Back to Journals » International Journal of Women's Health » Volume 15
Disease Burden of Dysmenorrhea: Impact on Life Course Potential
Authors MacGregor B, Allaire C, Bedaiwy MA, Yong PJ , Bougie O
Received 30 December 2022
Accepted for publication 13 March 2023
Published 3 April 2023 Volume 2023:15 Pages 499—509
DOI https://doi.org/10.2147/IJWH.S380006
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Brittany MacGregor,1 Catherine Allaire,1 Mohamed A Bedaiwy,1 Paul J Yong,1,* Olga Bougie2,*
1Department of Obstetrics and Gynaecology, University of British Columbia, BC Women’s Centre for Pelvic Pain and Endometriosis, Vancouver, Canada; 2Department of Obstetrics and Gynaecology, Queen’s University, Kingston, Canada
*These authors contributed equally to this work
Correspondence: Olga Bougie, 76 Stuart Street, Victory 4, Department of Obstetrics & Gynecology, Queen’s University, Kingston, Ontario, Canada, K7L 2V7, Email [email protected] Paul J Yong, Department of Obstetrics & Gynecology, University of British Columbia, FRCSC, F2 – 4500 Oak Street, Vancouver, British Columbia, V6H3N1, Canada, Email [email protected]
Abstract: Dysmenorrhea is the most common gynecologic condition among the female population and has a significant impact on life course potential. It has a widespread impact on a female’s mental and physical well-being, with longstanding impairments on quality of life, personal relationships, and education and career attainment. Furthermore, untreated dysmenorrhea can lead to hyperalgesic priming, which predisposes to chronic pelvic pain. Primary dysmenorrhea is pain in the lower abdomen that occurs before or during menses and in the absence of pelvic pathology. One possible mechanism is endometrial inflammation and increased prostaglandin release, resulting in painful uterine contractions. Dysmenorrhea may also occur secondary to pelvic pathology, such as endometriosis, adenomyosis or due to cyclic exacerbation of non-gynecologic pain conditions. A thorough patient evaluation is essential to differentiate between potential causes and guide management. Treatment must be tailored to individual patient symptoms. Pharmacologic management with non-steroidal anti-inflammatory medications and/or combined hormonal contraceptives is most common. Heat therapy, exercise, vitamins and dietary supplements have limited evidence and can be offered for patients seeking non-pharmacologic adjunctive or alternative options. Greater awareness for both health-care providers and patients allows for early intervention to reduce impact on quality of life and life course potential.
Keywords: primary dysmenorrhea, adolescents, chronic pain, health trajectory, women’s health
Introduction and Prevalence
Dysmenorrhea or pain in the lower abdomen experienced during menstruation is the most common reason to seek gynecologic care, occurring in 50–90% of the female population.1,2 In a systematic review and meta-analysis of studies including over 20,000 young women from 38 different countries, the prevalence of dysmenorrhea was 71.1%.3 Amongst adolescents, the most common cause of dysmenorrhea is primary dysmenorrhea, which is defined as menstrual pain in the absence of pelvic pathology.4 Primary dysmenorrhea typically presents 6–12 months following menarche and is thought to be mediated by uterine contractions as well as prostaglandin release.1,5 In Canada, a telephone survey of 2721 female individuals aged 18 years and older conducted by Burnett et al identified that 60% of the 1546 respondents who reported experiencing menstrual periods met the criteria for primary dysmenorrhea.6
The World Health Organization conducted a systematic review focusing on chronic pelvic pain, naming it “a neglected reproductive health morbidity.”7 Specifically looking at prevalence of dysmenorrhea in 106 studies, prevalence rates varied almost as far as statistically possible from 1.7% to 97%. Importantly, national estimates were missing for a large global portion. Looking at specific national examples, the rate of dysmenorrhea in the UK and other European countries was estimated between 45% and 97% in community-based studies and between 41% and 62% in hospital-based studies. In representatively sampled high-quality studies (n = 20), the rate of dysmenorrhoea was between 16.8% and 81%. This variation in prevalence may be related to differences in the definition of dysmenorrhea, including with respect to level of severity, between these studies.
Pathophysiology
Evidence for the pathophysiologic mechanisms of primary dysmenorrhea has been recently reviewed.8–11 It is thought that the drop of progesterone at the end of the luteal phase (with corpus luteal regression) results in destabilization of endometrial cellular lysosomes, resulting in phospholipase A2 release that produces arachidonic acid (from matrix metalloproteinase released phospholipids).8,12 Arachidonic acid is in turn converted by cyclooxygenase (COX) to prostaglandins such as PGE2 and PGF2alpha.8,11,12 Tissue breakdown at menstruation can further expose the uterus to higher prostaglandins.12 In particular, PGF2alpha is elevated in individuals with primary dysmenorrhea and correlated to level of pain by sensitizing nerve endings and inducing uterine contractions and vessel constriction that induce ischemia,8,12 though there is controversy about prostaglandin levels locally (eg in menstrual effluent) versus systemically.
Recently, real-time MRI characterized the uterine contractions contributing to the symptoms of primary dysmenorrhea.13 At the beginning of menstrual bleeding, participants used a squeeze bulb to indicate current episodes of cramping pain. Simultaneous continuous MRI monitored myometrial events (decreases in T2 signal intensity) thought to reflect sustained uterine contractions and/or vascular changes. These MRI myometrial events correlated with the participants’ bulb squeezes. Moreover, participants with dysmenorrhea were more likely to have myometrial events than controls. Amongst the dysmenorrhea participants, all subjects with primary dysmenorrhea had myometrial events, while some with endometriosis did not have myometrial events. The combination of patient-reported cramping and MRI assessment of the uterus provides a model for future studies of primary dysmenorrhea.
In addition to PGF-2alpha, other inflammatory pathways may be involved in primary dysmenorrhea. Arachidonic acid can be converted by 5-lipoxygenase to leukotrienes that may also be involved in dysmenorrhea.11 Other inflammation mediators that may be implicated in dysmenorrhea include vasopressin, CRP, VEGF, TNF-alpha, and IL-6.10 Moreover, while the relationship between PGF-2alpha expression and a drop in progesterone would suggest an association between primary dysmenorrhea and ovulatory cycles, recent work has also shown dysmenorrhea in anovulatory cycles identified by basal body temperature,14 suggesting involvement of other mechanistic pathways, although changes in progesterone (and estradiol) may still be seen to a lesser degree in anovulatory cycles.
Furthermore, there may be changes in pain processing in some individuals with primary dysmenorrhea, which leads to central nervous system sensitization that further amplifies pain and predisposes these individuals to other pain comorbidities.8,12 While quantitative sensory testing (QST) studies in subjects with dysmenorrhea have shown variable results, related to the type of stimulus, the body site tested, and timing in the menstrual cycle, the majority do suggest increased pain hypersensitivity associated with dysmenorrhea in multiple body sites and during the menstrual and non-menstrual times of the cycle.12 In addition, imaging has shown evidence for changes in brain structure and function in subjects with dysmenorrhea. For example, Vincent et al found that during the menstrual phase, subjects with dysmenorrhea exhibited less deactivation activity in response to a noxious stimulus compared to controls; while in the non-menstrual phase, subjects with dysmenorrhea showed increased entorhinal cortex activity in response to a noxious stimulus compared to controls.15 Psychological changes are also apparent, with primary dysmenorrhea subjects having greater state anxiety and depression scores during the menstrual phase compared to controls.16
Recent studies by Tu and Hellman have explored the role of bladder sensitivity as a sign of nervous system changes in dysmenorrhea. They have found that moderate-to-severe dysmenorrhea pain was correlated with greater pain and urgency at different bladder volumes as well as lower maximum bladder capacity.17,18 In a cohort of young women with dysmenorrhea, about one-quarter showed evidence of this bladder sensitivity, which may represent a subset of those with dysmenorrhea who have developed cross-organ sensitization (ie viscero-visceral convergence between the uterus and bladder) in the central nervous system.19,20
Finally, there is evidence for a genetic component to primary dysmenorrhea. Aouad et al recently performed a twin study for primary dysmenorrhea, where monozygotic twins showed twice the correlation for different pain phenotypes compared to dizygotic twins, with heritability estimates between 57% and 67%.21 A genome-wide association study found a relationship between primary dysmenorrhea and genomic variants at the rs76518691 locus associated with the ZMIZ1 gene (involved with autoimmune conditions) and at the rs7523831 locus associated with the NGF gene (involved with hyperalgesia).22
Primary Vs Secondary Dysmenorrhea
While the focus of this review is on primary dysmenorrhea, other causes of menstrual pain include secondary dysmenorrhea (eg endometriosis, adenomyosis and fibroids) and pain conditions that can be exacerbated at the time of menstruation (eg irritable bowel syndrome, painful bladder syndrome and myofascial pain).23 These latter conditions may also develop as comorbidities (overlapping conditions) in those with a history of primary dysmenorrhea, through central nervous system changes including viscero-visceral convergence (between the uterus and the bladder or bowel) and viscero-somatic convergence (between the uterus and pelvic myofascial structures) as part of cross-organ sensitization in the central nervous system.20,24 It has been proposed that recurrent dysmenorrhea results in “hyperalgesic priming” of the nervous system that predisposes to these cross-organ sensitization events and central nervous system sensitization more broadly.25
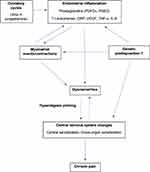
A summary of the pathophysiology of primary dysmenorrhea is depicted in Figure 1.
Patient Evaluation
The goal of the initial assessment is to acknowledge patients’ symptomatology, differentiate between primary and secondary dysmenorrhea and rule out conditions requiring prompt evaluation or referral. This involves a complete patient history with targeted exams and investigations.
Primary dysmenorrhea classically begins 1 to 2 days prior to menses and lasts 12 to 72 hours.26 Symptoms are consistent and predictable from one cycle to the next. Pain is most commonly described as midline and can be a fluctuating, crampy pain or a continuous dull ache. Patients may complain of radiation to the lower back or upper thighs. Owing to the prostaglandin mediated response, associated symptoms include nausea, vomiting and diarrhea. Symptoms begin with the start of ovulatory cycles, generally 6 to 12 months after menarche.
Patients presenting with secondary dysmenorrhea are typically older in age and may exhibit progressive symptoms over time (eg due to progressive endometriosis). Menstrual pain may be unilateral, and in addition to dysmenorrhea, patients may develop pain that is acyclic chronic pelvic pain or mid cycle pain. There may be associated gynecologic symptoms including dysuria, dyschezia or dyspareunia. Patients may have a history of infertility, abnormal or heavy vaginal bleeding.26 It is important to inquire about symptoms requiring urgent evaluation and treatment including infectious symptoms, signs of anemia and abnormal bleeding.
Unfortunately, patients often have a delay in presenting for care, in part due to normalization of period pains, lack of access to care, or embarrassment. In one =study, despite having significant dysmenorrhea, approximately half of the participants (51%) thought that their period was normal.3 It is important for health-care providers to validate the patients’ experience, select appropriate investigations, confirm the diagnosis in a timely fashion, and offer evidence-based treatment options.
Physical Exam
The objective of physical exam is to ascertain the degree and location of pain and help guide further investigations. Exams should be conducted keeping in mind the differential diagnosis based on the history attained, including potential causes of secondary dysmenorrhea, as well as overlapping chronic pain conditions related to central nervous system. A pelvic examination is often recommended at the initial appointment to screen for secondary causes of pelvic pain including adenomyosis, fibroids, endometriosis or myofascial pain. Findings such as a bulky, irregular or fixed uterus can prompt further imaging for fibroids or adenomyosis. Sexually transmitted infection (STI) and cancer screening can be done at the time of pelvic examination. A pelvic examination may be deferred for adolescent patients presenting with a classic history of primary dysmenorrhea with mild-to-moderate symptoms. An exam is recommended for patients presenting with new dysmenorrhea outside of adolescence, for those who have severe dysmenorrhea or symptoms of secondary dysmenorrhea, or patients who are sexually active.
Empirical treatment can be initiated for patients with a classic history of primary dysmenorrhea prior to imaging studies. For patients that do not respond to treatment or there is concern for a secondary cause, transvaginal or transabdominal ultrasound is a typical first-line imaging modality. Ultrasound is readily available, cost effective and well tolerated by patients. Imaging should be normal with primary dysmenorrhea. Ultrasound does not readily detect superficial endometriosis; however, routine ultrasound can accurately diagnose fibroids, adenomyosis and ovarian endometriomas.
Treatment
Goals of treatment include pain reduction and improvement in quality of life while avoiding adverse effects. Non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit cyclooxygenase activity and in turn the production of prostaglandins, are a typical first-line choice for primary dysmenorrhea as they are readily available and well tolerated. For patients with predictable symptoms, treatment can be started 1 to 2 days before the onset of menses.
A 2015 Cochrane review of 80 randomized trials concluded that NSAIDs were 4.5 times more effective than placebo for pain relief and more than twice as effective as acetaminophen.27 Approximately 18% of patients will be non-responders to NSAID therapy.11 Studies have not identified a significant difference between classes of NSAIDs. Consideration to a trial of a different class should be given if patients do not respond to empiric treatment. Extended use of NSAIDs can cause GI upset and increased risk of gastritis and ulcers. Patients may take these medications with food to avoid these concerns, however this can reduce absorption. Thorough discussion surrounding appropriate dosing is recommended.
Approximately 1 in 5 patients with dysmenorrhea will not experience a benefit from NSAID therapy, in which case a trial of combined hormonal contraceptives is recommended.28 This is a first-line treatment that can provide reliable contraception and target other symptoms such as menstrual migraines, mood symptoms and acne. COCs suppress ovulation and endometrial proliferation and in turn prostaglandin production. Hadara et al demonstrated a reduction in total dysmenorrhea score with COC use in a placebo-controlled randomized controlled trial including 115 patients with primary dysmenorrhea.29 Formulation and route of administration can be guided by patient preference, availability and tolerability. Patients should be encouraged to continue a three-month trial to determine effectiveness. For incomplete symptom relief, patients can switch to a reduced hormone-free interval or to continuous use. Several trials, as well as a Cochrane review, have demonstrated superiority of continuous and extended use of COC regiments compared to cyclical regiments for pain relief in dysmenorrhea.30–32
Progesterone-only methods have not been as extensively studied, however they have been demonstrated to reduce primary dysmenorrhea in multiple studies. The levonorgestrel IUS has been shown to have similar efficacy to the COC for relief of dysmenorrhea.33–35 Progesterone-only pills have also proven efficacy in management of endometriosis-related pain.36 Studies demonstrate a likely reduction in primary dysmenorrhea, however frequent unscheduled bleeding often limits tolerability.23
Gonadotropin releasing agonists or antagonists are not considered first line for management of primary dysmenorrhea. Due to side-effects such as hot flashes and bone loss, these are often reserved for refractory cases, or potentially as a therapeutic trial when secondary causes are suspected.
Patients may wish to try alternative and complementary therapy as an adjunct to, or in place of, pharmacologic options. There is modest evidence to support exercise in reducing primary dysmenorrhea, in addition to its other numerous health benefits.37 Application of heat is widely used as a primary or adjunctive treatment and studies have demonstrated a reduction in symptoms comparable to NSAID therapy.38 Transcutaneous electrical nerve stimulation (TENS) has been demonstrated to reduce pain, decrease use of analgesics and improve quality of life.39
There is a lack of high-quality studies for dietary supplements for dysmenorrhea.40 There is limited and low-quality evidence for fenugreek, valerian, zataria, zinc sulphate, calcium, magnesium, or vitamins B1 and B6.40,41 Vitamin D supplementation has been inconsistently shown to improve symptoms and is more likely to be effective in those with severe symptoms or low serum levels.42,43 There is limited evidence to support the benefits of Vitamin E alone or in combination with fish oil for treatment of primary dysmenorrhea.44–46
As most patients with primary dysmenorrhea will respond to a 3-to-6-month trial of NSAID or COC therapy, referral to a gynecologist for further work-up is recommended for those with ongoing or progressive symptoms. Up to 70% of adolescents who do not respond to first-line treatments have been demonstrated to have evidence of endometriosis on laparoscopy.47
Impact of Dysmenorrhea
Dysmenorrhea has a profound negative impact on an individual’s quality of life, through physical, social, psychological and emotional impairment.48–50 Over one’s life course, the accumulated impact of such ongoing challenges can limit achievement of one’s goals, including educational or career attainment, social relationships, and starting a family.
Health Impact
Symptom severity and impact of dysmenorrhea vary considerably between individuals. Approximately 30–50% of persons experiencing dysmenorrhea report severe symptoms.6,51 Pain intensity is influenced by a number of factors, including medical comorbidities and socioeconomic determinants of health.52–55 Age has been demonstrated to have an inverse relationship with severity of dysmenorrhea, with adolescents typically reporting more pronounced symptomatology.6,56,57 Up to one-third of adolescents suffering with dysmenorrhea report comorbidities such as gastrointestinal upset headache, fatigue, poor sleep, and depression/anxiety.52,58–60 The impact of dysmenorrhea on quality of life is exacerbated by the severity of pain and delay of diagnosis, which are modifiable factors that can be addressed by effective strategies for knowledge uptake.61 Psychological disorders, such as depression or anxiety, can exacerbate the impact of pain on an individual’s social and occupational function.62 There is also evidence to suggest that these psychological factors can impact one’s response to therapeutic interventions.63
A recent systematic review, including 33 studies, examined the relationship between mental health and primary dysmenorrhea.64 The authors identified that the most common conditions studied in patients with dysmenorrhea were depression, anxiety, and stress-related disorders. These were generally identified to have a high prevalence in this population. Other psychiatric disorders, such as attention-deficit disorder/attention-deficit hyperactivity disorders, panic attacks, phobias, obsessive-compulsive disorder, schizophrenia, bipolar disorder/manic depression, and eating disorders were only examined in one study.65 They were found to be increased in the patients with dysmenorrhea, however psychiatric disorders were not isolated but instead grouped as a collective entity. Although some have suggested a relationship between alcohol/substance use and dysmenorrhea,66 particularly as a self treatment during worsening symptoms, other studies do not support an increase in alcohol abuse in patients with dysmenorrhea.67,68
Impact on Social Relationships and Quality of Life
Dysmenorrhea, both primary and secondary types such as endometriosis, lead to a significant negative impact on patients’ quality of life,61,69 with patients reporting physical and psychosocial scores similar to those with other chronic conditions such as cystic fibrosis.69 When dysmenorrhea and menstrual disorders are not addressed, they continue to impact one’s productivity across the life span. Recent findings from the Performance Monitoring and Accountability 2020 (PMA2020) survey from Burkina Faso and Nigeria indicate that up to 1 in 5 responders who worked outside the home report missing work in the past month due to menstrual related disorders.70 Individuals experiencing dysmenorrhea also report a negative impact on their social function, including poor relationships with family and friends, as well as poor social and sports activities.3 One study of university health science students in Northern Ethiopia found that over 20% of students who experienced dysmenorrhea reported poor relationships due to this reason, and 78% practiced self-isolation.71
Rencz et al completed a study examining health-related quality of life values for severe and mild primary dysmenorrhea using 10-year time trade-off and willingness-to-pay methods.72 They studied 1836 participants who met the criteria for dysmenorrhea, originally recruited from a 2015 national convenience sample of internet survey responders in Hungary. Seventy percent of participants who experienced dysmenorrhea reported an impact on their work/study and social activities, with 16% reported extreme impact on these domains. For severe and mild dysmenorrhea, mean utility values were 0.85 and 0.94 and subjects were willing to pay a mean of €1127 and €142 for a complete cure, respectively. Quality-adjusted life year loss was comparable to type 1 diabetes, asthma, atopic eczema, or chronic migraine.
Economic Impact and Healthcare Utilization
Health-care utilization is increased in patients experiencing dysmenorrhea. In a health insurance database of Japanese women, Akiyama et found that health-care costs were 2.2 and 2.9 times higher for primary and secondary dysmenorrhea cohorts, compared with matched controls, after adjusting for baseline characteristics (both p<0.0001).73 A study examining inpatient cost (including surgery) of chronic pelvic pain in Canada identified an annual cost of $25 million, of which 30% was incurred by those with dysmenorrhea.74 It is important to emphasize that untreated or undertreated dysmenorrhea may lead to chronic pain over time (Figure 1). In a systematic review and meta-analysis by Li et al, dysmenorrhea was positively associated with the presence and severity of chronic pelvic pain: patients having a history of dysmenorrhea were 2.5 times more likely to develop chronic pelvic and non-pelvic pain.75
Life Course Potential Beginning in Adolescence
A recent narrative review examining the influence of dysmenorrhea and endometriosis on life-course potential identified that these conditions can significantly impact numerous aspects of one’s life, including hindering attainment of educational/career aspirations, social relationships, emotional well being, and starting a family.76 Given the chronic nature of dysmenorrhea, it is important to approach this condition through a cumulative life-course impact model, allowing a comprehensive understanding of an individual’s journey and identification of important factors that contribute to the cumulative effects of the chronic condition.77 Studying dysmenorrhea in young adulthood is important as this is a critical time in development, when adolescents establish social networks, identify life goals, and begin pursuing career-specific education. Recognizing the gaps in care specific to this population will facilitate targeting effective interventions specific to this population.
Primary dysmenorrhea and endometriosis (the most common cause of secondary dysmenorrhea) lead to a significant negative impact on adolescents’ quality of life,61,69 with young patients reporting physical and psychosocial scores similar to those with other chronic conditions such as cystic fibrosis.69 Adolescence and young adulthood can be a tumultuous time, marked by physical, mental, and emotional transitions. Recurrent or untreated dysmenorrhea can have a significant impact on the mental health of adolescents, with greater symptom severity associated with depression, anxiety, and impaired quality-of-life.60,78,79 Adolescents with dysmenorrhea also suffer from strained relationships with family and friends.80 At a time where the friend group is of high importance for emotional support, this can be particularly detrimental. Moreover, dysmenorrhea has been linked to increased impulsivity, as well as an increased risk of non-suicidal self-injury in adolescents.81 Adolescents with severe dysmenorrhea have also been shown to have increased rates of suicide attempts in the last year compared to those with no or mild-moderate dysmenorrhea.78
In adolescents, dysmenorrhea is the most common cause of recurrent short-term absenteeism from school.58,82,83 A previous systematic review demonstrated a significant academic impact of dysmenorrhea, with 20.1% reporting absence from school or university due to dysmenorrhea and 40.9% reporting classroom performance or concentration being negatively affected.51 Other studies have demonstrated that dysmenorrhea worsens during examination times in 50% of cases and can result in an increased rate of missing examinations.80,84
Adolescents with dysmenorrhea who are from lower socioeconomic strata are more likely to experience school absenteeism compared to those from more affluent backgrounds.58 Furthermore, the impact of dysmenorrhea may exacerbate in marginalized populations, including individuals who identify as racial/ethnic minorities, 2SLGBTQ+, or those living in poverty.82,85,86 There are identified research and clinical needs to examine and improve the therapeutic journey of these individuals experiencing dysmenorrhea.
Barriers to timely diagnosis and access to effective treatment are especially pronounced in adolescent and young adult population as young patients may lack information about when to seek medical attention87 and may lack the support necessary to effectively navigate the healthcare system.
A summary of the impact of dysmenorrhea on life course potential is illustrated in Figure 2.
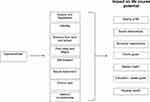 |
Figure 2 Impact of dysmenorrhea. This figure demonstrates possible impairments of dysmenorrhea and the impact on life course potential. |
Opportunities for Improvement
Therapeutic Journey
Early recognition of dysmenorrhea is important for validation of the patient’s experiences but also potentially to reduce the impacts discussed above and to prevent hyperalgesic priming and the long-term risk of chronic pain. Early recognition can be promoted in the family environment, in adolescent social circles, and in schools. When patients present to the healthcare system with dysmenorrhea, their symptoms should be acknowledged and appropriate assessment and investigations undertaken.
Education
There is a recognized need to improve education regarding dysmenorrhea, including the general public, health-care providers, and in the education system. For example, a menstrual health and endometriosis education program in secondary schools was potentially associated with younger adolescents seeking care.86 Such educational programs can be informative for all students regardless of sex/gender and help create environments where menstrual pain is de-stigmatized. It is essential that initiatives to raise awareness and increase knowledge about dysmenorrhea take into account an intersectional approach. These initiatives should utilize language that is inclusive of gender diversity and should be situated in the context of different cultures and values. To achieve this, such initiatives would benefit from a community participatory approach, which integrates stakeholders with lived experience into development and implementation.
Research
Among other research priorities in primary dysmenorrhea is the approach to phenotyping. As illustrated in Figure 1, there are several pathways to dysmenorrhea at the level of the endometrium, myometrium, hypothalamic-pituitary-ovary axis leading to ovulation, other pelvic organs (eg bladder), central nervous system sensitization, and genetic predisposition. For each patient with dysmenorrhea, future research should explore how these pathways can be characterized and thereby lead to phenotypic classification. For example, individuals with primarily endometrial prostaglandin origins of dysmenorrhea would be managed differently from individuals who have developed alterations of central nervous system pathways leading to co-morbid bladder sensitization. This phenotypic classification may then influence treatment response, as central sensitization could be a cause of non-response to NSAIDs.11 The role of myometrial events/contractions warrants more clinical trials into tocolysis, while endometrial inflammation also presents targets for anti-inflammatory drugs.
Conclusion
In conclusion, dysmenorrhea is a common symptom that has significant impact on life course potential. A systematic assessment can better characterize this presentation, differentiate between primary and secondary causes, and guide management. Greater awareness and education may reduce impact on life course potential and prevent long-term sequelae. More basic, translational and clinical research is needed into this understudied topic.
Disclosure
Dr. Catherine Allaire reports personal fees from Abbvie, personal fees from Ferring, outside the submitted work.
Dr. Mohamed Bedaiwy reports financial affiliations with Ferring Pharmaceuticals, AbbVie, and Baxter.
Dr Paul J Yong is supported by a Health Professional Investigator Award from Michael Smith Health BC and a Canada Research Chair (Tier 2) in Endometriosis and Pelvic Pain.
Dr. Olga Bougie reports financial affiliations with Hologic and AbbVie. Received Grant from SRI-Bayer.
The authors report no other conflicts of interest in this work.
References
1. Harel Z. Dysmenorrhea in adolescents and young adults: from pathophysiology to pharmacological treatments and management strategies. Expert Opin Pharmacother. 2008;9(15):2661–2672. doi:10.1517/14656566.9.15.2661
2. Al-Jefout M, Nawaiseh N. Continuous norethisterone acetate versus cyclical drospirenone 3 mg/Ethinyl Estradiol 20 mug for the management of primary dysmenorrhea in young adult women. J Pediatr Adolesc Gynecol. 2016;29(2):143–147.
3. Armour M, Parry K, Manohar N, et al. The prevalence and academic impact of dysmenorrhea in 21,573 young women: a systematic review and meta-analysis. J Womens Health. 2019;28(8):1161–1171.
4. Akerlund M. Pathophysiology of dysmenorrhea. Acta Obstet Gynecol Scand Suppl. 1979;87:27–32.
5. Rosenwaks Z, Seegar-Jones G. Menstrual pain: its origin and pathogenesis. J Reprod Med. 1980;25(4 Suppl):207–212.
6. Burnett MA, Antao V, Black A, et al. Prevalence of primary dysmenorrhea in Canada. J Obstet Gynaecol Can. 2005;27(8):765–770.
7. Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
8. Ferries-Rowe E, Corey E, Archer JS. Primary Dysmenorrhea: diagnosis and Therapy. Obstet Gynecol. 2020;136(5):1047–1058.
9. Szmidt MK, Granda D, Sicinska E, Kaluza J. Primary dysmenorrhea in relation to oxidative stress and antioxidant status: a systematic review of case-control studies. Antioxidants. 2020;9(10):994.
10. Barcikowska Z, Rajkowska-Labon E, Grzybowska ME, Hansdorfer-Korzon R, Zorena K. Inflammatory markers in dysmenorrhea and therapeutic options. Int J Environ Res Public Health. 2020;17(4):1191. doi:10.3390/ijerph17041191
11. Oladosu FA, Tu FF, Hellman KM. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol. 2018;218(4):390–400.
12. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778.
13. Hellman KM, Kuhn CS, Tu FF, et al. Cine MRI during spontaneous cramps in women with menstrual pain. Am J Obstet Gynecol. 2018;218(5):506.e1–506.e8.
14. Bann S, Goshtasebi A, Shirin S, Prior JC. A one-year observational cohort study of menstrual cramps and ovulation in healthy, normally ovulating women. Sci Rep. 2022;12(1):473.
15. Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152(9):1966–1975.
16. Tu CH, Niddam DM, Yeh TC, et al. Menstrual pain is associated with rapid structural alterations in the brain. Pain. 2013;154(9):1718–1724.
17. Tu FF, Epstein AE, Pozolo KE, Sexton DL, Melnyk AI, Hellman KM. A noninvasive bladder sensory test supports a role for dysmenorrhea increasing bladder noxious mechanosensitivity. Clin J Pain. 2013;29(10):883–890.
18. Hellman KM, Datta A, Steiner ND, et al. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. Am J Obstet Gynecol. 2018;219(1):84.e1–84.e8.
19. Tu FF, Datta A, Atashroo D, et al. Clinical profile of comorbid dysmenorrhea and bladder sensitivity: a cross-sectional analysis. Am J Obstet Gynecol. 2020;222(6):594.e1–594.e11.
20. Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149(3):660–672.
21. Aouad P, Bui M, Sarraf S, et al. Primary dysmenorrhoea in adolescents and young women: a twin family study of maternal transmission, genetic influence and associations. Aust N Z J Obstet Gynaecol. 2022;62(5):725–731.
22. Li Z, Chen J, Zhao Y, et al. Common variants in ZMIZ1 and near NGF confer risk for primary dysmenorrhoea. Nat Commun. 2017;8:14900.
23. Burnett M, Lemyre M. No. 345-Primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can. 2017;39(7):585–595.
24. Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain. 2016;17(9 Suppl):T93–T107.
25. Jarrell J, Arendt-Nielsen L. Evolutionary considerations in the development of chronic pelvic pain. Am J Obstet Gynecol. 2016;215(2):201.e1–201.e2014.
26. Proctor M, Farquhar C. Diagnosis and management of dysmenorrhoea. BMJ. 2006;332(7550):1134–1138.
27. Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;2015(7):CD001751.
28. Owen PR. Prostaglandin synthetase inhibitors in the treatment of primary dysmenorrhea. Outcome trials reviewed. Am J Obstet Gynecol. 1984;148(1):96–103.
29. Harada T, Momoeda M, Terakawa N, Taketani Y, Hoshiai H. Evaluation of a low-dose oral contraceptive pill for primary dysmenorrhea: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2011;95(6):1928–1931.
30. Edelman A, Micks E, Gallo MF, Jensen JT, Grimes DA. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014;2014(7):CD004695.
31. Dmitrovic R, Kunselman AR, Legro RS. Continuous compared with cyclic oral contraceptives for the treatment of primary dysmenorrhea: a randomized controlled trial. Obstet Gynecol. 2012;119(6):1143–1150.
32. Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol. 2002;186(6):1142–1149.
33. Bianchi P, Guo SW, Habiba M, Benagiano G. Utility of the levonorgestrel-releasing intrauterine system in the treatment of abnormal uterine bleeding and dysmenorrhea: a narrative review. J Clin Med. 2022;11(19):5836.
34. Bahamondes L, Petta CA, Fernandes A, Monteiro I. Use of the levonorgestrel-releasing intrauterine system in women with endometriosis, chronic pelvic pain and dysmenorrhea. Contraception. 2007;75(6 Suppl):S134–S139.
35. Lindh I, Milsom I. The influence of intrauterine contraception on the prevalence and severity of dysmenorrhea: a longitudinal population study. Hum Reprod. 2013;28(7):1953–1960.
36. Casper RF. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil Steril. 2017;107(3):533–536.
37. Armour M, Ee CC, Naidoo D, et al. Exercise for dysmenorrhoea. Cochrane Database Syst Rev. 2019;9(9):CD004142.
38. Jo J, Lee SH. Heat therapy for primary dysmenorrhea: a systematic review and meta-analysis of its effects on pain relief and quality of life. Sci Rep. 2018;8(1):16252.
39. Elboim-Gabyzon M, Kalichman L. Transcutaneous Electrical Nerve Stimulation (TENS) for primary dysmenorrhea: an overview. Int J Womens Health. 2020;12:1–10.
40. Pattanittum P, Kunyanone N, Brown J, et al. Dietary supplements for dysmenorrhoea. Cochrane Database Syst Rev. 2016;3(3):CD002124.
41. Saei Ghare Naz M, Kiani Z, Rashidi FF, Ghasemi V, Abed M, Ozgoli G. The effect of micronutrients on pain management of primary dysmenorrhea: a systematic review and meta-analysis. J Caring Sci. 2020;9(1):47–56.
42. Rahnemaei FA, Gholamrezaei A, Afrakhteh M, et al. Vitamin D supplementation for primary dysmenorrhea: a double-blind, randomized, placebo-controlled trial. Obstet Gynecol Sci. 2021;64(4):353–363.
43. Abdi F, Amjadi MA, Zaheri F, Rahnemaei FA. Role of vitamin D and calcium in the relief of primary dysmenorrhea: a systematic review. Obstet Gynecol Sci. 2021;64(1):13–26.
44. Alikamali M, Mohammad-Alizadeh-Charandabi S, Maghalian M, Mirghafourvand M. The effects of vitamin E on the intensity of `primary dysmenorrhea: a systematic review and meta-analysis. Clin Nutr ESPEN. 2022;52:50–59.
45. Kashanian M, Lakeh MM, Ghasemi A, Noori S. Evaluation of the effect of vitamin E on pelvic pain reduction in women suffering from primary dysmenorrhea. J Reprod Med. 2013;58(1–2):34–38.
46. Sadeghi N, Paknezhad F, Rashidi Nooshabadi M, Kavianpour M, Jafari Rad S, Khadem Haghighian H. Vitamin E and fish oil, separately or in combination, on treatment of primary dysmenorrhea: a double-blind, randomized clinical trial. Gynecol Endocrinol. 2018;34(9):804–808.
47. Laufer MR, Sanfilippo J, Rose G. Adolescent endometriosis: diagnosis and treatment approaches. J Pediatr Adolesc Gynecol. 2003;16(3 Suppl):S3–S11.
48. Hooker AB, van Moorst BR, van Haarst EP, van Ootegehem NA, van Dijken DK, Heres MH. Chronic pelvic pain: evaluation of the epidemiology, baseline demographics, and clinical variables via a prospective and multidisciplinary approach. Clin Exp Obstet Gynecol. 2013;40(4):492–498.
49. Tripoli TM, Sato H, Sartori MG, De Araujo FF, Girao MJ, Schor E. Evaluation of quality of life and sexual satisfaction in women suffering from chronic pelvic pain with or without endometriosis. J Sex Med. 2011;8(2):497–503.
50. Allyn K, Evans S, Seidman LC, Payne LA. ”Tomorrow, I’ll Be Fine”: impacts and coping mechanisms in adolescents and young adults with primary dysmenorrhoea. J Adv Nurs. 2020;76(10):2637–2647.
51. Armour M, Parry K, Al-Dabbas MA, et al. Self-care strategies and sources of knowledge on menstruation in 12,526 young women with dysmenorrhea: a systematic review and meta-analysis. PLoS One. 2019;14(7):e0220103.
52. Balik G, Ustuner I, Kagitci M, Sahin FK. Is there a relationship between mood disorders and dysmenorrhea? J Pediatr Adolesc Gynecol. 2014;27(6):371–374.
53. Ng TP, Tan NC, Wansaicheong GK. A prevalence study of dysmenorrhoea in female residents aged 15–54 years in Clementi Town, Singapore. Ann Acad Med Singap. 1992;21(3):323–327.
54. Alonso C, Coe CL. Disruptions of social relationships accentuate the association between emotional distress and menstrual pain in young women. Health Psychol. 2001;20(6):411–416.
55. Sundell G, Milsom I, Andersch B. Factors influencing the prevalence and severity of dysmenorrhoea in young women. Br J Obstet Gynaecol. 1990;97(7):588–594.
56. Pullon S, Reinken J, Sparrow M. Prevalence of dysmenorrhoea in Wellington women. N Z Med J. 1988;101(839):52–54.
57. Weissman AM, Hartz AJ, Hansen MD, Johnson SR. The natural history of primary dysmenorrhoea: a longitudinal study. BJOG. 2004;111(4):345–352.
58. Klein JR, Litt IF. Epidemiology of adolescent dysmenorrhea. Pediatrics. 1981;68(5):661–664.
59. Woosley JA, Lichstein KL. Dysmenorrhea, the menstrual cycle, and sleep. Behav Med. 2014;40(1):14–21.
60. Bahrami A, Sadeghnia H, Avan A, et al. Neuropsychological function in relation to dysmenorrhea in adolescents. Eur J Obstet Gynecol Reprod Biol. 2017;215:224–229.
61. Gallagher JS, DiVasta AD, Vitonis AF, Sarda V, Laufer MR, Missmer SA. The impact of endometriosis on quality of life in adolescents. J Adolesc Health. 2018;63(6):766–772.
62. Gagua T, Tkeshelashvili B, Gagua D. Primary dysmenorrhea: prevalence in adolescent population of Tbilisi, Georgia and risk factors. J Turk Ger Gynecol Assoc. 2012;13(3):162–168.
63. Granot M, Yarnitsky D, Itskovitz-Eldor J, Granovsky Y, Peer E, Zimmer EZ. Pain perception in women with dysmenorrhea. Obstet Gynecol. 2001;98(3):407–411.
64. Bajalan Z, Moafi F, MoradiBaglooei M, Alimoradi Z. Mental health and primary dysmenorrhea: a systematic review. J Psychosom Obstet Gynaecol. 2019;40(3):185–194.
65. Jones AV, Hockley JRF, Hyde C, et al. Genome-wide association analysis of pain severity in dysmenorrhea identifies association at chromosome 1p13.2, near the nerve growth factor locus. Pain. 2016;157(11):2571–2581.
66. Ju H, Jones M, Mishra GD. A U-Shaped relationship between body mass index and dysmenorrhea: a longitudinal study. PLoS One. 2015;10(7):e0134187.
67. Unsal A, Ayranci U, Tozun M, Arslan G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci. 2010;115(2):138–145.
68. Pejcic A, Jankovic S. Risk factors for dysmenorrhea among young adult female university students. Ann Ist Super Sanita. 2016;52(1):98–103.
69. Nur Azurah AG, Sanci L, Moore E, Grover S. The quality of life of adolescents with menstrual problems. J Pediatr Adolesc Gynecol. 2013;26(2):102–108.
70. Hennegan J, OlaOlorun FM, Oumarou S, et al. School and work absenteeism due to menstruation in three West African countries: findings from PMA2020 surveys. Sex Reprod Health Matters. 2021;29(1):1915940.
71. Yesuf TA, Eshete NA, Sisay EA. Dysmenorrhea among university health science students, northern Ethiopia: impact and associated factors. Int J Reprod Med. 2018;2018:9730328.
72. Rencz F, Pentek M, Stalmeier PFM, et al. Bleeding out the quality-adjusted life years: evaluating the burden of primary dysmenorrhea using time trade-off and willingness-to-pay methods. Pain. 2017;158(11):2259–2267.
73. Akiyama S, Tanaka E, Cristeau O, Onishi Y, Osuga Y. Evaluation of the treatment patterns and economic burden of dysmenorrhea in Japanese women, using a claims database. Clinicoecon Outcomes Res. 2017;9:295–306.
74. Chen I, Thavorn K, Shen M, et al. Hospital-associated costs of chronic pelvic pain in Canada: a population-based descriptive study. J Obstet Gynaecol Can. 2017;39(3):174–180.
75. Li R, Li B, Kreher DA, Benjamin AR, Gubbels A, Smith SM. Association between dysmenorrhea and chronic pain: a systematic review and meta-analysis of population-based studies. Am J Obstet Gynecol. 2020;223(3):350–371.
76. Missmer SA, Tu FF, Agarwal SK, et al. Impact of endometriosis on life-course potential: a narrative review. Int J Gen Med. 2021;14:9–25.
77. Burton-Jeangros C, Cullati S, Sacker A, Blane D. Introduction. In: Burton-Jeangros C, Cullati S, Sacker A, Blane D, editors. A Life Course Perspective on Health Trajectories and Transitions. Cham (CH); 2015:1–18.
78. Ambresin AE, Belanger RE, Chamay C, Berchtold A, Narring F. Body dissatisfaction on top of depressive mood among adolescents with severe dysmenorrhea. J Pediatr Adolesc Gynecol. 2012;25(1):19–22.
79. Sahin N, Kasap B, Kirli U, Yeniceri N, Topal Y. Assessment of anxiety-depression levels and perceptions of quality of life in adolescents with dysmenorrhea. Reprod Health. 2018;15(1):13.
80. Eryilmaz G, Ozdemir F, Pasinlioglu T. Dysmenorrhea prevalence among adolescents in eastern Turkey: its effects on school performance and relationships with family and friends. J Pediatr Adolesc Gynecol. 2010;23(5):267–272.
81. Liu P, Liu Y, Wang G, et al. Changes of functional connectivity of the anterior cingulate cortex in women with primary dysmenorrhea. Brain Imaging Behav. 2018;12(3):710–717.
82. Wong LP, Khoo EM. Dysmenorrhea in a multiethnic population of adolescent Asian girls. Int J Gynaecol Obstet. 2010;108(2):139–142.
83. Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258–265.
84. Al-Jefout M, Seham AF, Jameel H, et al. Dysmenorrhea: prevalence and impact on quality of life among young adult Jordanian females. J Pediatr Adolesc Gynecol. 2015;28(3):173–185.
85. Banikarim C, Chacko MR, Kelder SH. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Arch Pediatr Adolesc Med. 2000;154(12):1226–1229.
86. Sommer M, Schmitt M, Gruer C, Herbert A, Phillips-Howard P. Neglect of menarche and menstruation in the USA. Lancet. 2019;393(10188):2302.
87. Bush D, Brick E, East MC, Johnson N. Endometriosis education in schools: a New Zealand model examining the impact of an education program in schools on early recognition of symptoms suggesting endometriosis. Aust N Z J Obstet Gynaecol. 2017;57(4):452–457.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.