Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
COPD in Never-Smokers: BOLD Australia Study
Authors Ivey MA , Smith SM, Benke G, Toelle BG, Hunter ML , James AL, Maguire GP, Wood-Baker R, Johns DP, Marks GB , Abramson MJ
Received 3 October 2023
Accepted for publication 21 December 2023
Published 17 January 2024 Volume 2024:19 Pages 161—174
DOI https://doi.org/10.2147/COPD.S439307
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Marsha A Ivey,1,2 Sheree M Smith,3,4 Geza Benke,1 Brett G Toelle,5,6 Michael L Hunter,7 Alan L James,8 Graeme P Maguire,9 Richard Wood-Baker,10 David P Johns,11 Guy B Marks,5,12 Michael J Abramson1
1School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, 3004, Australia; 2School of Medicine, Faculty of Medical Sciences, The University of the West Indies, St Augustine, Trinidad and Tobago; 3School of Nursing and Midwifery, Campbelltown Campus, Western Sydney University, Penrith, NSW, 2751, Australia; 4Faculty of Medicine, University of Queensland, Brisbane, QLD, Australia; 5Respiratory and Environmental Epidemiology Group, Woolcock Institute of Medical Research, Sydney, NSW, 2037, Australia; 6Sydney Local Health District, Sydney, NSW, 2050, Australia; 7School of Population and Global Health, University of Western Australia, Perth, WA, 6009, Australia; 8Department of Pulmonary Physiology and Sleep Medicine, Sir Charles Gairdner Hospital and Medical School, University of Western Australia, Perth, WA, 6009, Australia; 9Curtin Medical School, Curtin University, Perth, WA, 6102, Australia; 10School of Medicine, University of Tasmania, Hobart, TAS, 7000, Australia; 11Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia; 12School of Clinical Medicine, University of New South Wales, Sydney, NSW, 2052, Australia
Correspondence: Michael J Abramson, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, 3004, Australia, Tel +61 3 9903 0573, Fax +61 3 9903 0556, Email [email protected]
Purpose: Tobacco smoking is the major risk factor for COPD, and it is common for other risk factors in never-smokers to be overlooked. We examined the prevalence of COPD among never-smokers in Australia and identified associated risk factors.
Methods: We used data from the Australia Burden of Obstructive Lung Disease (BOLD) study, a cross-section of people aged ≥ 40 years from six sites. Participants completed interviews and post-bronchodilator spirometry. COPD was primarily defined as an FEV1/FVC ratio < 0.70 and secondarily as the ratio less than the lower limit of normal (LLN).
Results: The prevalence of COPD in the 1656 never-smokers who completed the study was 10.5% (95% CI: 9.1– 12.1%) [ratio
Keywords: non-smokers, prevalence, spirometry, lower limit of normal, LLN, burden of obstructive lung disease, BOLD
Introduction
Ranked third among all causes of death globally, chronic obstructive pulmonary disease (COPD) remains one of the seven non-communicable diseases in the top 10 causes of death.1 In Australia, COPD is the fifth leading cause of mortality with 14.5% of the general population aged 40 years and over found to have COPD in a recent survey.2,3 Like other high-income countries, Australia has an ageing population with approximately 16% of the population aged 65 years and older.4 Tobacco smoking is the most recognisable risk factor. However, with declining rates of smoking and increasing aging of the population, it is important to recognize other important causes of COPD.
Often considered a disease that predominantly affects men, studies have shown that COPD prevalence is increasing in women.5 The convergence of risk factors, especially smoking and occupational exposures, are likely reasons for narrowing of the prevalence gap.5 As women have higher rates of survival, hospital visits and medication use, the role of gender in both COPD and asthma becomes important for health systems particularly for efficient resource utilization and clinical and public health interventions.6
All international guidelines, including the Australian COPD-X plan, highlight smoking and other risk factors as being important for COPD.7 As in other high-income countries, in Australia there has been a significant decline in smoking through a range of public health policies, such as increased taxation, plain packaging with graphic warnings, advertising restrictions and smoking bans. However, there has been limited focus on prevention of COPD among never-smokers through identification of non-smoking risk factors that may inform such prevention. Epidemiological studies have reported that the prevalence of COPD among persons who did not smoke, or were not exposed to smoking (although likely includes second-hand smoking), could range from 3% to 8% or even higher.8–13 Some risk factors identified in these studies include older age, being male, body mass index, asthma, and comorbidities.
It is important to understand the characteristics of never-smokers in an ageing population. The objective of this analysis was to assess, among never-smokers, the prevalence and risk factors for abnormal lung function consistent with COPD, by sex.
Materials and Methods
Study Design and Population
This analysis used data from BOLD-Australia, a multi-site, cross-sectional study of non-institutionalized adults aged 40 years or older.14 Six study centers, representing the sociodemographic and geographical composition of the population, were selected for sampling. Electoral rolls were used to obtain a sex-stratified random sample of participants, in all but one center which used a household census.14 A total of 3522 adults participated in the study providing complete questionnaire data and high-quality post-bronchodilator spirometry measurements. This sub-group analysis focuses on 1656 (47.0%) who self-reported as never-smokers of cigarettes (tobacco). Further details of the Australian BOLD study methodology have been outlined elsewhere.14,15
The BOLD-Australia Study was approved by the Human Research Ethics Committee of the University of Sydney (ref. no. 12-2006/9724) in accordance with the ethical standards of these local committees on human experimentation and with the revised Helsinki Declaration 2000. All participants provided written informed consent.
Measures
Questionnaire
The BOLD study core questionnaire collected information such as sex, age, education, respiratory symptoms (cough, phlegm, wheeze, breathlessness), history of comorbid conditions (heart disease, hypertension, diabetes), family history of respiratory diseases, hospitalization for breathing problems prior to age 10, exposures to second-hand smoke in the home, working in dusty jobs for ≥1 year and occupational exposures.
Anthropometric and Lung Function Measurements
Measurements of weight (kg) and height (cm) were recorded, and body mass index (BMI) calculated. Pre- and post-bronchodilator spirometry was performed according to the joint ATS/ERS guidelines to obtain measurements of the forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio and its associated lower limit of normal (LLN) was calculated using The Global Lung Initiative (version 1.3.4 build 4).16,17
Definitions
- Never-smokers: participants who responded “No” to the question “Have you ever smoked cigarettes?”
- Asthma: participant reported being informed by a doctor or other healthcare provider that they had “asthma, asthmatic bronchitis or allergic bronchitis”.
- SOB on moderate exertion: shortness of breath when hurrying on the level or walking up a slight hill.
- Family history: first-degree relative with a diagnosis of emphysema, chronic bronchitis, or COPD
- BMI: categorised as Low <20, Normal 20≤ BMI <25, or Overweight/Obese ≥25 kg/m2.
- Urbanicity: classified as rural, urban and remote according to participants’ reported postcodes and the Australian Bureau of Statistics (ABS) Statistical Area Level 4 classification.18
- Working in a dusty job: classified as working for more than one year in a dusty job.
- Vapours, Gases and Fumes: classified as working in occupations, 3 months or more, including chemical or plastics manufacturing, firefighting, or welding.
- COPD: defined according to the Global Initiative for Obstructive Lung Disease (GOLD) criteria of a fixed post-bronchodilator ratio of FEV1/FVC <0.70. This was further categorised according to FEV1 as a proportion of the predicted value into severity levels GOLD 1+ (FEV1/FVC <0.70 and any FEV1%predicted) and GOLD 2+ (FEV1/FVC <0.70 and any FEV1%predicted <80%).19 This paper focuses on COPD as non-reversible airway obstruction, GOLD 1+.
- LLN: calculated as below the 5th percentile or lower boundary of the expected ratio of FEV1/FVC. Global Lung Function Initiative data were used to calculate the LLN.
Statistical Analysis
Descriptive statistics for the never-smokers were calculated for demographic, health characteristics and the occupational exposures of participants and to obtain prevalence estimates for the outcomes COPD and ratio<LLN. Sex differences in demographics (age, ethnicity, education, BMI, and geographical location) and health characteristics (respiratory symptoms, history of respiratory conditions, comorbidities, and lung function) and occupational exposures (working in a dusty job for one year or more and vapors, gases, and fumes) were examined using chi-square and t-tests. Associations with COPD were assessed using chi-square tests and odds ratios (OR).
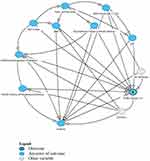
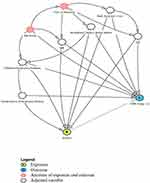
A directed acyclic graph (DAG) was constructed, using the DAGitty.v3.2 software,20 to examine the relationship between the covariates and COPD inclusive of unmeasured exposures of genetics and air pollution (Figure 1). DAGs are diagrams used to help understand the relationships and influences between variables.21 In this analysis, each covariate shown in the DAG, was selected one-at-a-time, and treated as an exposure to generate the minimum adjustment sets necessary for estimating the direct causal effects of risk factors for COPD. Logistic regression was then used to obtain odds ratios (aOR) adjusted for this minimum adjustment set of covariates. Potential covariates included: sex, age, education, BMI, Asthma, childhood breathing problems before age 10, family history of respiratory disease, presence of second-hand smoke in the home, vapors gases and fumes (>3 months exposure) and having worked a year or more in a dusty job. The DAG identified covariates for adjustment (example shown in Figure 2) and the results are presented Table 1 and Supplemental Table S1. This analysis was conducted separately for men and for women (Table 2).
 |
Table 1 Characteristics of Never-Smokers and Adjusted Odds Ratios for Selected Exposure Variables by COPD (GOLD 1+), BOLD Australia |
 |
Table 2 Adjusted and Unadjusted Odds Ratios by Sex for Selected Exposure Variables and COPD Among Never Smokers, BOLD Australia |
The Global Lung Initiative equations (version 1.3.4 build 4) were used to calculate the LLN for FEV1/FVC ratio.17 Level of statistical significance was set at α<0.05, and SPSS (ver25, IBM, Armonk, NY) was used to conduct the analysis on unweighted data.
Results
Demographic Characteristics of Never Smokers
The demographic characteristics of the never-smokers, classified by sex, are shown in Table 3. Most of the participants were Caucasian (90.9%) and had 12 years or less of education (70.5%). Females predominated (57.1%) and were significantly older than males (60 years vs 58 years, p=0.011). The mean BMI was 27.8 [SD=5.0] kg/m2. Over two-thirds (68.3%) of the participants were overweight/obese and significantly more males than females (73.7% vs 64.3%, p<0.001), while more females were underweight (3.5% vs 1.4%, p<0.001). There were significantly more males than females residing in urban areas (45.9% vs 40.6%) and more females in rural areas (45.8% vs 39.8%) (p=0.046). Less than 1 in 7 lived in remote areas.
 |
Table 3 Characteristics of Never-Smokers by Sex, BOLD-Australia Study |
Health and Occupational Exposure Characteristics of Never Smokers
The most prevalent respiratory symptoms were cough (21.4%), shortness of breath (SOB) (23.4%) and wheeze (21.6%) (Table 3). There were no sex differences for wheeze, but more females than males reported usually having a cough (30.8% vs 22.8%, p<0.001) and being SOB on moderate exertion (30.9% vs 13.6%, p<0.001), while more males reported phlegm (16% vs 12.3%, p=0.029).
Twenty-three percent of never-smokers reported a history of some respiratory condition, more commonly among females than males (p<0.001) (Table 3). Asthma (21.5%) and a reported family history of COPD (18.1%) were most frequently reported and significantly more by females than males (p<0.001). No significant differences by sex were observed for those who reported hospitalization due to breathing problems before the age of 10 years.
The most frequently reported occupational exposure was working in a dusty job for one year or more (27.8%), more commonly in males (p<0.001), with similar findings for inorganic dust, organic dust or vapors, gases, and fumes (p<0.001). No sex differences were seen for exposure to second-hand smoke within the home.
The prevalence of COPD was 10.5% (95% CI: 9.1–12.1%) when defined as FEV1/FVC ratio <0.70 and 4.6% (3.7–5.8%) when defined as the ratio < LLN. There were no significant sex differences observed for either definition (Table 3). Doctor diagnosed COPD was reported by 4.1% of participants and significantly more females than males reported a diagnosis (5.5% vs 2.3%, p<0.001). Out of 212 pairs (13%) where people had different COPD diagnoses (spirometry vs self-reported doctor diagnoses), 75% with FEV1/FVC ratio < 0.70 did not report a doctor’s diagnosis of COPD while 25% with FEV1/FVC ratio > 0.70 (ie with spirometric evidence of no COPD) did report doctor diagnosed COPD (McNemar’s test, p=0.002).
Lung Function Profiles
Overall Profile – All Persons
Never-smokers with COPD defined as FEV1/FVC ratio <0.70 were significantly older than those without COPD [mean age 68 (SD 11) vs mean age 58 (11 years), p<0.001].
In Table 1, we examined exposure variables to identify significant uOR and aORs overall and by sex. As age increased the odds of COPD increased 4-fold among those 60–69 and nearly 9-fold for those 70 and older compared to those aged 40–49 years, after controlling for covariates listed in Table 1 (aOR=4.11 and 8.73, respectively) (Table 1). Those with a family history of respiratory disease (aOR = 1.51) and who experienced hospitalization due to breathing problems before the age of 10 years (aOR=2.70) were at increased risk of COPD, compared to those without. Controlling for the covariates did not substantially alter (<10% increase) the odds (Table 1).
Among never-smokers with more than 12 years of education, the odds of COPD were 43% lower (uOR = 0.57) than for those with less education, but the aOR was not significant after adjusting for the covariates (Table 1). The odds of COPD among overweight/obese never-smokers were 36% lower (aOR=0.64) compared to those with normal weight, after adjusting for the relevant covariates (see Table 1). No associations were observed for second-hand smoke in the home, nor for occupational exposures.
COPD- Profiles for Males and Females
When the data were examined by sex (Table 2), males who never smoked had a near 6-fold increase in odds of COPD among those aged 60–69 years and an 11-fold increase among those aged 70 years and older (aOR=5.88 and 11.05, respectively) compared to the 40–49-year age group, after controlling for covariates. Among male never-smokers, after controlling for covariates, the odds of COPD also increased among those with asthma (aOR=2.45), a family history of respiratory disease (aOR=2.15), and a history of hospitalization due to breathing problems prior to age 10 years (aOR=2.67). There were no observed associations for BMI, years of education, exposure to second-hand smoke and working in a dusty job for more than a year.
For females who never smoked, the odds of COPD increased for those aged 60–69 years (aOR=3.32) and 70 years and over (aOR=7.87) compared with those aged 40–49 years There was no substantial change in the odds after controlling for the covariates in Table 2. The odds of COPD also increased among female never-smokers with asthma (aOR=1.85) and those with history of hospitalization due to breathing problems prior to age 10 (aOR=2.49), after controlling for covariates. There were no observed associations for BMI or a family history of respiratory disease.
Ratio FEV1/FVC < LLN Profile
Odds ratios are presented in Supplemental Table S1 for selected independent variables of ratio <LLN1+, after controlling for the potential confounding effects identified by the DAGs. Compared to never-smokers aged 40–49 years, the risk of ratio <LLN1+ increased 2-fold among those aged ≥70 years, after adjusting for relevant covariates. Increased odds of COPD remained among those with asthma (aOR=5.00) and breathing problems before the age of 10 years (aOR=4.52) after adjusting for covariates. There were no observed associations for BMI, years of education, family history of respiratory disease, and exposure to second-hand smoke in the home, working in a dusty job, or vapors, gases, and fumes.
Discussion
In this study of Australian never-smokers aged 40 years and older, the prevalence of mild to very severe airflow limitation was observed to be 10.5%, as defined by COPD GOLD 1+, and 4.6% when based on the lower limit of normal. A substantial proportion of never-smokers were either misdiagnosed or underdiagnosed by their physicians for COPD. The analysis identified several independent predictors of COPD. These included older age, fewer years of education, a history of asthma, a family history of respiratory disease, and experiencing hospitalization due to breathing difficulties before the age of 10 years. Notably, the predictors common to both males and females were older age, asthma, and a history of hospitalization for breathing difficulties before age 10. Family history of respiratory disease was a further predictor of COPD in men but overweight/obesity in men presented as being protective.
In the main BOLD-Australia study approximately one in every three of those with COPD (33%) had never smoked, and this finding was consistent with other studies, yet global estimates for COPD indicate 22–51% were never smokers.22–24 In this sub-analysis, the 10.5% prevalence of mild to severe COPD (GOLD Stage 1+) observed among never-smokers was approximately half that of ever-smokers (21.1%). In contrast to never smokers, Toelle et al reported a 14.5% weighted prevalence of COPD (smokers and never ever smokers) among the general Australian population.3 In general, the prevalence of COPD among never-smokers in our study was relatively consistent with a reported prevalence of 12.2% for 14 BOLD country sites and within a wide range of 1.1% to 40% in other reports.8,9,24–26 The variability observed across these studies may be explained by the different methods and definitions used for COPD, socioeconomic status, demographics, or tobacco control measures.
Although COPD is a leading cause of mortality and morbidity globally, it is often underdiagnosed.27–29 This underdiagnosis varied from 10% to 95% across studies. A study that utilized data from four large population-based surveys, including BOLD-International, reported that 81.4% of spirometry-defined COPD cases were under-diagnosed.30 Our study had similar findings, with a high rate of underdiagnosis in never-smokers; three in four spirometry-confirmed COPD patients. This could be related to the low use of spirometry by health providers, poor awareness of COPD symptoms among patients, or a low suspicion of the disease, as these were never-smokers. The greater number of females with physician-diagnosed COPD could be because they are more likely to seek medical attention than men, although the higher count of older women compared to men might indicate that women sought medical care when the disease was more advanced.31
Studies of respiratory symptoms in never-smokers have shown that they are less likely to have common COPD symptoms such as dyspnea and wheezing and a lower prevalence of cough and sputum than smokers.8,24 In our analysis of only never-smokers, we found that the symptoms associated with COPD were wheezing in the previous 12 months and dyspnea. Similar to the Austrian BOLD study, never-smokers were mostly older females.13 We found that respiratory conditions, such as chronic bronchitis and asthma, were more commonly reported by females and were also associated with COPD in never-smokers. Chronic bronchitis in the presence of exacerbations and asthma overlapping with COPD are considered clinical phenotypes of COPD in smokers.32 However, this disease overlap was not as clear in never-smokers. Similar to other studies, we also report a few cases of chronic bronchitis among never-smokers.22,24 Furthermore, more than one in three with COPD, post-bronchodilator, had self-reported asthma.
Salvi and Barnes showed that a low socioeconomic status (SES) was associated with COPD and proposed it as a risk factor.23 The present analysis showed that people with less education, as a proxy for low SES, were more likely to have COPD. This association between low SES and disease is consistent with unhealthy lifestyle practices, higher environmental and occupational exposures, and biological risk factors, resulting in reduced lung function.9,19 COPD is also known to develop over a long period of time, typically manifesting later in life at 40 years or older. Our study showed that COPD increased with age in both males and females, and age ≥60 years was a strong predictor of COPD. Disease manifestation is mainly due to the cumulative effect of airway injuries early in life and the natural decline in lung function due to aging. This indicates that the presence of COPD in never-smokers could also be linked to different lung function trajectories that could be observed at an early stage in life.33
Early lung damage or respiratory conditions during childhood contribute to the development of COPD in adulthood. In our study, early childhood breathing problems were associated with an increased risk of COPD in both sexes. Adverse events manifesting in childhood (eg, viral infections, pneumonia, bronchitis) could lead to the development of COPD later in life.34 A further complication is the age-related decline in lung function. This highlights the importance of establishing baseline peak lung function, usually achieved in the early twenties, as a risk marker for COPD in the future. Our study also found that a family history of first-degree relatives with a diagnosis of emphysema, chronic bronchitis, or COPD was an independent predictor of COPD in never-smokers. Previous studies reporting family history yielded mixed results,10,35 and the Tunisian BOLD study found no associations.10,12,35 There was also a strong association between COPD and family history in men in our study, but none was detected in women. The association in men could have an epigenetic basis with family history being a proxy for the interaction between genetic predisposition and environmental exposures.36 Since genetic factors are known to play a role in the development of COPD, it is likely that the predisposition for COPD could be more prominent or that the interaction with the environment is greater in men than in women.
An inherent characteristic of the sample was the BMI profile of the participants. In this study, overweight/obesity appeared to reduce the risk of COPD, particularly among men. However, obesity is recognized widely as a known risk factor for many other chronic conditions. Nonetheless, our findings align with other research studies, underscoring overweight/obesity can be protective although BMI is associated with lung function decline.37,38 This paradox may require further research since the observed association could be due to the complex relationship between BMI and lung vital capacity. Vital capacity is known to decrease with age which would further be compounded by the added weight from being overweight/obese, reducing lung compliance.
A strength of this study is the large sample size of never-smokers provided robust findings, and the use of quality-controlled spirometry served to objectively define COPD, aligning with the aim to establish comparable statistics with other studies. However, some limitations should be acknowledged. The cross-sectional design of the study limits our ability to infer causation. The significance of some associations might not have persisted following the analysis stratified by socioeconomic status due to insufficient statistical power. Most risk factors, like occupational exposures, relied on self-report, introducing potential recall bias. While we also presented COPD as defined by the lower limit of normal, it should be noted that some misclassification could be present, given the greater use of the GOLD criteria up to now. Notably, GOLD estimates might potentially overestimate COPD due to the inclusion of mild airflow limitation, particularly among older age groups.
Conclusion
The observed prevalence of COPD among never-smokers was greater than expected. These findings underscore the complex interplay of diverse factors in the development of COPD among never-smokers. Despite generally healthier status compared to smokers, never-smokers account for a notable proportion of COPD cases across a range of severity among older individuals. The study highlights that epigenetic effects are likely at work, particularly in cases of family history of respiratory disease and childhood respiratory issues, amplifying COPD risk. Further exploration of these aspects remains pivotal. Routine screening by establishing baseline lung function in early twenties, followed by periodic measurements for monitoring changes should be implemented. Such an approach would aid early detection and prevention strategies.
Acknowledgments
The authors acknowledge investigators from our BOLD-Australia study-sites who are not co-authors on this paper: Christine Jenkins, David Atkinson, Haydn Walters, Deborah Burton. We thank the research staff and participants at each study site for their important contribution. In particular, the following site members, Busselton: Bill Musk (deceased), Elspeth Inglis and Peta Grayson; Broome/Kimberly: David Reeve, Matthew Yap, Mary Lane, Nathania Cooksley, Sally Young and Wendy Cavilla; Melbourne: Angela Lewis, Joan Raven and Joan Green; Rural New South Wales: Phillipa Southwell, Melanie Heine, Cassanne Eccleston, Julie Cooke, Bruce Graham, Brian Spurrell and Robyn Paton; Sydney: Tessa Bird, Wei Xuan, Kate Hardaker and Paola Espinel; Tasmania: Carol Phillips and Loren Taylor. We also acknowledge the support provided to Graeme Maguire and Guy Marks by NHMRC Practitioner Fellowships and to Graeme Maguire by the Margaret Ross Chair in Indigenous Health.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the National Health and Medical Research Council (NHMRC) of Australia, grant number 457385 and supported by the Thoracic Society of Australia and New Zealand, The Robert Pierce Grant-in-Aid for Indigenous Lung Health (2010); Air Liquide, AstraZeneca; GlaxoSmithKline; and Boehringer Ingelheim (2005).
Disclosure
Guy B Marks has provided independent medical service on an advisory board for Astra Zeneca. Michael J Abramson holds investigator-initiated grants for unrelated research from Pfizer, GSK, Boehringer-Ingelheim and Sanofi and he has also conducted an unrelated consultancy for Sanofi. He has also received a speaker’s fee from GSK. The other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
The other authors declare no conflicts of interest.
References
1. WHO Global Health Estimates. The top 10 causes of death. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2. Australian Institute of Health and Welfare. Deaths in Australia Australian institute of health and welfare. Available from: https://www.aihw.gov.au/reports/life-expectancy-death/deaths/data.
3. Toelle BG, Ampon RD, Abramson MJ, et al. Prevalence of chronic obstructive pulmonary disease with breathlessness in Australia: weighted using the 2016 Australian census. Internal Med J. 2021;51(5):784–787. doi:10.1111/imj.15325
4. Australian Bureau of Statistics. Twenty years of population change (feature article); 2019. Available from: https://www.abs.gov.au/ausstats/[email protected]/0/1cd2b1952afc5e7aca257298000f2e76.
5. Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176(12):1179–1184. doi:10.1164/rccm.200704-553CC
6. Lisspers K, Larsson K, Janson C, et al. Gender differences among Swedish COPD patients: results from the arctic, a real-world retrospective cohort study. NPJ Prim Care Respir Med. 2019;29(1):1–8. doi:10.1038/s41533-019-0157-3
7. Yang IA, Brown JL, George J, et al. COPD-X Australian and New Zealand guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 2017 update. Med J Aust. 2017;207(10):436–442. doi:10.5694/mja17.00686
8. Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers. Chest. 2011;139(4):752–763. doi:10.1378/chest.10-1253
9. Lee SH, Hwang ED, Lim JE, et al. The risk factors and characteristics of COPD among nonsmokers in Korea: an analysis of KNHANES IV and V. Lung. 2016;194(3):353–361. doi:10.1007/s00408-016-9871-6
10. Hagstad S, Ekerljung L, Lindberg A, Backman H, Rönmark E, Lundbäck B. COPD among non-smokers – report from the Obstructive Lung Disease in Northern Sweden (OLIN) studies. Respir Med. 2012;106(7):980–988. doi:10.1016/j.rmed.2012.03.010
11. Terzikhan N, Verhamme KMC, Hofman A, Stricker BH, Brusselle GG, Lahousse L. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam study. Eur J Epidemiol. 2016;31(8):785–792. doi:10.1007/s10654-016-0132-z
12. Denguezli M, Daldoul H, Harrabi I, et al. COPD in nonsmokers: reports from the Tunisian population-based burden of obstructive lung disease study Behrens T, ed. PLoS One. 2016;11(3):e0151981. doi:10.1371/journal.pone.0151981
13. Lamprecht B, Schirnhofer L, Kaiser B, Buist S, Studnicka M. Non-reversible airway obstruction in never smokers: results from the Austrian BOLD study. Respir Med. 2008;102(12):1833–1838. doi:10.1016/j.rmed.2008.07.007
14. Toelle BG, Xuan W, Bird TE, et al. Respiratory symptoms and illness in older Australians: the Burden of Obstructive Lung Disease (BOLD) study. Med J Aust. 2013;198(3):144–148. doi:10.5694/mja11.11640
15. Buist AS, Vollmer WM, Sullivan SD, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD. 2005;2(2):277–283. doi:10.1081/COPD-57610
16. Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
17. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312
18. Australian Bureau of Statistics. Australian Statistical Geography Standard (ASGS): Volume 1. Main structure and greater capital city statistical areas; 2016.
19. GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2021 report. GOLD. 2021;176:532–55.
20. Textor J, Van Der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH. Robust causal inference using directed acyclic graphs: the R package ‘dagitty. Int J Epidemiol. 2017;dyw341. doi:10.1093/ije/dyw341
21. Lederer DJ, Bell SC, Branson RD, et al.; Control of Confounding and Reporting of Results in Causal Inference Studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann ATS. 2019;16(1):22–28. doi:10.1513/AnnalsATS.201808-564PS
22. Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax. 2015;70(9):822–829. doi:10.1136/thoraxjnl-2015-206938
23. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:9691):733–743. doi:10.1016/S0140-6736(09)61303-9
24. Yang IA, Jenkins CR, Salvi SS. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. 2022;10(5):497–511. doi:10.1016/S2213-2600(21)00506-3
25. Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128(3):1239–1244. doi:10.1378/chest.128.3.1239
26. Bang KM. Chronic obstructive pulmonary disease in nonsmokers by occupation and exposure: a brief review. Curr Opin Pulm Med. 2015;21(2):149–154. doi:10.1097/MCP.0000000000000135
27. Diab N, Gershon AS, Sin DD, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(9):1130–1139. doi:10.1164/rccm.201804-0621CI
28. Ho T, Cusack RP, Chaudhary N, Satia I, Kurmi OP. Under- and over-diagnosis of COPD: a global perspective. Breathe. 2019;15(1):24–35. doi:10.1183/20734735.0346-2018
29. Petrie K, Toelle BG, Wood-Baker R, et al. Undiagnosed and misdiagnosed chronic obstructive pulmonary disease: data from the BOLD Australia study. COPD. 2021;16:467–475. doi:10.2147/COPD.S287172
30. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148(4):971–985. doi:10.1378/chest.14-2535
31. Gut-Gobert C, Cavaillès A, Dixmier A, et al. Women and COPD: do we need more evidence? Eur Respir Rev. 2019;28(151):180055. doi:10.1183/16000617.0055-2018
32. Miravitlles M, Soler-Cataluña JJ, Calle M, Soriano JB. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur Respir J. 2013;41(6):1252–1256. doi:10.1183/09031936.00118912
33. Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6(7):535–544. doi:10.1016/S2213-2600(18)30100-0
34. Duan P, Wang Y, Lin R, et al. Impact of early life exposures on COPD in adulthood: a systematic review and meta-analysis. Respirology. 2021;26(12):1131–1151. doi:10.1111/resp.14144
35. Hagstad S, Backman H, Bjerg A, et al. Prevalence and risk factors of COPD among never-smokers in two areas of Sweden - Occupational exposure to gas, dust or fumes is an important risk factor. Respir Med. 2015;109(11):1439–1445. doi:10.1016/j.rmed.2015.09.012
36. Benincasa G, DeMeo DL, Glass K, Silverman EK, Napoli C. Epigenetics and pulmonary diseases in the horizon of precision medicine: a review. Eur Respir J. 2021;57(6):2003406. doi:10.1183/13993003.03406-2020
37. Park HJ, Cho JH, Kim HJ, Park JY, Lee HS, Byun MK. The effect of low body mass index on the development of chronic obstructive pulmonary disease and mortality. J Internal Med. 2019;286(5):573–582. doi:10.1111/joim.12949
38. Sun Y, Milne S, Jaw JE, et al. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. 2019;20(1):236. doi:10.1186/s12931-019-1209-5
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.