outcome, process and cost attributes in cancer treatment: a systematic review of discrete choice experiments. Patient. 2017;10(5):553–565. doi:10.1007/s40271-017-0235-y
26. Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–1399. doi:10.2147/PPA.S101821
27. Matza LS, Cutts KN, Stewart KD, Norrbacka K, García-Pérez LE, Boye KS. Health state utilities associated with treatment process for oral and injectable GLP-1 receptor agonists for type 2 diabetes. Qual Life Res. 2021;30(7):2033–2043. doi:10.1007/s11136-021-02808-2
28. Heckman BW, Mathew AR, Carpenter MJ. Treatment burden and treatment fatigue as barriers to health. Curr Opin Psychol. 2015;5:31–36. doi:10.1016/j.copsyc.2015.03.004
29. Datapharm Ltd. Monofer 100mg/mL solution for injection/infusion: patient information leaflet (PIL). emc; 2021. Available from: https://www.medicines.org.uk/emc/product/5676/pil.
30. Datapharm Ltd. Ferinject (ferric carboxymaltose): patient information leaflet (PIL). emc; 2022. Available from: https://www.medicines.org.uk/emc/product/5910/pil.
31. Datapharm Ltd. Venofer (iron sucrose) 20 mg iron/mL, solution for injection or concentrate for solution for infusion: patient information leaflet (PIL). emc; 2020. Available from: https://www.medicines.org.uk/emc/product/5911/pil.
32. Market Research Society. Code of conduct: October 2019. London, UK: Market Research Society; 2019. Available from: https://www.mrs.org.uk/pdf/MRS-Code-of-Conduct-2019.pdf.
33. ESOMAR, Global Research Business Network (GRBN). ESOMAR/GRBN online research guideline. Amsterdam: ESOMAR and Global Research Business Network (GRBN); 2015. Available from: https://www.esomar.org/uploads/public/knowledge-and-standards/codes-and-guidelines/ESOMAR-GRBN-Online-Research-Guideline-October-2015.pdf.
34. National Health and Family Planning Commission. 涉及人的生物医学研究伦理审查办法 [Measures for ethical review of biomedical research involving humans]. Chinese; 2016. Available from: http://www.nhc.gov.cn/fzs/s3576/201610/84b33b81d8e747eaaf048f68b174f829.shtml.
35. Yang Z, Busschbach J, Timman R, Janssen MF, Luo N. Logical inconsistencies in time trade-off valuation of EQ-5D-5L health states: whose fault is it? PLoS One. 2017;12(9):e0184883. doi:10.1371/journal.pone.0184883
36. Attema AE, Krol M, van Exel J, Brouwer WBF. New findings from the time trade-off for income approach to elicit willingness to pay for a quality adjusted life year. Eur J Health Econ. 2018;19(2):277–291. doi:10.1007/s10198-017-0883-9
37. Welch BL. The generalisation of student’s problems when several different population variances are involved. Biometrika. 1947;34(1–2):28–35. doi:10.1093/biomet/34.1-2.28
38. Boye KS, Matza LS, Stewart KD, et al. Health state utilities associated with weight loss in type 2 diabetes and obesity. J Med Econ. 2022;25(1):14–25. doi:10.1080/13696998.2021.2002062
39. Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645–2650. doi:10.1007/s11136-014-0712-x
40. Smith G. Modeling (optional). In: Essential Statistics, Regression, and Econometrics.
41. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. Available from: https://www.R-project.org/.
42. Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. doi:10.21105/joss.01686
43. Boerma T, Hosseinpoor AR, Verdes E, Chatterji S. A global assessment of the gender gap in self-reported health with survey data from 59 countries. BMC Public Health. 2016;16(1):675. doi:10.1186/s12889-016-3352-y
44. Cherepanov D, Palta M, Fryback DG, Robert SA. Gender differences in health-related quality-of-life are partly explained by sociodemographic and socioeconomic variation between adult men and women in the US: evidence from four US nationally representative data sets. Qual Life Res. 2010;19(8):1115–1124. doi:10.1007/s11136-010-9673-x
45. Tegenge MA, Belov A, Moncur M, Forshee R, Irony T. Comparing clotting factors attributes across different methods of preference elicitation in haemophilia patients. Haemophilia. 2020;26(5):817–825. doi:10.1111/hae.14119
46. Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–230. doi:10.1007/s10198-010-0224-8
47. Igarashi A, Bekker Hansen B, Langer J, et al. Preference for oral and injectable GLP-1 RA therapy profiles in Japanese patients with type 2 diabetes: a discrete choice experiment. Adv Ther. 2021;38(1):721–738. doi:10.1007/s12325-020-01561-1
48. Mansfield C, Ndife B, Chen J, Gallaher K, Ghate S. Patient preferences for treatment of metastatic melanoma. Future Oncol. 2019;15(11):1255–1268. doi:10.2217/fon-2018-0871
49. Matza LS, Boye KS, Stewart KD, Davies EW, Paczkowski R. Health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. BMC Health Serv Res. 2017;17(1):774. doi:10.1186/s12913-017-2648-7
50. Van Wyck DB, Roppolo M, Martinez CO, Mazey RM, McMurray S. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68(6):2846–2856. doi:10.1111/j.1523-1755.2005.00758.x
51. Moran K, Null K, Huang Z, Lissoos T, Kane S. Retrospective claims analysis indirectly comparing medication adherence and persistence between intravenous biologics and oral small-molecule therapies in inflammatory bowel diseases. Adv Ther. 2019;36(9):2260–2272. doi:10.1007/s12325-019-01037-x
52. Ziller V, Kostev K, Kyvernitakis I, Boeckhoff J, Hadji P. Persistence and compliance of medications used in the treatment of osteoporosis--analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther. 2012;50(5):315–322. doi:10.5414/cp201632
53. da Costa DiBonaventura M, Copher R, Basurto E, Faria C, Lorenzo R. Patient preferences and treatment adherence among women diagnosed with metastatic breast cancer. Am Health Drug Benefits. 2014;7(7):386–396.
54. Galloway R, McGuire J. Determinants of compliance with iron supplementation: supplies, side effects, or psychology? Soc Sci Med. 1994;39(3):381–390. doi:10.1016/0277-9536(94)90135-X
55. Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with type 2 diabetes: a retrospective cohort study. Diabet Med. 2002;19(4):279–284. doi:10.1046/j.1464-5491.2002.00689.x
56. Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32(4):341–355. doi:10.1007/s12325-015-0199-z
57. Horii T, Momo K, Yasu T, Kabeya Y, Atsuda K. Determination of factors affecting medication adherence in type 2 diabetes mellitus patients using a nationwide claim-based database in Japan. PLoS One. 2019;14(10):e0223431. doi:10.1371/journal.pone.0223431
58. Matza LS, Stewart KD, Lloyd AJ, Rowen D, Brazier JE. Vignette-based utilities: usefulness, limitations, and methodological recommendations. Value Health. 2021;24(6):812–821. doi:10.1016/j.jval.2020.12.017
59. Arnesen TM, Norheim OF. Quantifying quality of life for economic analysis: time out for time trade off. Med Humanit. 2003;29(2):81–86. doi:10.1136/mh.29.2.81
60. Lugnér AK, Krabbe PFM. An overview of the time trade-off method: concept, foundation, and the evaluation of distorting factors in putting a value on health. Expert Rev Pharmacoecon Outcomes Res. 2020;20(4):331–342. doi:10.1080/14737167.2020.1779062
61. Attema AE, Edelaar-Peeters Y, Versteegh MM, Stolk EA. Time trade-off: one methodology, different methods. Eur J Health Econ. 2013;14(Suppl 1):53–64. doi:10.1007/s10198-013-0508-x
62. Stolk E, Ludwig K, Rand K, Hout B, Ramos-Goñi JM. Overview, update, and lessons learned from the international EQ-5D-5L valuation work: version 2 of the EQ-5D-5L valuation protocol. Value Health. 2019;22(1):23–30. doi:10.1016/j.jval.2018.05.010
63. Heerwegh D, Loosveldt G. Face-to-face versus web surveying in a high-internet-coverage population: differences in response quality. Public Opin Q. 2008;72(5):836–846. doi:10.1093/poq/nfn045
64. Boye KS, Matza LS, Stewart KD, et al. Patient preferences and health state utilities associated with dulaglutide and semaglutide injection devices among patients with type 2 diabetes in Italy. J Med Econ. 2019;22(8):806–813. doi:10.1080/13696998.2019.1609482
65. Matza LS, Cong Z, Chung K, et al. Utilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastases. Patient Prefer Adherence. 2013;7:855–865. doi:10.2147/PPA.S44947
66. Matza LS, Paramore LC, Stewart KD, Karn H, Jobanputra M, Dietz AC. Health state utilities associated with treatment for transfusion-dependent β-thalassemia. Eur J Health Econ. 2020;21(3):397–407. doi:10.1007/s10198-019-01136-0
67. Jizhe N. Main data of the seventh national population census: news release. Beijing: National Bureau of Statistics of China; 2021. Available from: http://www.stats.gov.cn/english/PressRelease/202105/t20210510_1817185.html.
68. Hu S, Liu L, Pollock RF, Pöhlmann J, Wu D, Zhang Y. Intravenous iron for the treatment of iron deficiency anemia in China: a patient-level simulation model and cost-utility analysis comparing ferric derisomaltose with iron sucrose. J Med Econ. 2022;25(1):561–570. doi:10.1080/13696998.2022.2065092
69. Pollock RF, Biggar P. Indirect methods of comparison of the safety of ferric derisomaltose, iron sucrose and ferric carboxymaltose in the treatment of iron deficiency anemia. Expert Rev Hematol. 2020;13(2):187–195. doi:10.1080/17474086.2020.1709437
70. Bellos I, Frountzas M, Pergialiotis V. Comparative risk of hypophosphatemia following the administration of intravenous iron formulations: a network meta-analysis. Transfus Med Rev. 2020;34(3):188–194. doi:10.1016/j.tmrv.2020.07.002
71. Auerbach M, Macdougall I. The available intravenous iron formulations: history, efficacy, and toxicology. Hemodial Int. 2017;21(S1):S83–S92. doi:10.1111/hdi.12560
72. Pollock RF, Muduma G. A patient-level cost-effectiveness analysis of iron isomaltoside versus ferric carboxymaltose for the treatment of iron deficiency anemia in the United Kingdom. J Med Econ. 2020;23(7):751–759. doi:10.1080/13696998.2020.1745535
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





